肿瘤干细胞的分子机制和调控通路
2016-02-16涂艳阳张永生第四军医大学唐都医院实验外科唐都医院唐都医院脑外科陕西西安7008
涂艳阳,王 震,张永生,王 樑(第四军医大学:唐都医院实验外科,唐都医院,唐都医院脑外科,陕西西安7008)
肿瘤干细胞的分子机制和调控通路
涂艳阳1,王 震1,张永生2,王 樑3(第四军医大学:1唐都医院实验外科,2唐都医院,3唐都医院脑外科,陕西西安710038)
恶性肿瘤是人类健康和生命最严重的威胁之一,肿瘤治疗仍然是今天人类面临的一个大问题.传统的肿瘤治疗方法包括手术、化疗、放疗等方法,但是很多肿瘤仍然会复发,无法做到根治,最根本的原因是肿瘤转移和复发的机制不清楚.近年来,肿瘤细胞表面标记、肿瘤细胞增殖和肿瘤发生能力的研究让人们提出肿瘤干细胞(CSC)的理论.肿瘤干细胞属于肿瘤细胞中一类具有无限自我更新能力和异质免疫缺陷动物致瘤能力的干细胞.肿瘤干细胞在肿瘤的发生、发展和转移过程中扮演重要角色.肿瘤干细胞理论的提出为肿瘤治疗提供了新思路.本文中,我们将总结肿瘤干细胞理论形成和发展的过程,讨论肿瘤干细胞的生成,表面标志物,自我更新和调控途径.这些肿瘤干细胞的理论机制可能为未来恶性肿瘤靶向治疗提供帮助.
恶性肿瘤;干细胞;调控通路;靶向治疗
0 引言
恶性肿瘤是当今人类面临的主要威胁之一.传统的消除肿瘤细胞治疗肿瘤的方法包括手术、化疗、放疗等.但恶性肿瘤的复发和转移是肿瘤难治愈的主要原因之一,并且恶性肿瘤患者死亡率高,使得肿瘤治疗成为人们面临的最大挑战之一.尽管肿瘤相关的生物学理论研究越来越深,生物学技术越来越进步,但至今肿瘤发生发展、复发和转移的根本原因还不清楚.因为肿瘤细胞就像干细胞一样具有自我更新和分化的能力,这导致研究人员提出了肿瘤干细胞理论.肿瘤干细胞理论认为一部分肿瘤细胞具有干细胞的特性,具有自我更新和分化的能力,这是肿瘤干细胞的存在是肿瘤复发和转移的主要原因.
肿瘤干细胞样的细胞也称为肿瘤起源细胞,是肿瘤细胞中一类像正常干细胞一样具有自我更新能力并能够诱导肿瘤发生的细胞[1].肿瘤干细胞最先在白血病中被发现.人们将急性髓细胞性白血病细胞(acute myelogenous leukemia,AML)移植到严格联合免疫缺陷(severe cembined immunodeficient,SCID)老鼠身上.AML细胞根据细胞表面标志物分选得到,注射到SCID小鼠体内的白血病起始细胞能够产生大量克隆状的CD34+CD38-祖细胞,这种体内模型很类似于AML祖细胞,并且定义了一种新的相比克隆形成细胞不太成熟的白血病起始细胞[2].从此之后,肿瘤干细胞在多种实体肿瘤中被发现,如乳腺癌[3]、脑肿瘤[4]、结肠癌[5]、肺癌[6]和其他肿瘤组织[7].很多研究也报道了脑肿瘤干细胞的发现和鉴定过程[5,8-11].肿瘤干细胞被认为是肿瘤化疗和放疗抵抗的主要原因,并且缺氧条件下可以诱导肿瘤血管生成和肿瘤发生[12-15].因此,在多种肿瘤中,肿瘤干细胞被视为潜在治疗靶点,但肿瘤干细胞干性维持的关键分子机制至今仍未明确.目前的研究成果表明肿瘤干细胞收到一些信号通路的调控,其他的调控方式还有microRNA,肿瘤微环境和许多其他多种因素.
1 肿瘤干细胞分子标志物
几乎所有类型的肿瘤干细胞都有自己的特定的表面标志物.根据特定的肿瘤干细胞的表面标志物可以对特定类型的肿瘤进行精准治疗.很长一段时间,许多研究人员都致力于寻找肿瘤干细胞的表面标志物.肿瘤干细胞表面标志物的研究最开始是在血液瘤中进行的.Lapido等人首次报道了CD34+CD38-表型的急性髓系白血病(AML)细胞[2].分离得到的CD34+CD38-表型的细胞可以在小鼠体内诱发类似于人类的白血病的,但是CD34CD38+表型的细胞就没有这种致肿瘤能力.另外CD96+也是急性髓系白血病AML干细胞的特异性标记,因为在CD96+细胞内发现了人类CD45+细胞,而CD96-细胞内则没有[16].其他的干细胞标志物还包括,CD133+和nestin被证实是脑肿瘤干细胞特异性标记[17],而CD44+被验证为胃癌干细胞标记.CD44、CD133 CD166和EpCAM被证实是结肠癌肿瘤干细胞表面标志物[18].报告Lin-ESZ+CD44+CD24-/low和ALDH1+是乳腺癌干细胞表面标记[19].周等认为CD133+喉肿瘤干细胞的标志之一,而最新研究表明ALDH1也是特定的头部和颈部肿瘤干细胞的标志物[20-21].ABCG2、ALDH1、MCM2、SCA-1和p63已经被证实是视网膜母细胞瘤细胞的干细胞表面标记物[22-23].Levina等用化疗药物处理后,发现高表达CD133、CD117和OCT4的肺癌细胞能存活下来,这些分子被认为是肺癌干细胞标志物[24].此外,CD44、CD24、ESA和CD133+可以用于标记胰腺癌干细胞[25-26].CD44+/CD133+/α-2β1hi是前列腺癌干细胞表面标记,CD133、CD90用于标记肝癌干细胞[27-28].其他肿瘤干细胞标志物如表1所示.
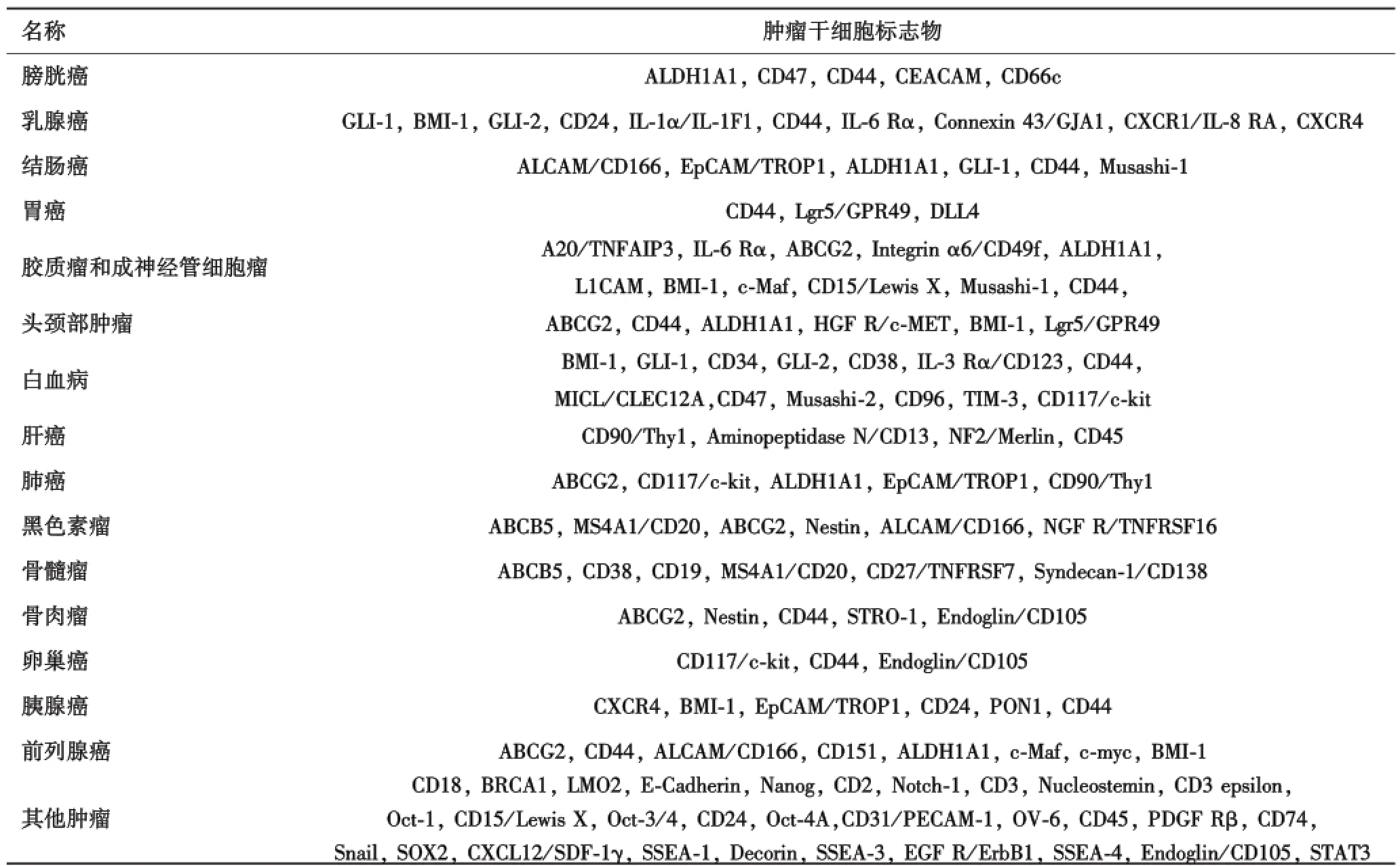
表1 不同类型肿瘤及其干细胞标志物列表
2 肿瘤干细胞的调控机制
目前为止,肿瘤干细胞的分子调控机制尚不完全清楚.如今关于肿瘤干细胞调控机制的研究主要集中在信号通路调控、转录因子表达异常、肿瘤微环境和microRNA和其他表观遗传调控方面.
2.1 信号通路调节异常
2.1.1 Wnt通路 Wnt信号通路负责调控脊椎动物和无脊椎动物的胚胎发育,视网膜干细胞、肠道、乳腺
癌、胚胎干细胞和其他各种干细胞的自我更新都非常重要[29-33].Wnt/β-catenin信号通路对正常干细胞的增殖和分化也非常重要[34-35].近年来在结肠直肠癌、肝癌、胰腺癌、子宫内膜癌、卵巢癌、甲状腺癌、前列腺癌、肾肿瘤和一些其他类型的肿瘤中均发现wnt信号通路是突变或激活的状态,表明wnt信号通路在肿瘤发生过程中起到调控的作用.同时一些研究也证实了wnt通路中的分子在肿瘤干细胞中的重要作用.例如wnt-1和β-catenin老鼠4T1乳腺癌细胞系和NXS2神经母细胞瘤细胞系中高表达[36-37].wnt信号的过度激活导致干细胞过度增殖,进而转化成肿瘤干细胞.但wnt信号在分化细胞中没有功能.原因可能是高表达分化细胞中含有wnt信号抑制分子,抑制wnt信号通路分子的活性,导致下游分子β-catenin不能激活[38].这些研究表明wnt信号通路中肿瘤干细胞的发生和命运都至关重要.
2.1.2 Notch通路 Notch信号通路主要调控正常干细胞的增殖、分化、细胞凋亡和细胞间通讯.Notch信号通路在果蝇的遗传研究中首次被发现,因为一些Notch的等位基因的诱导产生切口翅膀而得名(notched wings).Notch信号通路对脊椎动物和无脊椎动物的细胞增殖、细胞凋亡、神经系统发育和器官的形成都非常重要.此外,Notch通路在肿瘤发生过程中也很关键.一些Notch信号通路分子在正常干细胞中高表达[39-40],说明Notch信号通路与干细胞的自我更新密切相关.研究表明,Notch信号通路的激活会促进促进神经干细胞、脑垂体干细胞和乳腺癌干细胞的增殖[39-41],同时能促进乳腺癌微球的形成[42].然而也有研究表明Notch信号可以防止干细胞的过度增殖导致的干细胞恶性增加[43-44].研究表明,的MCF-7乳腺癌细胞系中notch1高表达.然而过表达Notch2细胞内结构域(NICD2)会导致测亚群增加.抑制Notch信号能增加肿瘤干细胞的凋亡率,但分化细胞不受影响.动物实验表明,抑制Notch信号能降低细胞的致瘤能力[45].Notch信号能够促进一些恶性肿瘤的转移,另一方面Notch信号在其他肿瘤组织又能抑制其转移[46-48],说明Notch信号通路对肿瘤干细胞的调控具有组织特异性,其分子机制需要进一步研究.
2.1.3 Hedgehog通路 Hedgehog基因编码的分泌蛋白Hh主要调控细胞增殖、分化和形态学自分泌或旁分泌的方式.已知的Hedgehog通路分子包括Desert、Indian和Sonic.Sonic Hedgehog信号分子Gli能够通过Bmi1抑制p14和p16,进而保护cyclinD/CDK4并抑制p53.Hedgehog信号在乳腺癌、胰腺癌、前列腺癌等肿瘤组织中异常表达[49].Hedgehog信号是一个比较经典的干细胞调控途径,Hedgehog信号通路参与果蝇卵巢干细胞、原始造血干细胞、肠道祖细胞和乳腺干细胞等干细胞的调控.此外,Hedgehog信号对各种干细胞的自我更新也非常重要.Shh受体的激活能够促进人类表皮干细胞的增殖,而Shh抑制剂能够抑制干细胞增殖[50].在神经系统中,敲除Shh能够引起神经微球损伤[51],而组成性激活Shh和c-myc能够促进神经祖细胞的增殖,进而导致成神经管细胞瘤的形成[52].因此,Hedgehog信号通路对肿瘤干细胞的调控十分重要.
2.2 转录因子表达异常转录因子的异常表达对肿瘤干细胞增殖和自我更新至关重要,许多转录因子与肿瘤干细胞的形成和维持密切相关.转录因子SOX2、c-myc、Klf4、Oct4和Lin28的表达水平都被证明与肿瘤干细胞的自我更新和多向分化能力相关.研究发现这些转录因子在许多人类肿瘤组织中高表达,其表达水平与肿瘤的发展及预后密切相关[53].肺癌干细胞的自我更新和上皮间充质转变(EMT)被证明与Oct4和Nanog的异常表达有关[54].转录因子Twist和Zeb也参与乳腺癌上皮间充质转变(EMT)过程,导致肿瘤细胞具有干细胞的特性[55].转录因子对肿瘤干细胞的调控不是独立的,而是多种转录因子的异常表达共同调控相关肿瘤干细胞的维持.
2.3 MicroRNAmicroRNA通常在转录后水平上调节基因的表达.microRNA对细胞的增殖、分化、发育、衰老和凋亡都非常重要,而且microRNA在肿瘤形成、生长、分化和发展过程中都扮演重要的调控角色[56-58].同一种MicroRNA可以调控多个基因,同时多种microRNA也可以共同调控同一种基因,microRNA通过排列和组合可以对基因表达实现精确调控[59-60].研究表明,EMT相关的转录因子像Twist1、Snail1、Zeb1、microRNA和Zeb2都可以被microRNA调控,所以microRNA是EMT过程的重要参与因素[61].例如,microRNA中的mir-200家族[62-65]像mir-200a/b/c、mir-141和mir-200家族的其他成员通过抑制Zeb1的表达调控EMT过程,这种调控使得细胞维持上皮样表型并减少EMT的发生[63].其他很多microRNA也参与调控EMT,例如过表达miR-29b可以逆转EMT过程,抑制细胞侵入性表型的出现[66].MiR-30可以调控Snail1的表达水平抑制TGF-β的表达进而诱导EMT的发生[67].mir-661和mir-491-5p则通过减弱细胞连接抑制EMT的发生[68-69].因此microRNA对于肿瘤中的EMT过程至关重要.
在干细胞方面,microRNA很早就被已经被证实参与调控胚胎干细胞、成体干细胞和肿瘤干细胞.microRNA对正常胚胎干细胞自我更新和细胞分化能力有很重要的调控作用[70].mir-290簇可以调控干细胞的细胞周期,包括mir-291-3p、mir-294和mir-295能增强KLF4、OCT4、SOX2的表达并诱导细胞多能效率[71].研究表明mir-145在自我更新的胚胎干细胞中低表达,而在分化细胞中高表达.mir-145与它的靶基因包括OCT4、SOX2和KLF4共同抑制人类胚胎干细胞并诱导分化[72].研究还表明,过表达let-7抑制小鼠体内肿瘤的形成和转移[73].这些发现表明,microRNA对干肿瘤干细胞的维持和细胞分化的调控至关重要,研究microRNA肿瘤干细胞理论的完善很有意义.
2.4 表观遗传调控MicroRNA属于表观遗传调控的方式之一,除此之外,表观遗传的调控的分子机制还包括DNA甲基化与去甲基化、组蛋白修饰和染色质重塑[74-76].肿瘤干细胞可能起源于正常干细胞/祖细胞,也可能来自分化细胞的重编程.表观遗传学在体细胞重编程过程中扮演重要角色,预示着其在干细胞形成发展中的重要地位[77-79].肿瘤干细胞中常常出现信号转导通路异常和转录因子的表达异常引起的表观遗传变化,这些表观遗传变化进而导致一系列的基因变化.Pellacani发现CD133阳性的前列腺癌肿瘤干细胞受到浓缩染色质的动态调控[80].DNA甲基化剂5-Aza-Dc和组蛋白乙酰化抑制剂SAHA可以通过miR-34抑制胰腺癌干细胞的Notch信号通路,降低胰腺癌肿瘤细胞的自我更新和增殖能力,降低EMT和侵袭能力[81].表观遗传学的机制和方法已被应用于肿瘤的预防、诊断和治疗中.甲基化特异性PCR被应用于患者的体液和活细胞甲基化表达的检测,将成为肿瘤诊断的有力工具.如果能进一步明确肿瘤干细胞表观遗传学的调控机制及其在肿瘤发生及发展中的作用,将为未来的肿瘤治疗提供有益参考.
2.5 肿瘤干细胞微环境肿瘤干细胞微环境对肿瘤发生、侵袭及转移过程都有很重要的意义.肿瘤干细胞微环境主要包括细胞因子、间充质细胞、免疫细胞、血管和细胞外基质等.不同的肿瘤干细胞微环境特征主要包括缺氧、邻血管、炎症反应和上皮间充质转变等.这些微环境之间相互关联,共同调控相关的肿瘤干细胞[82].
肿瘤干细胞微环境的特征之一就是缺氧状态.研究表明,肿瘤干细胞的适应性反应是受低氧诱导因子(HIF)的调控,Oct4、c-myc和Notch都是低氧诱导因子(HIF)的直接或间接的靶标.此外,低氧诱导因子还调节肿瘤干细胞表型的形成过程[83].调节干细胞的氧化应激微环境被证明对慢性粒细胞性白血病具有一定的治疗效果[84].邻血管在位置和功能上都与肿瘤干细胞密切相关[53].邻血管微环境的很多特征都可以调控肿瘤干细胞并产生抗肿瘤功能,例如抑制肿瘤血管生成,破坏肿瘤血管,抑制一氧化氮分子作用和改变肿瘤干细胞邻近血管微环境等[53],这些因素表明邻近血管微环境是肿瘤干细胞的重要调控方式[85].炎性反应肿瘤干细胞微环境包括各种各样的间充质细胞和免疫细胞分泌的炎症因子的,如IL-6、IL-8、TNF-α、和MFG等.这些炎症因子可以通过激活NFκB、Stat3、Hedgehog和Notch信号通路的方式调控肿瘤干细胞[86-87].这些炎症因子可以作为调节肿瘤干细胞微环境靶标,最终达到治疗肿瘤的目的.
3 展望
肿瘤干细胞理论为恶性肿瘤的治疗提供了新的思路.肿瘤干细胞与分化的肿瘤细胞最大的不同在于肿瘤干细胞通常具有放疗和化疗抵抗性.这意味着虽然放疗和化疗可以杀死大多数肿瘤细胞,但是关键致瘤性的肿瘤干细胞依然能够存活下来.这部分细胞是肿瘤复发和转移的根源,所以明确肿瘤干细胞的发生机制和调控途径对肿瘤治疗具有非常重要的意义.本文论述了肿瘤干细胞的生成机制,总结了肿瘤干细胞的表面标志物和肿瘤干细胞的主要调控途径.然而,目前关于肿瘤干细胞的研究仍处于起步阶段,仍有许多问题有待解决.例如,只有部分特定肿瘤干细胞的标记物被发现并验证,仍有更多的肿瘤干细胞特异性标志物有待发现.肿瘤干细胞的放疗和化疗抵抗性的具体分子机制需要进一步的研究.肿瘤干细胞各种信号转导途径和调控通路的核心机理尚不清楚.即使关于肿瘤干细胞仍有如此多的问题等待解决,我们相信随着肿瘤干细胞发生机制和调控通路研究的不断深入,肿瘤干细胞理论在恶性肿瘤靶向治疗中的一定会发挥更加重要的作用.
[1]Yoon CH,Kim MJ,Kim RK,et al.c-Jun N-terminal kinase has a pivotal role in the maintenance of self-renewal and tumorigenicity in glioma stem-like cells[J].Oncogene,2012,31(44):4655-4666.
[2]Lapidot T,Sirard C,Vormoor J,et al.A cell initiating human acute myeloid leukaemia after transplantation into SCID mice[J].Nature,1994,367(6464):645-648.
[3]Al-Hajj M,Wicha MS,Benito-Hernandez A,et al.Prospective identification of tumorigenic breast cancer cells[J].Proc Natl Acad Sci USA,2003,100(7):3983-3988.
[4]Singh SK,Hawkins C,Clarke ID,et al.Identification of human brain tumour initiating cells[J].Nature,2004,432(7015):396-401.
[5]Ricci-Vitiani L,Lombardi DG,Pilozzi E,et al.Identification and expansion of human colon-cancer-initiating cells[J].Nature,2007,445(7123):111-115.
[6]Eramo A,Lotti F,Sette G,et al.Identification and expansion of the tumorigenic lung cancer stem cell population[J].Cell Death Differ,2008,15(3):504-514.
[7]Visvader JE,Lindeman GJ.Cancer stem cells in solid tumours:accumulating evidence and unresolved questions[J].Nat Rev Cancer,2008,8(10):755-768.
[8]Galli R,Binda E,Orfanelli U,et al.Isolation and characterization of tumorigenic,stem-like neural precursors from human glioblastoma[J].Cancer Res,2004,64(19):7011-7021.
[9]Houman D,Hemmati IN,Jorge A,et al.Cancerous stem cells can arise from pediatric brain tumors[J].PNAS,2003,100(25):5.
[10]Salmaggi A,Boiardi A,Gelati M,et al.Glioblastoma-derived tumorospheres identify a population of tumor stem-like cells with angiogenic potential and enhanced multidrug resistance phenotype[J].Glia,2006,54(8):850-860.
[11]Yi L,Zhou ZH,Ping YF,et al.Isolation and characterization of stem cell-like precursor cells from primary human anaplastic oligoastrocytoma[J].Mod Pathol,2007,20(10):1061-1068.
[12]Murat A,Migliavacca E,Gorlia T,et al.Stem Cell-Related“Self-Renewal”Signature and High Epidermal Growth Factor Receptor Expression Associated With Resistance to Concomitant Chemoradiotherapy in Glioblastoma[J].J Clin Oncol,2008,26(18):3015-3024.
[13]Bao S,Wu Q,Mclendon RE,et al.Glioma stem cells promote radioresistance by preferential activation of the DNA damage response[J].Nature,2006,444:756-760.
[14]Li Z,Bao S,Wu Q,et al.Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells[J].Cancer Cell,2009,15(6):501-513.
[15]Bao S,Wu Q,Sathornsumetee S,et al.Stem Cell-like Glioma Cells Promote Tumor Angiogenesis through Vascular Endothelial Growth Factor[J].Cancer Res,2006,66:7843-7848.
[16]Hosen N,Weissman IL,et al.CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia[J].Proc Natl Acad Sci USA,2007,104(26):11008-11013.
[17]Jordan CT.Cancer stem cell biology:from leukemia to solid tumors[J].Curr Opin Cell Biol,2004,16(6):708-712.
[18]Dalerba P,Dylla SJ,Park IK,et al.Phenotypic characterization of human colorectal cancer stem cells[J].Proc Natl Acad Sci USA,2007,104(24):10158-10163.
[19]Himuro T,Horimoto Y,Arakawa A,et al.Activated Caspase 3 Expression in Remnant Disease After Neoadjuvant Chemotherapy May Predict Outcomes of Breast Cancer Patients[J].Ann Surg Oncol,2016,23(7):2235-2241.
[20]Ginestier C,Min HH,Charafe-Jauffret E,et al.ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome[J].Cell Stem Cell,2007,1(5):555-567.
[21]Zhou L,Wei X,Cheng L,et al.CD133,one of the markers of cancer stem cells in Hep-2 cell line[J].Laryngoscope,2007,117(3):455-460.
[22]Fang D,Nguyen TK,Leishear K,et al.A tumorigenic subpopulation with stem cell properties in melanomas[J].Cancer Res,2005,65(20):9328-9337.
[23]Seigel GM,Campbell LM,Narayan M,et al.Cancer stem cell characteristics in retinoblastoma[J].Mol Vis,2005,11:729-737.
[24]Levina V,Marrangoni AM,Demarco R,et al.Drug-selected human lung cancer stem cells:cytokine network,tumorigenic and metastatic properties[J].PLoS One,2008,3(8):e3077.
[25]Heidt DG,Li C,Mollenberg N,et al.Identification of pancreatic cancer stem cells[J].Cancer Res,2007,67(3):1030-1037.
[26]Hermann PC,Huber SL,Herrler T,et al.Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer[J].Cell Stem Cell,2007,1(3):313-323.
[27]Nagaki A,Tsukada M,Osawa Y,et al.CD133 as a putative marker of cancer stem/progenitor cells in hepatocellular carcinoma[J].J Gastroen Hepatol,2007(22):A192.
[28]Ma S,Chan KW,Hu L,et al.Identification and characterization of tumorigenic liver cancer stem/progenitor cells[J].Gastroenterology,2007,132(7):57-60.
[29]He B,Barg RN,You L,et al.Wnt signaling in stem cells and nonsmall-cell lung cancer[J].Clin Lung Cancer,2005,7(1):54-60.
[30]Schepers A,Clevers H.Wnt signaling,stem cells,and cancer of the gastrointestinal tract[J].Cold Spring Harb Perspect Biol,2012,4(4):a007989.
[31]He B,Jablons DM.Wnt signaling in stem cells and lung cancer[J].Ernst Schering Found Symp Proc,2006(5):27-58.
[32]Bisson I,Prowse DM.WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics[J].Cell Res,2009,19(6):683-697.
[33]Basu S,Haase G,Ben-Ze'ev A.Wnt signaling in cancer stem cells and colon cancer metastasis[J].F1000 Res,2016:5.
[34]Nayak L,Bhattacharyya NP,De RK.Wnt signal transduction pathways:modules,development and evolution[J].BMC Syst Biol,2016,10(Suppl 2):44.
[35]Yang J,Fang Z,Wu J,et al.Construction and application of a lung cancer stem cell model:antitumor drug screening and molecular mechanism of the inhibitory effects of sanguinarine[J].Tumour Biol,2016:1-13.
[36]Patrawala L,Calhoun T,Schneiderbroussard R,et al.Side population is enriched in tumorigenic,stem-like cancer cells,whereas ABCG2+and ABCG2-cancer cells are similarly tumorigenic[J].Cancer Res,2005,65(14):6207-6219.
[37]Kruger JA,Kaplan CD,Luo Y,et al.Characterization of stem celllike cancer cells in immune-competent mice[J].Blood,2006,108(12):3906-3912.
[38]Yang Y,Cheng Z,Tang H,et al.Neonatal Maternal Separation Impairs Prefrontal Cortical Myelination and Cognitive Functions in Rats Through Activation of Wnt Signaling[J].Cereb Cortex,2016.
[39]Chen J,Crabbe A,Van DV,et al.The notch signaling system ispresent in the postnatal pituitary:marked expression and regulatory activity in the newly discovered side population[J].Mol Endocrinol,2006,20(12):3293-3307.
[40]Challen GA,Bertoncello I,Deane JA,et al.Kidney side population reveals multilineage potential and renal functional capacity but also cellular heterogeneity[J].J Am Soc Nephrol,2006,17(7):1896-1912.
[41]Androutsellis-Theotokis A,Hoeppner DJ,Rueger MA,et al.Notch signalling regulates stem cell numbers in vitro and in vivo[J].Nature,2006,442(7104):823-826.
[42]Dontu G,Jackson KW,Mcnicholas E,et al.Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells[J].Breast Cancer Res,2004,6(6):1-11.
[43]Wang XD,Leow CC,Zha J,et al.Notch signaling is required for normal prostatic epithelial cell proliferation and differentiation[J].Dev Biol,2006,290(1):66-80.
[44]Thelu J,Rossio P,Favier B.Notch signalling is linked to epidermal cell differentiation level in basal cell carcinoma,psoriasis and wound healing[J].BMC Dermatol,2002,2:7.
[45]Fan X,Matsui W,Khaki L,et al.Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors[J].Cancer Res,2006,66(15):7445-7452.
[46]Braune EB,Lendahl U.Notch-a goldilocks signaling pathway in disease and cancer therapy[J].Discov Med,2016,21(115):189-196.
[47]Allenspach EJ,Maillard I,Aster JC,et al.Notch signaling in cancer[J].Cancer Biol Ther,2006,6(8):905-918.
[48]Alketbi A,Attoub S.Notch Signaling in Cancer:Rationale and Strategies for Targeting[J].Curr Cancer Drug Targets,2015,15(5):364-374.
[49]Merchant AA,Matsui W.Targeting Hedgehog-a cancer stem cell pathway[J].Clini Cancer Res,2010,16(12):3130-3140.
[50]Zhou JX,Jia LW,Liu WM,et al.Role of sonic hedgehog in maintaining a pool of proliferating stem cells in the human fetal epidermis[J].Hum Reprod,2006,21(7):1698-1704.
[51]Palma V,Ruiz IAA.Hedgehog-GLI signaling regulates the behavior of cells with stem cell properties in the developing neocortex[J].Development,2004,131(2):2252-2259.
[52]Rao G,Pedone CA,Coffin CM,et al.c-myc enhances sonic hedgehog-induced medulloblastoma formation from nestin-expressing neural progenitors in mice[J].Neoplasia,2003,5(3):198-204.
[53]Li Y,Laterra J.Cancer stem cells:distinct entities or dynamically regulated phenotypes?[J].Cancer Res,2012,72(3):576-580.
[54]Chiou SH,Wang ML,Chou YT,et al.Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiation[J].Cancer Res,2010,70(24):10433-10444.
[55]Mani SA,Guo W,Liao MJ,et al.The epithelial-mesenchymal transition generates cells with properties of stem cells[J].Cell,2008,133(4):704-715.
[56]De Sano JT,Xu L.MicroRNA regulation of cancer stem cells and therapeutic implications[J].AAPS J,2009,11(4):682-692.
[57]Rybicka A,Mucha J,Majchrzak K,et al.Analysis of microRNA expression in canine mammary cancer stem-like cells indicates epigenetic regulation of transforming growth factor-beta signaling[J].J Physiol Pharmacol,2015,66(1):29-37.
[58]Garzia L,Andolfo I,Cusanelli E,et al.MicroRNA-199b-5p impairs cancer stem cells through negative regulation of HES1 in medulloblastoma[J].PLoS One,2009,4(3):e4998.
[59]Subramanyam D,Blelloch R.From microRNAs to targets:pathway discovery in cell fate transitions[J].Curr Opin Genet Dev,2011,21(4):498-503.
[60]Liu C,Tang DG.MicroRNA regulation of cancer stem cells[J].Cancer Res,2011,71(18):5950-5954.
[61]Ma L,Weinberg RA.Micromanagers of malignancy:role of microRNAs in regulating metastasis[J].Trends Genet,2008,24(9):448-456.
[62]Burk U,Schubert J,Wellner U,et al.A reciprocal repression between ZEB1 and members of the mir-200 family promotes EMT and invasion in cancer cells[J].Embo Reports,2008,9(6):582-589.
[63]Gregory PA,Bert AG,Paterson EL,et al.The mir-200 family and mir-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1[J].Nat Cell Biol,2008,10(5):593-601.
[64]Korpal M,Lee ES,Hu G,et al.The mir-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2[J].J Biol Chem,2008,283(22):14910-14914.
[65]Park SM,Gaur AB,Lengyel E,et al.The mir-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2[J].Genes&Development,2008,22(7):894-907.
[66]Ru P,Steele R,Newhall P,et al.miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling[J].Mol Cancer Ther,2012,11(5):1166-1173.
[67]Zhang J,Zhang H,Liu J,et al.miR-30 inhibits TGF-beta1-induced epithelial-to-mesenchymal transition in hepatocyte by targeting Snail1[J].Biochem Biophys Res Commun,2012,417(3):1100-1105.
[68]Vetter G,Saumet A,Moes M,et al.mir-661 expression in SNAI1-induced epithelial to mesenchymal transition contributes to breast cancer cell invasion by targeting Nectin-1 and StarD10 messengers[J].Oncogene,2010,29(31):4436-4448.
[69]Zhou Q,Fan J,Ding X,et al.TGF-{beta}-induced MiR-491-5p expression promotes Par-3 degradation in rat proximal tubular epithelial cells[J].J Biol Chem,2010,285(51):40019-40027.
[70]Marson A,Levine SS,Cole MF,et al.Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells[J].Cell,2008,134(3):521-533.
[71]Judson RL,Babiarz JE,Venere M,et al.Embryonic stem cell-specific microRNAs promote induced pluripotency[J].Nat Biotechnol,2009,27(5):459-461.
[72]Xu N,Papagiannakopoulos T,Pan G,et al.MicroRNA-145 regulates OCT4,SOX2,and KLF4 and represses pluripotency in human embryonic stem cells[J].Cell,2009,137(4):647-658.
[73]Yu F,Yao H,Zhu P,et al.let-7 regulates self renewal and tumorigenicity of breast cancer cells[J].Cell,2007,131(6):1109-1123.
[74]Lim YY,Wright JA,Attema JL,et al.Epigenetic modulation of the mir-200 family is associated with transition to a breast cancer stemcell-like state[J].J Cell Sci,2013,126(10):2256-2266.
[75]van Vlerken LE,Hurt EM,Hollingsworth RE.The role of epigenetic regulation in stem cell and cancer biology[J].J Mol Med-Jmm,2012,90(7):791-801.
[76]Widschwendter M,Fiegl H,Egle D,et al.Epigenetic stem cell signature in cancer[J].Nature Genetics,2007,39(2):157-158.
[77]Patel S,Shah K,Mirza S,et al.Epigenetic regulators governing cancer stem cells and epithelial-mesenchymal transition in oral squamous cell carcinoma[J].Curr Stem Cell Res Ther,2015,10(2):140-152.
[78]Marquardt JU.Epigenetic Modulation selects unique cancer stem cell population within the Sp fraction of human hcc[J].Hepatology,2008,48(4):983a.
[79]Balch C,Nephew KP,Huang TH,et al.Epigenetic“bivalently marked”process of cancer stem cell-driven tumorigenesis[J].Bioessays,2007,29(9):842-845.
[80]Pellacani D,Packer RJ,Frame FM,et al.Regulation of the stem cell marker CD133 is independent of promoter hypermethylation in human epithelial differentiation and cancer[J].Mol Cancer,2011,10(7):1-14.
[81]Nalls D,Tang SN,Rodova M,et al.Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells[J].Plos One,2011,6(8):e24099.
[82]Cabarcas SM,Mathews LA,Farrar WL.The cancer stem cell nichethere goes the neighborhood?[J].Inte J Cancer,2011,129(10):2315-2327.
[83]Garvalov BK,Acker T.Cancer stem cells:a new framework for the design of tumor therapies[J].J Mol Med(Berl),2011,89(2):95-107.
[84]Ito K,Bernardi R,Morotti A,et al.PML targeting eradicates quiescent leukaemia-initiating cells[J].Nature,2008,453(7198):1072-1078.
[85]Eyler CE,Wu Q,Yan K,et al.Glioma stem cell proliferation and tumor growth are promoted by nitric oxide synthase-2[J].Cell,2011,146(1):53-66.
[86]Jinushi M,Chiba S,Yoshiyama H,et al.Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells[J].Proc Natl Acad Sci USA,2011,108(30):12425-12430.
[87]Wang H,Lathia JD,Wu Q,et al.Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth[J].Stem Cells,2009,27(10):2393-2404.
The molecular mechanism and regulatory pathway of cancer stem cell
TU Yan-Yang1,WANG Zhen1,ZHANG Yong-Sheng2,WANG Liang3
Fourth Military Medical University:1Department of Experimental Surgery,Tangdu Hospital;2Tangdu Hospital;3Department of Brain Surgery,Tangdu Hospital,Xi'an 710038,China
Malignant tumor is one of the most harmful diseases that threat human health and life.Traditional methods for the cancer therapy include surgery,chemotherapy,radiotherapy and other methods to remove existing cancer cells.The treatment of tumor is still a big problem today,and the fundamental reason is that we are unclear about the mechanism of cancer metastasis and recurrence.In recent years,the researches on the tumor cell surface markers,tumor cell proliferation and tumorigenic ability make people put forward the theory of cancer stem cell(CSC).Cancer stem cell is a small population of tumor cells that has unlimited self-renewal ability and heterogeneous immunodeficiency animals tumorigenic ability of stem cells in the tumor cells.They play a key role in the tumor growth and metastasis.Cancer stem cell theory provides a new way for tumor therapy.In this paper,we will summarize the formation and development process of tumor stem cell theory,we discuss the cancer stem cell generation,surface makers,self-renewal and relative regulatory pathways.These theory mechanisms may provide help for malignant tumor targeting therapy.
malignant tumor;cancer stem cell;regulatory pathway;targeting therapy
R730.3
A
2095-6894(2016)11-01-07
2016-10-08;接受日期:2016-10-18
国家自然科学基金资助项目(81272419,81572983,81402081)
涂艳阳.副主任医师,副教授.E-mail:Tu.fmmu@gmail.com
张永生.教授,主任医师,院长.E-mail:zhangys@fmmu.edu.cn
