α和γ晶型尼龙6的可控合成
2016-03-09黄映珊姜锋李杰彭舒敏任茜
黄映珊++姜锋++李杰++彭舒敏++任茜++罗玉航++易春旺����


摘 要 报导了可控晶型尼龙6的制备与表征.DSC结果显示γ晶型在Tm1为210 ℃左右出现一个尖锐的熔融峰,同时在Tm2为219 ℃左右出现一个小肩峰.当温度高于70 ℃,反应时间大于8 h时可以制备稳定的α晶型,α晶型显示双熔融峰.FTIR结果进一步表明所获得的样品为γ晶型、α晶型和混晶.SEM结果显示γ晶型表面是由孔径大小不一样的小孔组成,α晶型表面是由不同厚度的片晶堆积而成.
关键词 PA6;α晶型;γ晶型;可控晶型
Poly(ecaprolactam)s, known as polyamide6 (PA6) or nylon 6, is a kind of typical polycrystalline or semicrystalline polymers with the main chain containing amide groups[12]. There are strong hydrogen bonds among adjacent polymer chains in both crystalline and amorphous regions, which is the determining factor that nylon6 have good chemical stability and mechanical properties. Because of this, the structure and morphology of nylon6 have been of widespread study and use in plastics and synthetic fiber industry [38]. Currently, αphase and γphase structures are widely recognized as major crystalline nylon6[912]. The αphase, which was first characterized by Holmes[13], has a monoclinic structure, with hydrogen bonds formed between molecular chains in parallel arrangement[9,14]. It is generally believed that the αphase structure is thermodynamically more stable and can be observed by solution crystallization, annealing, crystallization at high temperatures, or slow cooling of nylon6 melt. In addition, the γphase structure is most commonly observed by melt rapid cooling, high speed spinning, phosphoric acidammonia solution steam precipitation method and treating in aqueous potassium iodideiodine solution[1521]. The γphase also has monoclinic structure that arranges in parallel between the molecular chains in the (200) plane to form hydrogen bonds and twists at an angle between the amide group and carbonitride molecular chain backbone methylene[2224]. Changes of conditions such as heat, pressure, or solvent can transform the γphase into the αphase. Based on the understanding from the current literature[2530], there are many ways to prepare a single crystal form, but these methods can only be suitable for the preparation of the αphase or only for forming the γphase. For example, nylon6 powders precipitated from a formic acid solution trend to form a highly crystalline αphase, without the trait of the γphase. Henceforth, a highly efficient and controllable method for the preparation of both α and γ phases of nylon6 is desirable.
In this paper, by using the phosphoric acidammonia solution method, we prepared αphase and γphase structures through adjusting the preparation temperature and duration time, and then the crystalline structures and melting behavior of nylon6 are studied carefully by Xray diffraction (XRD) and differential scanning calorimetry (DSC). Our results showed that this method is not only highly efficient, facile and greatly reducing the reaction time, but also suitable for the controllable formation of single crystal phases.
1 Experimental Section
1.1 Materials
Nylon 6 pellets with a relative viscosity of 2.50 were obtained from Guangdong Xinhui Meida Nylon Co.,Ltd (Guangdong, China); Phosphate acid(A.R.) were purchased from Beijing Yili Fine Chemicals Co.,Ltd (Beijing, China); Ammonia solution(A.R.) were purchased from Sinopharm Chemical Reagent Co.,Ltd. All other agents used in this study are commercially available.
1.2 Preparation of the α and γ phases of nylon6
Firstly, a solution of phosphate acid was added to nylon6 pellets to form the concentration of 6% clear solution, which was placed in a digital electric heated water bath maintained at 80 ℃ for 2 days. Then 15 g Nylon6/phosphate acid solution was shifted in a 50 mL beaker with a sufficient amount of ammonia solution added into a 100 mL beaker. The two beakers were put in a sealed container. Finally the sealed container was placed in a water bath, where the vapor of the ammonia solution diffuses into the nylon6/phosphate acid solution and induces the expected crystallization of nylon6 through controlling the water temperature and diffusing time. The precipitate was filtered, washed with ammonia solution and distilled by water three times. Then the product was ovendried in vacuo at 50 ℃ for 24 h.
1.3 Characterization of the α and γ phases of nylon6
XRD experiments were carried out on a PANalytical XPert Pro MPD Xray diffraction with nickelfiltered CuKα radiation (λ=1.54 ) at 45 kV and 40 mA. Xray scanning was collected in the range 5°<2θ<50° with a scan rate at 2° (2θ)/min. DSC measurements of the α and γ phases of nylon6 samples were performed on a DSC 8000 Instrument. The samples were heated from room temperature to 240 ℃ at a constant heating rate of 20 ℃/min under a nitrogen atmosphere. FTIR analysis was conducted on a Nicolet Magna IS10 spectrometer at a resolution of 4 cm-1 and 32 cm-1 scans. The surface morphology was examined by a scanning electron microscopy (JSM 6306).
2 Results and Discussion
2.1 Crystal structures of the samples
For the α phase structure, its characteristic peaks appear at about 20° and 24°, corresponding to (200) and (002)/(202) reflections. The characteristic peaks of the γ phase are assigned at about 10° and 21.5° and is attributed to (001) reflections[30].
Figures 1~6 display the XRD patterns of the prepared α and γ phases of nylon6 samples. As are shown in Figs. 1 and 2, it is clear that the crystal form is mainly γphase crystals of nylon6, with two diffraction peaks at about 10.9° and 21.6°, which can be assigned to the (001) reflections of γphase[30]. We do not see any notable diffraction peaks at 20.0° and 23.6°, which are allocated to the (200) and (002)/(202) reflections of the αphase. The XRD patterns at 60 ℃ shown in Fig.3 indicate that there are two distinctive peaks at 20.0° and 23.6°, with a diffraction peak at 21.6° with a shoulder at 10.9°, implying that there is the coexistence of both αphase and γphase structures at 60 ℃. With an increasing preparation time, the intensity of αphase diffraction characteristic peaks gradually increases, while the diffraction peaks characteristic of γphase the gradually decreases. The longer the preparation time was, the more the content of αphase formed. As shown in Fig.4, the peaks of αphase diffraction peaks become smaller than those of γphase diffraction at 70 ℃ for 6 h. At 70 ℃ and longer than 8 h, γphase trends to be transformed to αphase, and, as a result, the diffraction peaks of γphase are disappeared. From Fig.5, the intensity of αphase diffraction peaks are stronger than that of γphase with 80 ℃ and 6h. It can be seen that there was only αphase at 80 ℃ above 8h.The XRD patterns from 90 ℃ at different reaction times are shown in Fig.6. From the Figure, we can see that γphase is almost all transformed into αphase at 90 ℃.
The presence of crystals form as a function of the preparation temperature and reaction duration time is summarized in Tab.1. It has been well known that, in general, temperature is one of the most important factors affecting the crystal formation of nylon 6. The γphase structure could be obtained when the temperature is below 60 ℃ and αphase is formed when temperature is over 70 ℃. Under the same reaction time, γphase is more likely to be transformed into αphase as temperature increases, suggesting that αphase is thermodynamically more stable. Tab.1 also reveals that the reaction time plays a vastly important role during the course of crystal formation. For example, both α and γphases are formed in 6h at temperatures of 70 ℃ and 80 ℃. However, by elongating the preparation time to 8 h, only αphase is left over.
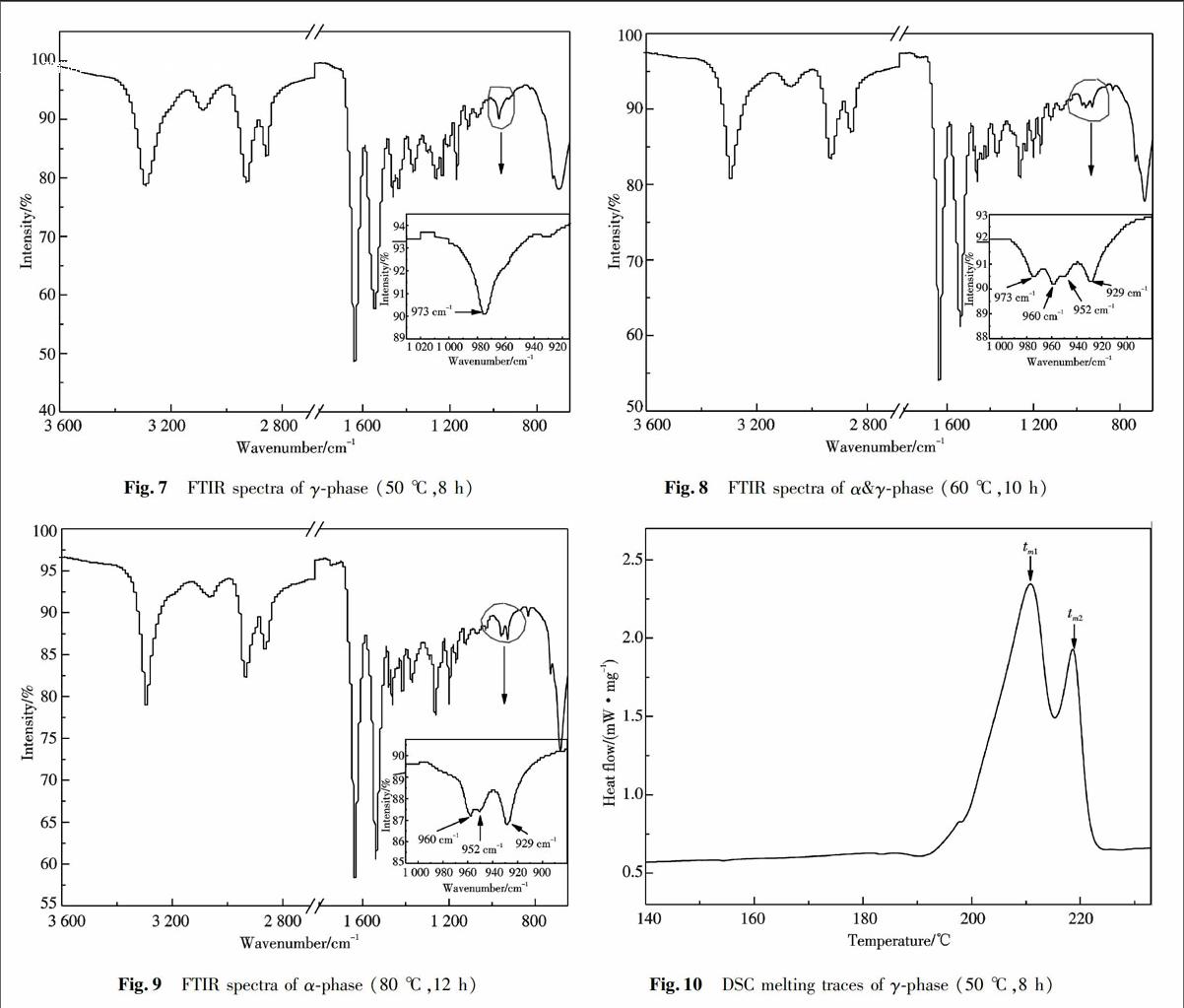
FTIR spectra can be used to distinguish between γ and α phase. Further study using FTIR was conducted for γphase (50 ℃,8 h),α & γphase (60 ℃,10 h) and αphase (80 ℃,12 h).
As are apparent in the FTIR spectra presented in Figs.(7~9), it can be clearly seen that there exist significant differences of characteristic absorption peaks for each crystalline phase of nylon6 between 900 and 1 050 cm-1, which is the most important range for the popular marker bands of α and γphases. The spectra for the single crystalline phase prepared at different conditions are significantly similar, so only one of them was selected to show in above Figures. The corresponding areas where characteristic peaks are located are marked by a red circle and magnified in the lower right corner of the Figure. Taking Fig.7 as an example, we find one forced marker band at 973 cm-1 that belongs to the γphase. Nevertheless, three strong marker bands at 929, 952 and 960 cm-1 are attributed to αphase (see also Fig.9). Some similar marker bands were found as the evidence of the coexistence of both α & γ phases shown in Fig.8, where the bands were found at 929, 952, 960 cm-1 and 973 cm-1 for the αphase and γphase, respectively[19,31]. These FTIR results confirm that the formation of single α and γphases could be controlled by adjusting preparation temperature and time.
Fig.7 FTIR spectra of γphase (50 ℃,8 h) Fig.8 FTIR spectra of α&γphase (60 ℃,10 h)
Fig.9 FTIR spectra of αphase (80 ℃,12 h) Fig.10 DSC melting traces of γphase (50 ℃,8 h)
2.2 Melting behavior of samples
The thermal behavior of γphase (50 ℃,8 h)、α & γphase (60 ℃,10 h) and αphase (80 ℃,12 h) were further investigated using DSC. This is an clearcut to distinguish between γ and α phase structures.
The DSC analysis was treated like FTIR with only one representative curve for each type of crystalline phase shown in the Figures. The DSC heating curve of γphase (50 ℃,8 h) in Fig.10 shows that there is only one main melting peak at about 210 ℃ (tm1), with one shoulder melting peak at about 219 ℃ (tm2). According to the literature[30,3235], the reason for these two melting peaks of γphase can be interpreted as (1) the coexistence of the αphase and γphase and (2) a crystal with different degrees of perfection. However, as can be seen from the results of XRD and FTIR, the sample of nylon6 prepared at 50 ℃ for 8 h only exhibits characteristic diffraction peaks of γphase in Figs.2 and 7. Figures 1~6 and Tab.1 clearly demonstrate the impact of temperature on the transformation of nylon6 crystal phases and a good agreement has been obtained with the experimental findings. That is, part of the γphase was irreversibly converted to the αphase at 60 ℃ for 6 h. In addition, according to the literature report, the transformation from γphase to αphase should be below the melting temperature[28,38]. Therefore, at a scan rate of 20 ℃/min, the instability of γphase might lead to a partial irreversible conversion of γphase into αphase as temperature increases. Based on this analysis, we suggest that tm1(210.8 ℃) can be attributed to the melting point of γphase(50 ℃,8 h) and tm2(219 ℃) is the melting peak of αphase. Furthermore, the DSC heating curve of α & γphase (60 ℃,10 h) in Fig.11 manifested the existence of double melting peaks at 209 ℃(tm1)and 216 ℃(tm2). The difference between the two melting peaks was only 10 ℃ so the two melting peaks overlap with each other. As shown by the XRD pattern of this sample obtained at 60 ℃,10 h in Fig.4, it has been known that there are two distinctive peaks at 20.0° and 23.6°, and a diffraction peak at 21.6° with a shoulder at 10.9°, indicating that αphase and γphase were coexisted at 70 ℃ for 6 h. The DSC heating cure of αphase (80 ℃,12 h) is shown in Fig.12, with the main melting peak at 217.5 ℃(tm1)coming from αphase, which is consistent with the literature report[2930]. Because no obvious γphase characteristic diffraction peaks at about 10.9° and 21.6° were observed in Fig.5, the shoulder melting peak at 202.2 ℃ (tm2) might be contributed to αphase with different thickness of the crystalline. These results further confirm that the obtained αphase is thermodynamically more stable and the transformation from γ to α occurs below the melting point.
Fig.11 DSC melting traces of α&γphase (60 ℃,10 h) Fig.12 DSC melting traces of αphase (80 ℃,12 h)
2.3 Morphological Characterization
SEM experiments was also carried out for the same samples, γphase (50 ℃,8 h), α&γphase (60 ℃,10 h) and αphase (80 ℃,12 h).
Fig.13 SEM images of γphase(a and b), α&γphase(c and d) and αphase(e and f) with various morphologies
The morphology of α and γ phases of nylon 6 samples are examined by SEM, whose images are shown in Fig13. From these SEM images, it is clear that γphase is composed of many holes with different diameters. These holes are stacked assembly with very smooth surface (shown in Fig.13 (a and b)). Fig.13 (c and d) present the coexistence morphology of α & γphase. It is interesting that not all holes with different diameters can clearly be seen in those images and a portion of the irregular lamellae with stack assembly also can be observed in them. Fig.13(e and f) are SEM images of αphase, which are irregular lamellae with stack assemble. These results suggest that the morphology of αphase and γphase are markedly different, with the appearance of γphase vesicular and αphase as the accumulation of irregular lamellae.
3 Conclusions
Based on the results obtained from this work, we presented a highly efficient and facile method for the preparation of α and γ phases of nylon6. By using this controllable formation, single α and γ phases of nylon6 could be prepared. Based on the information from XRD, FTIR and DSC analysis, the temperature is one of the most important factors affecting the crystal formation of nylon 6. The γphase was obtained below 60 ℃ by the vapor of the ammonia solution diffuses into nylon6/phosphate acid solution, which can be transformed to αphase above 60 ℃. The coexistence of the αphase and γphase was found at 60 ℃ and 70 ℃ by extending the preparation duration time. With the time increased, the content of the αphase gradually increased, while the content of the γphase gradually reduced. Finally, all γphase could be transformed into αphase as long as the preparation time is long enough. Besides, at a certain temperature, the higher the preparation temperature, the shorter the transition time. As for αphase, it is more thermalstable and could be formed above 70 ℃ for 8 h. From the images of SEM, the γphase is composed of holes with many different sizes, but the αphase is the accumulation of irregular lamellae. These morphology features are important in the sense that the pore structure of γphase could facilitate the penetration of colorings in nylon6 and improve dying properties. In the study ensued, we will focus on the impact of αand γphase structures on the dying.
References:
[1] LI Y, GODDARD W A. Nylon 6 crystal structures, folds, and lamellae from theory[J]. Macromolecules, 2002,35(22):84408455.
[2] PENELPIERRON L, DEPECKER C, SEGUELA R, et al. Structural and mechanical behavior of nylon 6 films part I. Identification and stability of the crystalline phases[J]. J Polym Sci Part B: Polym Phys, 2001,39(5):484495.
[3] LIU T X, LIU Z H, MA K X, et al. Morphology, thermal and mechanical behavior of polyamide 6/layeredsilicate nanocomposites[J]. Compos Sci Technol, 2003,63(3):331337.
[4] DASGUPTA S, HAMMOND W B, GODDARD W A. Crystal structures and properties of nylon polymers from theory[J]. J Am Chem Soc, 1996,118(49):1229112301.
[5] ABUISA I. αγ transition in nylon 6[J]. J Polym Sci Part A: Polym Chem, 1971,9(1):199216.
[6] HATFIELD G R, GLANS J H, HAMMOND W B. Characterization of structure and morphology in nylon 6 by solidstate carbon13 and nitrogen15 NMR[J]. Macromolecules, 1990,23(6):16541658.
[7] WEI M, DAVIS W, URBAN B, et al. Manipulation of Nylon6 crystal structures with its α Cyclodextrin inclusion complex[J]. Macromolecules, 2002,35(21): 80398044.
[8] MURTHY N S. Hydrogen bonding, mobility, and structural transitions in aliphatic polyamides[J]. J Polym Sci Part B: Polym Phys, 2006,44(13):17631782.
[9] HOLMES D R, BUNN C W, SMITH D J. The crystal structure of polycaproamide: Nylon 6[J]. J Polym Sci, 1955,17(84):159177.
[10] ARIMOTO H. αγ Transition of nylon 6[J]. J Polym Sci Part A: Polym Chem, 1964,2(5):22832295.
[11] ARIMOTO H, ISHIBASHI M, HIRAI M, et al. Crystal structure of the γform of nylon 6[J]. J Polym Sci Part A: Polym Chem, 1965,3(1):317326.
[12] MURTHY N S, BRAY R G, CORREALE S T, et al. Drawing and annealing of nylon6 fibres: studies of crystal growth, orientation of amorphous and crystalline domains and their influence on properties[J]. Polymer, 1995,36(20):38633873.
[13] BRILL R. On relations between the structure of polyamids and of silk fibroin[J]. Phys Chem B, 1943,53(2):6174.
[14] WALLNER L G. Uber den einfluss der kristallitlange auf die rontgeninterferenzen der polyamide[J]. Monatsh Chem, 1948,79(34):279295.
[15] MURTHY N S, AHARONI S M, SZOLLOSI A B. Stability of the γ form and the development of the α form in nylon 6[J]. J Polym Sci Part B: Polym Phys, 1985,23(12):25492565.
[16] WANG X, HOU W, ZHOU J, et al. Melting behavior of lamellae of isotactic polypropylene studied using hotstage atomic force microscopy[J]. Colloid Polym Sci, 2007,285(4):449455.
[17] MIYASAKA K, et al. Effects of temperature and water on the γ→ α crystalline transition of nylon 6 caused by stretching in the chain direction[J]. J Polym Sci Part A2: Polym Phys, 1968,6(7):13171329.
[18] MURTHY N S, WANG Z G, HISAO B S. Interactions between crystalline and amorphous domains in semicrystalline polymers: smallangle Xray scattering studies of the Brill transition in nylon 6, 6[J]. Macromolecules, 1999,32(17):55945599.
[19] QUARTI C, MILANI A, CIVALLERI B, et al. Ab initio calculation of the crystalline structure and IR spectrum of polymers: nylon 6 polymorphs[J]. J Phys Chem B, 2012,116(28):82998311.
[20] HABERKORN H, HAHN K, BREUER H, et al. On the neck‐like deformation in high‐speed spun polyamides[J]. J Appl Polym Sci, 1993,47(9):15511579.
[21] RAMESH C, GOWD E B. Hightemperature Xray diffraction studies on the crystalline transitions in the α and γ forms of nylon6[J]. Macromolecules, 2001,34(10):33083313.
[22] TSURUTA M, ARIMOTO H, ISHIBASHI M. The appearance of the new crystal structures in nylon 6[J]. Kobunshi Kagaku, 1958,15(162):619627.
[23] BALDRIAN J. Structural changes in α → γ phase transformations of polycaprolactam[J]. Cechoslovackij Fiziceskij Zurnal B, 1965,15(11):838847.
[24] KINOSHITA Y. An investigation of the structures of polyamide series[J]. Makromolekulare Chemie, 1959,33(1):120.
[25] MURTHY N S, CURRAN S A, AHARONI S M, et al. Premelting crystalline relaxations and phase transitions in nylon 6 and 6, 6[J]. Macromolecules, 1991,24(11):32153220.
[26] MURTHY N S. Structure of iodide ions in iodinated nylon 6 and the evolution of hydrogen bonds between parallel chains in nylon 6[J]. Macromolecules, 1987,20(2):309316.
[27] MATHIAS L J, DAVIS R D, et al. Observation of α and γ crystal forms and amorphous regions of nylon 6clay nanocomposites using solidstate 15N nuclear magnetic resonance[J]. Macromolecules, 1999,32(23):79587960.
[28] MEDELLINRODRIGUEZ F J, LARIOSLOPEZ L, et al. Melting behavior of polymorphics: molecular weight dependence and steplike mechanisms in nylon6[J]. Macromolecules, 2004,37(5):17991809.
[29] LI H, WU Y, SATO H, et al. A new facile method for preparation of Nylon6 with high crystallinity and special morphology[J]. Macromolecules, 2009,42(4):11751179.
[30] ZHANG Y, ZHANG Y, LIU S, et al. Phase stability and melting behavior of the α and γ phases of nylon 6[J]. J Appl Polym Sci, 2011,120(4):18851891.
[31] VASANTHAN N, SALEM D R. FTIR spectroscopic characterization of structural changes in polyamide‐6 fibers during annealing and drawing[J]. J Polym Sci Part B: Polym Phys, 2001,39(5):536547.
[32] ZHAO X. Thermal history dependence of polymorphic transformation of polyamide 6/silicate nanocomposites[J]. Polym Int, 2009,58(5):469474.
[33] MIRI V, ELKOUN S, PEURTON F, et al. Crystallization kinetics and crystal structure of nylon6clay nanocomposites: combined effects of thermomechanical history, clay content, and cooling conditions[J]. Macromolecules, 2008,41(23):92349244.
[34] CHENG L P, LIN D J, YANG K C. Formation of micaintercalatedNylon 6 nanocomposite membranes by phase inversion method[J]. J Membr Sci, 2000,172(1):157166.
[35] YEBRARODRIGUEZ A, ALVAREZLLORET P, RODRIGUEZNAVARRO A B, et al. ThermoXRD and differential scanning calorimetry to trace epitaxial crystallization in PA6/montmorillonite nanocomposites[J]. Mater Let, 2009,63(13):11591161.
(编辑 WJ)
