The Application of Acidic Electrolyzed Water to Grape Cultivation in the Southern Regions
2016-01-12,,,,
, , , ,
1. College of Science, Jiangxi Agricultural University, Nanchang 330045, China; 2. Jiangxi Research Institute of Agricultural Machinery, Nanchang 330047, China; 3. Jiangxi Xinyu Modern Agricultural Science Park, Xinyu 380007, China
1 Introduction
Over the past decade, the table grape cultivation in the southern regions has become a characteristic fruit industry, and the varieties mainly include Kyoho, Xiahei, Gold Finger, Manicure Finger, Yongyou 1, Benifuji, Jumeigui, Shine-Muscat, Zuijinxiang, Rosario Bianco, Flame Seedless and Heibaladuo. The southern regions have rich heat resources for grape cultivation, but there is also a rainy and humid climate, easily causing frequent virus damage, which will increase the application of pesticides and pesticide residues, thereby having a direct impact on the economic benefit of farmers and industrial development. For example, the grape cultivation base in Jiangxi Xinyu National Agricultural Science and Technology Park is a local table grape cultivation base, which researches and develops AEW technology to control virus damage and achieve pollution-free cultivation, and it has achieved significant virus control effect.
2 AEW’s virus damage prevention and control mechanism
AEW (Acidic Electrolyzed Water) is also known as the electrolyzed functional water[1]. 0.1% sodium chloride (potassium) was added to purified water to be dissolved into the electrolyte solution having high conductivity. The electrolyzed reaction was generated in the electrolyzing device equipped with platinum electrodes, and it was electrolyzed into the sodium hypochlorite acid water with high oxidizing potential (ORP of +900-+ 1200mV). The pH value was 2-3, and the available chlorine content (ACC) was 60-70 mg.L-1. It also generated sodium hydroxide solution with strong reduction potential (ORP of -800 mV), and the pH value was 10 to 13. The principle of AEW in preventing and controlling virus damage and killing virus lies in the physicochemical properties of acid[2-5]. The physical property is that it has high oxidation potential, and once it is in contact with bacteria and fungi or viruses, it acquires electrons from the biofilm with coerciveness and changes the normal potential and permeability of cell membrane, so that the permeability of the lipid membrane is damaged. It is like a hole through the cell membrane, leading to cellular content leakage. The chemical process is to rebuild a strong acid environment unsuitable for bacteria, thereby strongly inhibiting the bacterial development. Most of bacteria require pH of 3 or more, and the strong acid can have pH of 2.7 or less, so as to achieve bacteriostatic effects. The chlorine ion in hypochlorous acid is also a bactericide. After years of research, AEW has been gradually favored by people due to high efficiency, non-residue and non-pollution[6-17].
3 Materials and experimental procedure
3.1Materials,instrumentsandequipmentsThe grape varieties for experiment include Xiahei, Gold Finger, Yongyou 1, Zuijinxiang, and Rosario Bianco, and the instruments can be shown in Table 1.
3.2Experimentalprogram
3.2.1AEW preparation and determination of physicochemical propertiesThe electrolyzed water was taken by the XY_150 device. The electrolysis voltage was 13V. The concentration of sodium chloride (NaCl) was 1% (prepared from pure water). pH value of the electrolyzed water was measured by the PHS-2C laboratory pH meter. The oxidation-reduction potential was provided by the equipment or measured by the ORP-501 oxidation-reduction potentiometer (PHSJ-4A). Based on GB/T601-2002, GB19106-2003, the available chlorine concentration (ACC) was measured by the RC-3F available chlorine detector. The physicochemical parameters of electrolyzed water can be shown in Table 2.
Table1Instrumentsandequipmentsusedintheexperiments
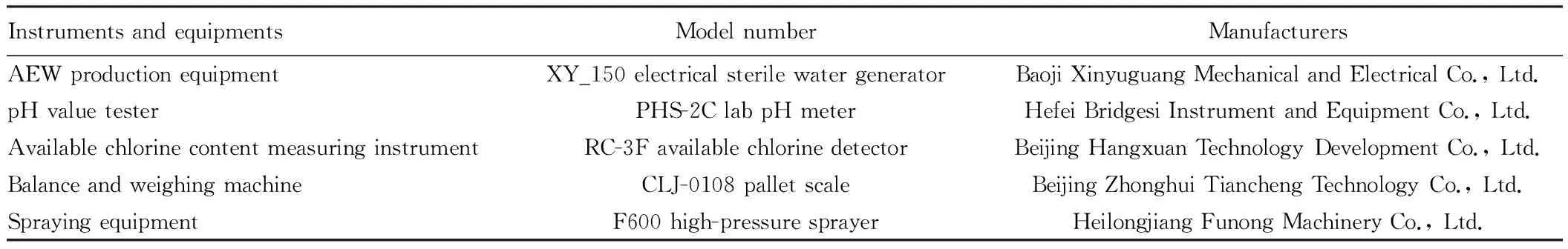
InstrumentsandequipmentsModelnumberManufacturersAEWproductionequipmentXY_150electricalsterilewatergeneratorBaojiXinyuguangMechanicalandElectricalCo.,Ltd.pHvaluetesterPHS-2ClabpHmeterHefeiBridgesiInstrumentandEquipmentCo.,Ltd.AvailablechlorinecontentmeasuringinstrumentRC-3FavailablechlorinedetectorBeijingHangxuanTechnologyDevelopmentCo.,Ltd.BalanceandweighingmachineCLJ-0108palletscaleBeijingZhonghuiTianchengTechnologyCo.,Ltd.SprayingequipmentF600high-pressuresprayerHeilongjiangFunongMachineryCo.,Ltd.
Table2Physicochemicalparametersofelectrolyzedwaterandtapwater
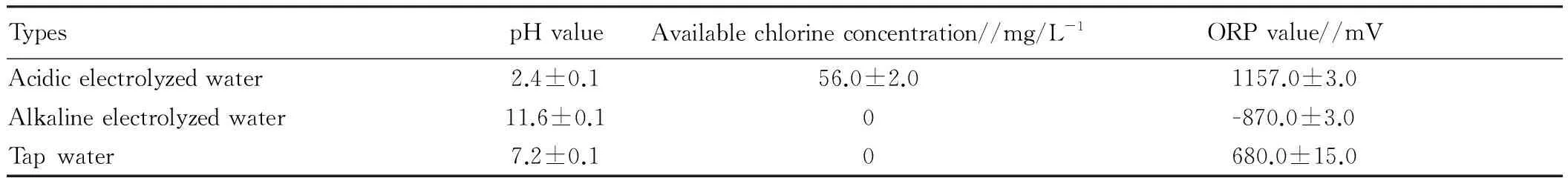
TypespHvalueAvailablechlorineconcentration//mg/L-1ORPvalue//mVAcidicelectrolyzedwater2.4±0.156.0±2.01157.0±3.0Alkalineelectrolyzedwater11.6±0.10-870.0±3.0Tapwater7.2±0.10680.0±15.0
3.2.2Experimental design and spraying method. The experiment was carried out from April to August 2015 in the grape cultivation base in Jiangxi Xinyu National Agricultural Science and Technology Park. Grape germinated in early April and was picked at the end of August. The vineyard uses the greenhouse shelter structure, and each greenhouse is 50 m long and 6 m wide. 25 plants were cultivated. There are five treatments: acidic electrolyzed water; alkaline electrolyzed water; alkaline electrolyzed water after acidic electrolyzed water; acidic electrolyzed water after alkaline electrolyzed water; bio-pesticides (control). There are five replications. The control experiment was conducted on five grape varieties. The hand-held high-pressure sprayer was used to spray electrolyzed water or biological pesticide on grape leaves and fruits from April 20 to August 26, and the spraying amount was 50 kg per mu. It was sprayed every 7-10 days, and when the grape virus damage was serious, it was sprayed every 3-5 days. It was sprayed with acidic electrolyzed water first and then alkaline electrolyzed water or with alkaline electrolyzed water first and then acidic electrolyzed water, with average interval of 30 minutes.
3.2.2Control effect standard of grape virus damage.The grape leaf sprouted in early April, the leaf was infected with the Botrytis cinerea, downy mildew and brown spot in May 20, and the fruit ear was naturally infected with anthracnose and grape Alternaria spot in June 10. Three points were randomly selected in each greenhouse before spraying electrolyzed water, in order to determine the infection area and viral species about the grape leaves. According to GB/T17980.30-2000 Field Efficacy Test Standard, the bactericide was used to prevent and control grape Botrytis cinerea, downy mildew, brown spot, anthracnose and grape Alternaria spot, and the disease index and control effect were calculated, respectively. Leaf virus damage grading standard is as follows: Level 0 (no scab); Level 1 (scab area accounting for less than 5% of total leaf area); Level 3 (scab area accounting for less than 6%-10% of total leaf area); Level 5 (scab area accounting for less than 11%-20% of total leaf area); Level 7 (scab area accounting for less than 21%-40% of total leaf area); Level 9 (scab area accounting for more than 40% of total leaf area). Fruit virus damage grading standard is as follows: Level 0 (no diseased fruit); Level 1 (diseased fruits accounting for less than 5% of total fruits); Level 3 (diseased fruits accounting for 6%-10% of total fruits); Level 5 (diseased fruits accounting for 11%-20% of total fruits); Level 7 (diseased fruits accounting for 21%-40% of total fruits); Level 9 (diseased fruits accounting for more than 40% of total fruits).
3.2.3Determination of main grape growth indicators.The grape plant height, rhizoma diameter, leaf area and leaf form were determined. Six grape plants were randomly selected at the beginning of experiment, and ruler and caliper were used to measure the plant height and rhizoma diameter weekly until the end of the experiment. Before spraying electrolyzed water each time, six tender, middle and old leaves were randomly selected from six grape plants, respectively, and measured. The regression equation is as follows:
A=0.64L2+0.47W+0.63W2-0.62L·W
whereLis the maximum leaf length;Wis the leaf width;Ais the leaf area.
The mean ofAwas also calculated, and the chlorophyll content of leaf was measured at the same time.
3.2.4Grape plant yield and quality measurement. After the grape picking period, the number of fruits per plant as well as the weight and number of fruits per string of grapes was calculated. The sugar meter was used to measure the sugar degree and alcoholic strength of the grape randomly selected.
3.3DataanalysisEXCEL 2010 was used for experimental data processing and statistical analysis.
4 Experimental results and analysis
4.1InvestigationofAEWpreventionandcontroleffectIn the maturity period of grapes, the virus damage prevention and control effect was investigated once in the demonstration plot and control plot, respectively. 2 grape growing fields were randomly selected, and 5 sampling points were set in each field. 5 plants were investigated at each point, 20 leaves and 5 strings of ear were investigated per plant, a total of 25 plants, 500 leaves and 75 strings of ear. The number of leaves and fruit ears infected with downy mildew, Botrytis cinerea, anthracnose, grape Alternaria spot and anthrachose of grape was calculated, and leaf disease incidence, disease index and fruit ear disease incidence were also calculated. Throughout the demonstration process, the grape growth, growers’ pesticide application frequency and rate, grape production and benefit were strictly recorded in the demonstrate plot and control plot, respectively.
4.2AEW’sgrapeviruspreventionandcontroleffectAfter AEW treatment of grape in the demonstration plot, the spread and infection of some diseases (such as downy mildew, Botrytis cinerea, anthracnose, grape Alternaria spot, and anthrachose of grape) was greatly reduced or avoided, as shown in Fig.1. The experiment effectively controlled the occurrence and spread of grape virus damage in the demonstration plot. According to the investigation in mid-July (grape maturity period) (Table 3), the leaf disease incidence, disease index and fruit ear disease incidence concerning grape downy mildew in the demonstration plot were 84.0%, 37.9% and 80% lower than in the control plot, respectively, with control effect of 94%, 93.5% and 88%, respectively; the leaf disease incidence, disease index, fruit ear disease incidence concerning Botrytis cinerea in the demonstration plot were 79.4%, 33.3% and 52% lower than in the control plot, respectively, with control effect of 95.4%, 96.2% and 92%, respectively; the leaf disease incidence, disease index, fruit ear disease incidence concerning grape anthracnose in the demonstration plot were 69.8%, 38.4% and 80% lower than in the control plot, respectively, with control effect of 97.6%, 93.2% and 84%, respectively; the leaf disease incidence, disease index, fruit ear disease incidence concerning grape Alternaria spot in the demonstration plot were 48%, 40.3% and 64% lower than in the control plot, respectively, with control effect of 100%, 100% and 84%, respectively; the leaf disease incidence, disease index, fruit ear disease incidence concerning anthrachose of grape were 89.8%, 39.5% and 56% lower than in the control plot, respectively, with control effect of 90.8%, 87.9% and 72%, respectively.
Table3AEW’sgrapeviruspreventionandcontroleffectinXinyuCityduringmid-July2015
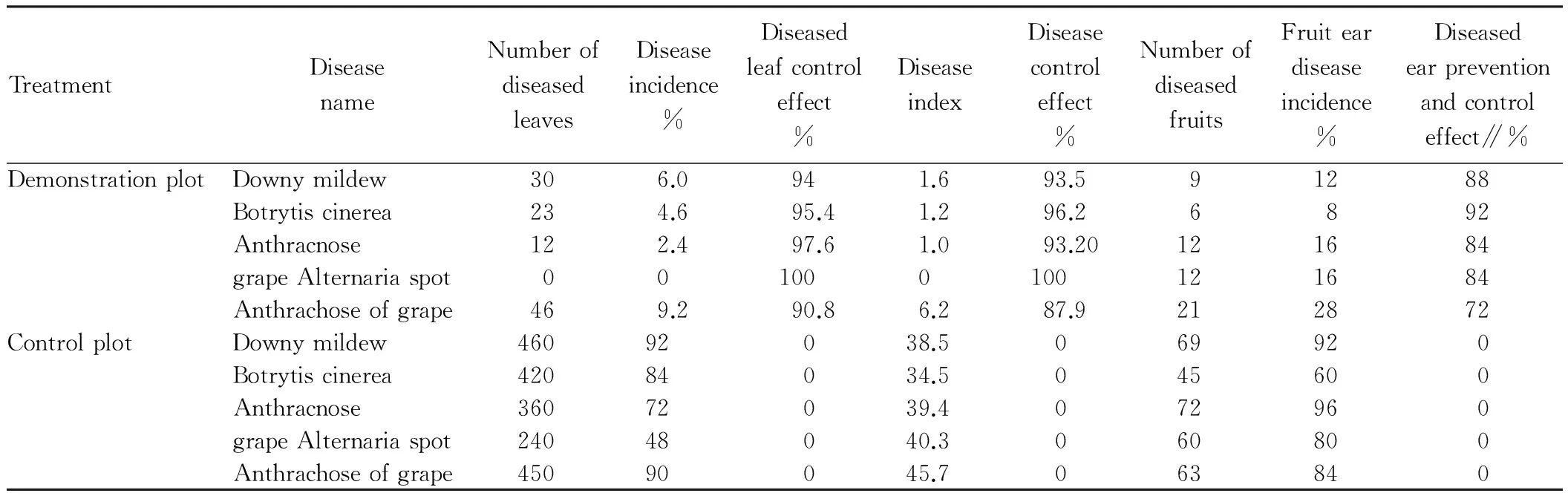
TreatmentDiseasenameNumberofdiseasedleavesDiseaseincidence%Diseasedleafcontroleffect%DiseaseindexDiseasecontroleffect%NumberofdiseasedfruitsFruiteardiseaseincidence%Diseasedearpreventionandcontroleffect∥%DemonstrationplotDownymildew306.0941.693.591288Botrytiscinerea234.695.41.296.26892Anthracnose122.497.61.093.20121684grapeAlternariaspot001000100121684Anthrachoseofgrape469.290.86.287.9212872ControlplotDownymildew46092038.5069920Botrytiscinerea42084034.5045600Anthracnose36072039.4072960grapeAlternariaspot24048040.3060800Anthrachoseofgrape45090045.7063840
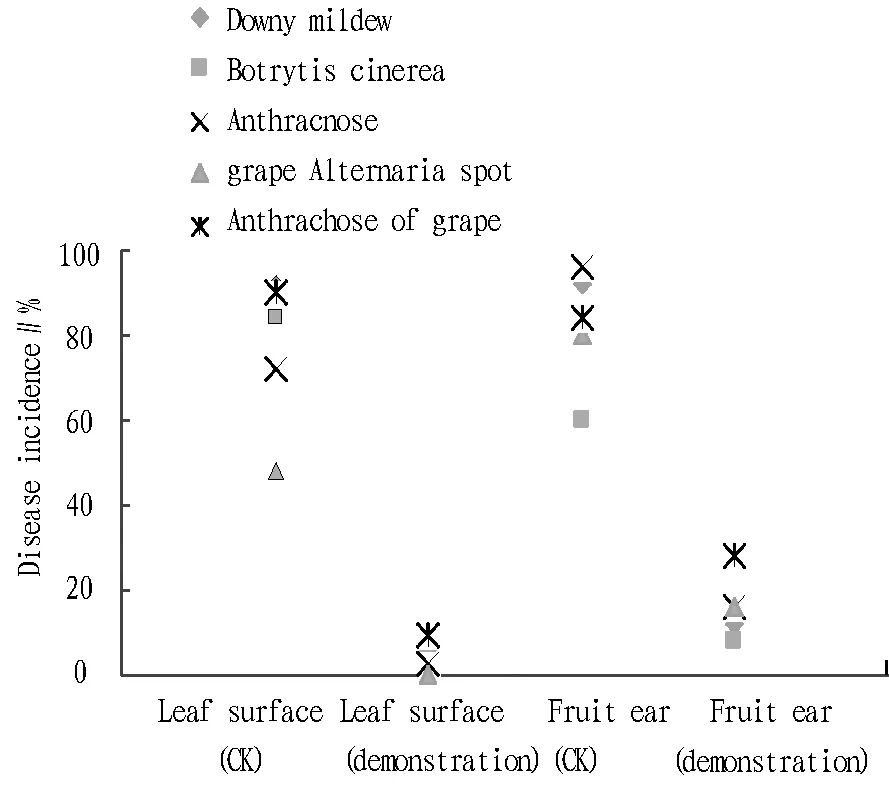
Fig.1 AEW’s virus damage control effect
4.3GrapeviruscontroleffectunderthreetreatmentsIn order to study the electrical functional water control effect, three treatments (spraying acidic electrolyzed water; spraying alkaline electrolyzed water after spraying acidic electrolyzed water; spraying acidic electrolyzed water after spraying alkaline electrolyzed water) for comparative experiment, and it was compared with the treatment of spraying biopesticides. In view of the frequent downy mildew and Botrytis cinerea and serious disease characteristics during the grape cultivation in the southern regions, when the grape was infected with downy mildew or Botrytis cinerea, the bio-pesticides and three electrolyzed water treatments were employed for study. It was found that there were significant differences in the final control effect of different treatments on grape downy mildew and Botrytis cinerea (June 23, 2015). As shown in Fig.2, applying pesticide, applying alkaline electrolyzed water after applying acidic electrolyzed water, and applying acidic electrolyzed water, had good control effect, with disease index of 2.1, 2.8 and 2.2, respectively. The disease index of applying acidic electrolyzed water after applying alkaline electrolyzed water was 4.8. In this experiment, there was no significant difference in control effect of grape downy mildew and Botrytis cinerea between bio-pesticides and applying acidic electrolyzed water or applying alkaline electrolyzed water after applying acidic electrolyzed water, while it was greatly different from applying alkaline electrolyzed water after applying acidic electrolyzed water. It can be found that applying acidic electrolyzed water, and applying alkaline electrolyzed water after applying acidic electrolyzed water, can have good control effect on grape downy mildew and Botrytis cinerea. In the prevention and control, there is a need to reduce the application of pesticides or avoid the use of pesticides, in order to reduce pesticide residues and environmental pollution. In addition, applying acidic electrolyzed water after alkaline electrolyzed water has ordinary control effect, and the reason needs to be further studied.
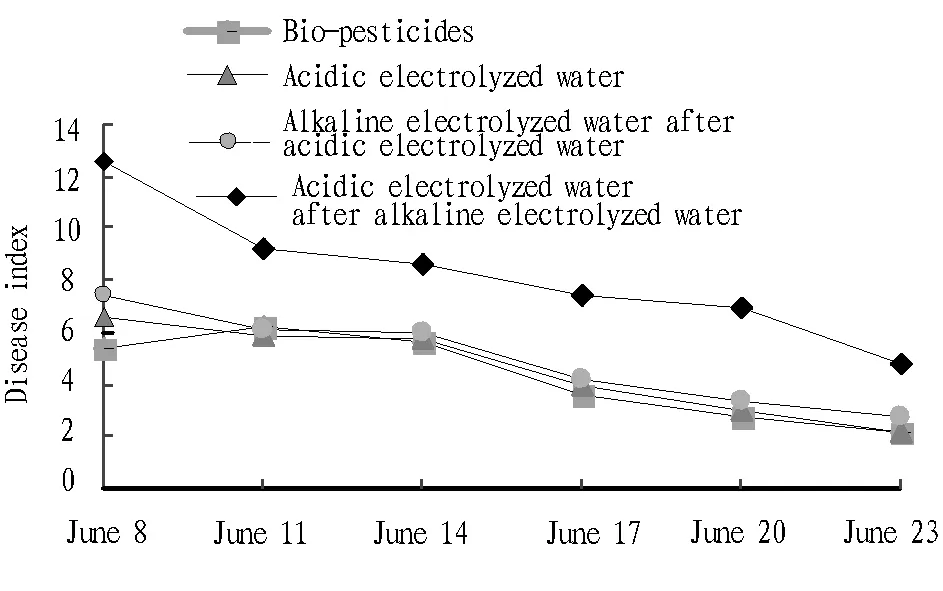
Fig.2 Control effect comparison of three treatments and bio-pesticides
4.3EffectofelectrolyzedwaterongrapeweightandsugardegreeFor about every seven days, the Xiahei grape ripe during the same period is picked, and by sampling comparison, it is found that under the treatment of applying acidic electrolyzed water, the average weight or sugar degree of Xiahei grape are significantly higher than that of traditional varieties. As shown in Fig. 3, the average grape weight of Xiahei increases by 11.2%, and sugar degree increases by 9.6%, 1.8 points higher than that of normal varieties. It suggests that acidic electrolyzed water can promote grape growth and improve the quality of grape.
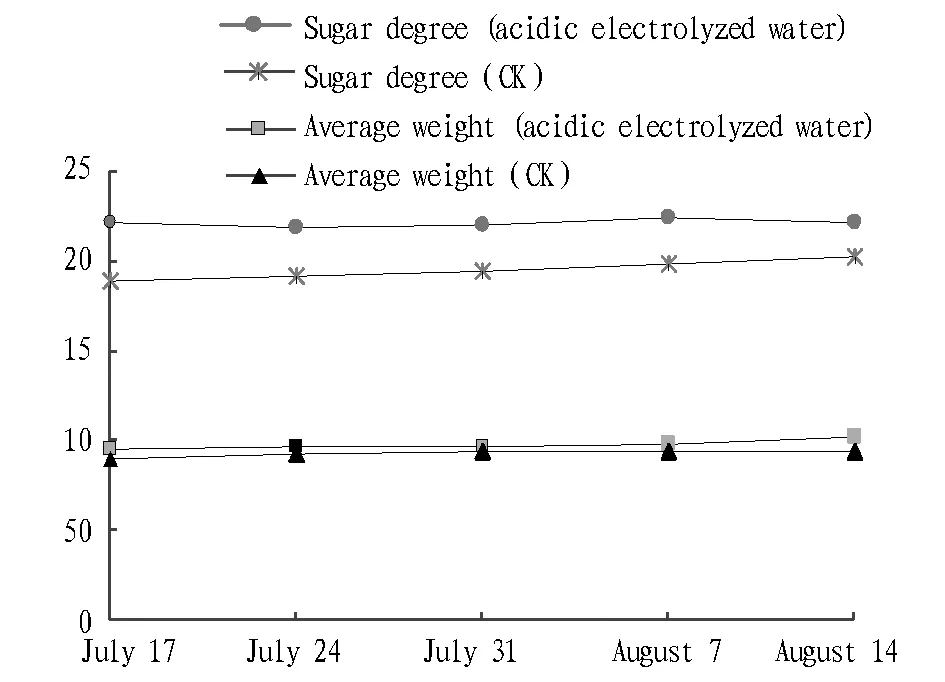
Fig.3 Effect of electrolyzed water on average grape weight and sugar degree
5 Conclusions and discussions
The experimental results show that applying acidic electrolyzed water, and applying alkaline electrolyzed water after applying acidic electrolyzed water have good effect in controlling some diseases such as grape downy mildew, Botrytis cinerea, anthracnose, grape Alternaria spot and anthrachose of grape (94% and 79.2%, respectively), and can replace pesticides. Acidic electrolyzed water can increase average grape weight and sugar degree, so acidic electrolyzed water can be safely applied in the greenhouse grape cultivation. The shortcoming in this experiment is that it lacks in-depth study of grape’s Vc, soluble sugar, soluble protein content, and it will be addressed at the next stage. In this study, 150 L/h acidic electrolyzed water can be obtained from the water electrolysis device, and l h of water output can meet the application needs of five greenhouses (300 m2). The equipment is simple, and has low energy consumption and low electrolyte cost, with good promotion value. However, the electrolyzed water equipment has a high demand on water quality, and some areas with poor water quality need to be equipped with water softener. Studies have shown that acidic electrolyzed water plays a strong role in killing various types of microbes, and has good fungicidal properties[18-19]. Compared with other chemical disinfectants, acidic electrolyzed water can be completely restored into non-toxic and non-residual ordinary water after sterilization, and it does not damage the ecological environment. The production cost of acidic electrolyzed water is low, and it is simple and easy to use, so it can be used as a kind of safe fungicide or disinfectant.
[1] GUAN DS, LI LT. The preparation of strong acidification water and its sterilizing effect[J]. Journal of China Agricultural University, 1997, 2(2): 109-113. (in Chinese).
[2] ZHI SH.The preparation of strong oxidized water and its application in sterilizing[J]. Chinese Journal of Disinfection,1998,15(3):179-180.(in Chinese).
[3] WU L, XIAO WH, LI LT,etal. The preservation of fresh grape by the acidic electrolyzed water[J]. Food Science and Technology, 2004, 39(9):81-83.(in Chinese).
[4] WEI XP, DONG Y, LUAN GZ,etal. Effect of electrolyzed water on controlling cucumber powdery mildew and cucumber growth and quality[J].China Plant Protection, 2015,35(2):8-12.(in Chinese).
[5] KUMAR SV, GABRIEL OE, YENCON H,etal. Efficacy of electrolyzed oxidizing water for inactivating Escherichia coli O157:H7, Salmonella enteritidis, and Listeria monocytogenes[J]. Applied and Environmental Microbiology, 1999,65(9): 4276-4279.
[6] LI LT. Agriculture application research and prospect of functional water engendered from electricity[J].China Agricultural Information,2006(1):15-16.(in Chinese).
[7] SHIGETO S, KOKI M, HIROMOTO Y. Acidic electrolyzed water in the disinfection of the ocular surface[J]. Experimental Eye Research, 2000, 70: 1-6.
[8] ABDULSUDI IZ, YOSHINORI K, NAMI M,etal. Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for decontamination of fresh ready-to-eat vegetables and sprouts [J]. Food Control, 2011, 22(3-4): 601-607.
[9] TOYOAKL A, MAKOTO M. Continuous flow determination of free chlorine in water[J]. Analytical Chemistry, 1983, 55: 209-212.
[10] LIU YY, LIU HJ, TATSUMI E,etal. The application of UV spectrophotometry in the measuring of slightly acid electrolyzed water[J].Modern Food Science and Technology, 2011, 27(11):1393-1397.(in Chinese).
[11] HAO JX, LI LT. Study on removal of pesticide residues on vegetables with electrolyzed functional water[J].Science and Technology of Food Industry,2006,27(5):164-166.(in Chinese).
[12] LI HZ, ZHENG SF, SONG SH,etal. Study on sterilization and preservation of fruits and vegetables using acidic electrolyzed oxidizing water[J].Modern Food Science and Technology, 2011, 27(3):361-365.(in Chinese).
[13] XIAO WD, LI LT, LI ZG. Assay study on strawberries storage treated by electrolyzed acid water[J].Food Science, 2003, 24(5):152-155.(in Chinese).
[14] SOO-VOON L, YEN-CON H, MARILYN E,etal. Ultraviolet spectrophotometric characterization and bactericidal properties of electrolyzed oxidizing water as influenced by amperage and pH[J]. Journal of Food Protection, 2000,63 (11):1534-1537.
[15] XIAO WH, LI LT, LI ZG. Tests of functional water produced by electrolytic action against powdery mildew disease on cucumber[J].Plant Protection,2003,29(2):50-51.(in Chinese).
[16] SEYMOUR S, BLOCK. Disinfection, sterilization, and preservation, 3rd ed[M]. Philadelphia: Lea and Febiger, 1983.
[17] FABRIZIO KA, CUTTER CN. Application of electrolyzed oxidizing water to reduce Listeria monocytogenes on ready-to-eat meats[J]. Meat Science,2005, 71:327-333.
[18] LUO XX. The effect analysis of acidic electrolyzed water on controlling cucumber powdery mildew[J]. Journal of Anhui Agricultural Sciences, 2015,43(36):207-209.(in Chinese).
[19] GAO XH, LIU ZH, LI XL,etal. Sterilization mechanism and application of strong acidic electrolyzed water[J]. Chinese Agricultural Science Bulletin, 2008,24(7):393-399.(in Chinese).
杂志排行
Asian Agricultural Research的其它文章
- How to Improve the Teaching Quality of Plant Physiology?
- Overview of Ecological Toxicity of Potassium Chlorate Pollution
- Path Choice of Rural Land Transfer in China
- A Study on the Reform of China’s Agricultural Administration System
- Recent Advance in Division of Carbohydrate and Protein Fractions of Ruminant Feed and Their Metabolism in Digestive Tract
- Current Situation and Protection Measures concerning the Wetland Resources in Yunnan Province
