An immortalized rat pancreatic stellate cell line RP-2 as a new cell model for evaluating pancreatic fibrosis, inflammation and immunity
2015-12-24
Changchun, China
An immortalized rat pancreatic stellate cell line RP-2 as a new cell model for evaluating pancreatic fibrosis, inflammation and immunity
Rong-Li Piao, Ming Xiu, David R Brigstock and Run-Ping Gao
Changchun, China
BACKGROUND: Pancreatic stellate cells (PSCs) play a critical role in the pathogenesis of pancreatic fibrosis and have emerging functions as progenitor cells, immune cells or intermediaries in pancreatic exocrine secretion. Increasing evidence has shown that desmin as an exclusive cytoskeleton marker of PSC is only expressed in part of these cells. This study was to establish a desmin-positive PSC cell line and evaluate its actions on pancreatic fibrosis, inflammation and immunity.
METHODS: The presence of cytoskeletal proteins, integrin α5β1or TLR4, was determined by immunocytochemistry while the production of desmin, collagen I, MMP-1, MMP-2, TIMP-2, or CD14 was evaluated by Western blotting. The levels of desmin, collagen I, IL-1 and IL-6 mRNA were determined by real-time quantitative PCR. The secretion of cytokines was detected by ELISA. Cell function was assessed using adhesion, migration, or proliferation assays.
RESULTS: A stable activated rat PSC cell line (designated as RP-2) was established by RSV promoter/enhancer-driven SV40 large T antigen expression. RP-2 cells retained typical PSC properties, exhibited a myofibroblast-like phenotype and persistently produced desmin. The cells produced collagen I protein, matrix metalloproteinases and inhibitors thereof. RP-2 cells demonstrated typical PSC functions, including proliferation, adherence, and migration, the latter two of which occurred in response to fibronectin and were mediated by integrin α5β1. TLR4 and its response genes including proinflammatory cytokines (IL-1, IL-6, TNF-α) and chemotactic cytokines (MCP-1, MIP-1α, Rantes) were produced by RP-2 cells and activated by LPS. LPS-induced IL-1 or IL-6 mRNA expression in this cell line was fully blocked with MyD88 inhibitor.
CONCLUSION: RP-2 cells provide a novel tool for analyzing the properties and functions of PSCs in the pathogenesis of fibrosis, inflammation and immunity in the pancreas.
(Hepatobiliary Pancreat Dis Int 2015;14:651-659)
pancreatic stellate cell;
pancreatic fibrosis;
Toll-like receptor 4;
inflammation;
immunity
Introduction
Pancreatic fibrosis is a constant pathological feature of chronic pancreatitis and pancreatic cancer.[1-4]Pancreatic stellate cells (PSCs) are a major cell type responsible for pancreatic fibrosis.[1,5,6]In the normal pancreas, PSCs are found in periacinar and periductular location and contain vitamin A in cytoplasmic lipid droplets. During pancreatic injury, quiescent PSCs become transcriptionally active in response to paracrineacting cytokines and undergo a phenotypic transformation into activated fibroblast-like or myofibroblast-like cells which express the cytoskeletal proteins α-smooth muscle actin (α-SMA) and glial fibrillary acidic protein (GFAP), together with variable production of desmin.[5]
In vitrostudies have shown that activated PSCs have the capacity to proliferate, adhere, migrate, and synthesize type I collagen and other extracellular matrix (ECM) proteins.[1,5,7,8]Additionally, activated PSCs synthesize and secrete cytokines which influence PSC function via autocrine pathways. They also produce matrix degrading enzymes (matrix metalloproteinases, MMPs) and their inhibitors (tissue inhibitor of metalloproteinases, TIMPs),resulting in a shift from the normal ECM turnover of the healthy pancreas to a condition in which ECM production is enhanced and ECM degradation is suppressed, leading to pathological fibrosis during chronic pancreatic disease.[1,9]Accumulating evidence suggests that PSCs may function as immune cells that express Tolllike receptors (TLR2, 3, 4, 5) which recognize foreign pathogen-associated molecular patterns (PAMPs).[1,10,11]Lipopolysaccharide (LPS) is a well-characterized PAMP for TLR4 and exerts a synergistic effect with alcohol on PSC activation.[10]
Primary PSC culture is a useful tool for studying mechanisms of pancreatic fibrogenesis. A variety of methods have been described for their isolation, including enzymatic tissue digestion and density gradient centrifugation or outgrowth of PSC from pancreatic tissue.[7,12,13]However, these methods may yield a heterogenous mixture of activated PSCs with other pancreatic cell types, including α-SMA-negative fibroblasts and/or vascular smooth muscle cells.[5]The isolation of PSCs is time consuming and yields are modest. Comparison of results from different batches of PSC may be challenging because isolates from rodent or human pancreatic tissues vary between preparations and even the same preparation may produce a variable response depending on the passage conditions. In our earlier studies of connective tissue growth factor (CTGF) in primary rat PSC,[7,14]we found that the most reproducible data were obtained at passages 2-4.
Several immortalized PSC lines (designated as SIPS or LTC-14 and SAM-K) have been developed either through spontaneous generation or by murine leukemia retrovirus-mediated simian virus 40 (SV40) antigen transfer.[15-17]While such cell lines can potentially address some of the drawbacks of primary cultures, they have not been broadly embraced and are in stark contrast to the development of a variety of hepatic stellate cell (HSC) lines that have been widely accepted and provided considerable traction in the field of hepatic fibrosis.[18]
Since the Rous Sarcoma virus (RSV) promoter/enhancer contains stronger regulatory elements than SV40 or Vlambda1 for expression of genes in lymphoid cell lines,[19]we established a new stable rat PSC cell line (RP-2) by immortalization of primary rat PSC by RSV promoter/enhancer-driven expression of SV40 large T antigen. Extensive characterization showed that RP-2 cells are smaller in size of myofibroblast-like phenotype than primary activated PSCs and persistently produce desmin, the latter of which is different from the previously described LTC-14 or SAM-K PSC cell lines.[16,17]RP-2 cells provide a new model for studying the pathogenesis of pancreatic fibrosis, inflammation and immunity.
Methods
Isolation and culture of PSC
All rats, weighing 180-200 g, were obtained from the Experimental Animal Center of Jilin University. Rat PSCs were isolated by the method described previously[7]using a protocol that was approved by the Ethics Committee on Animal Experiments of the First Hospital of Jilin University. Freshly isolated PSCs were cultured in DMEM supplemented with 25 mmol/L Hepes buffer, 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained at 37 ℃ in a humidified atmosphere of 5% CO2/95% air. Activated PSCs were split every 3 days at a ratio of 1:3 and used in the experiments at passages 2 to 4.
Transfection and immortalization of rat PSC
Primary PSCs at passage 3 were plated in 6-well culture plates at a density of 40% confluence. After incubation for 12 hours, the medium was exchanged with fresh medium and the cells were transiently transfected for 24 hours with X-tremeGENE HP DNA Transfection Reagent (Roche, Mannheim, Germany), containing a cDNA that mediated the expression RSV-driven SV40 large T antigen. The cell culture subsequently underwent eight passages. Multiple cell clones were selected and evaluated for their transformation phenotypes. A single cell clone, termed RP-2, which demonstrated a stable phenotype closely resembling characteristics of activated PSC, was selected and studied for over 120 generations. RP-2 cell line used in the experiments at passages 35 to 120.
Immunocytochemistry
RP-2 cells were cultured in 4-well Lab-Tek® chamber slides. At the end of culture, slides were washed in PBS, and fixed for 30 minutes in -20 ℃ acetone. Thereafter, slides were preincubated with 0.3% Triton-X100 in PBS for 30 minutes. Incubations with mouse monoclonal antibodies to α-SMA (1:300), vimentin (1:100), or desmin (1:100) (Boster, Wuhan, China) were performed at room temperature for 1 hour, followed by FITC- or TRITC-labeled goat anti-mouse antibodies for 30 minutes. For SV40 large T antigen or GFAP staining, slides were successively incubated with mouse anti-SV40 Tag monoclonal antibody (Pab 101, 1:50) (Santa Cruz, San Francisco, USA) or rabbit anti-GFAP polyclonal antibody (1:50) (ProteinTech, USA) or rabbit anti-TLR4 polyclonal antibody (Boster, Wuhan, China) for 1 hour, followed by biotinylated anti-IgG and streptavidin-HRP. Development of the chromogenic color reaction was accomplished using 3-amino-9-ethylcarbazole or 3, 3'-diaminobenzidine tetrahydrochloride substrate. Primary PSCs werestained with oil red O to detect cytoplasmic fat droplets. All slides were examined under an Olympus BX51 TRF fluorescent/light microscope (Olympus, Tokyo, Japan).
Real-time quantitative PCR analysis
Total RNA was extracted from activated PSCs or RP-2 cells using Trizol reagent according to the manufacturer's instructions (Dingguo, Beijing, China). Levels of mRNA were determined by real-time quantitative PCR (qPCR). Briefly, 1 μg RNA was reverse transcribed to cDNA using a PrimeScript® RT reagent kit (Takara, Dalian, China). Real-time qPCR analysis was performed using power SYBR Green PCR Master Mix (Life Technologies, Warrington, UK) with primers shown on the Applied Biosystems 7500 Sequence Detection System (Table 1). Data were normalized to β-actin, and fold change in target gene expression converted to Ctvalues using the Delta-Delta Ctmethod. Real-time qPCR assays were repeated three times. Fold changes were compared using one-way ANOVA followed by the Newman-Keuls test.
Western blotting analysis
RP-2 cells were placed in 6-well culture plates at a density of 2.5×105cells/well and incubated for 12 hours in 1% FBS medium. The medium was then exchanged for 0.5% FBS medium and the cells were allowed to incubate for 1 hour, after which they were treated with or without 20 ng/mL TGF-β1or 10 ng/mL platelet-derived growth factor (PDGF) and allowed to incubate for additional 24 hours. The cells were then washed with cold PBS prior to addition of 60 μL cell lysis buffer containing six broad-spectrum protease inhibitors (Sigma) with rocking at 4 ℃ for 30 minutes. Protein samples (15 μg each) were separated on 10% SDS-PAGE, and transferred to nitrocellulose. After washing with Tris buffer saline with Tween 20 (TBS/T) and blocking with 2.5% non-fat milk, the membranes were separately incubated with rabbit polyclonal anti-desmin, anti-collagen I, antiβ-actin, or anti-MMP-1, or anti-MMP-2, anti-TIMP-2 antibodies, or mouse monoclonal anti-CD14 (Protein-Tech, USA) at 4 ℃ overnight. Blots were incubated for 1 hour with HRP-linked goat anti-rabbit IgG (Beyotime), and washed extensively with TBS/T before detection using the ECL system (Thermo, USA).
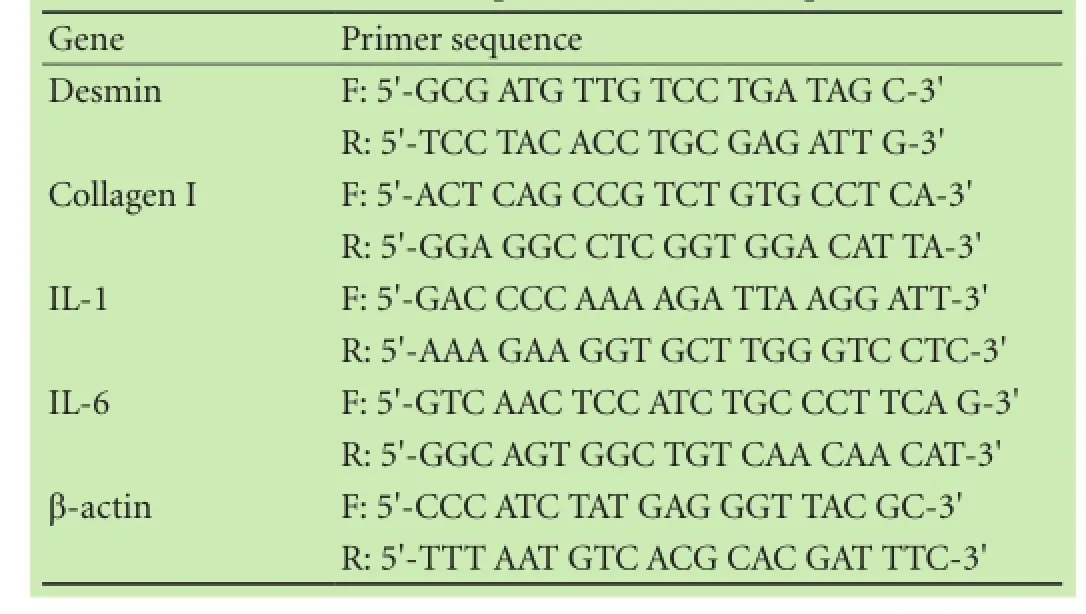
Table 1. Real-time quantitative PCR sequences
Cell adhesion assay
96-well microtiter plates (Becton Dickinson, Franklin Lakes, NJ) were pre-coated with 2 μg/mL fibronectin (FN) for 16 hours at 4 ℃, followed by blocking with 1% BSA at room temperature for 1 hour. Each well then received 50 μL of RP-2 cells (2.5×105cells/mL) that had been detached from stock plates using 1 mmol/L EDTA PBS and resuspended in DMEM/0.5% BSA. After incubation at 37 ℃ for 25 minutes, adherent cells were fixed with 10% formalin, stained with methylene blue, and quantified by dye extraction and measurement of absorbance at 620 nm.
Cell migration assay
RP-2 cells were suspended in cell culture inserts (2.5 × 104cells/insert) that were contained within individual wells of a 12-well companion plate. The ability of RP-2 cells to migrate to the underside of the insert (8 μm pore size) in response to the presence of PDGF (10 ng/mL), TGF-β1(20 ng/mL) or FN (100 ng/mL) was assessed after a 6-hour incubation. RP-2 cells on the upper and lower surfaces of the membrane were counted in 10 random 400× fields.[7]
Cell proliferation assay
A total of 1×104RP-2 cells were placed in each well of a 96-well plate and cultured in DMEM containing 1% FBS for 6 hours. The medium was replaced with 0.1 mL of DMEM/0.5% FBS in the presence or absence of 10 ng/ mL PDGF, 20 ng/mL TGF-β1or 100 ng/mL FN, and the cells were then incubated for 24 hours. Ten μL of CCK-8 reagent was added to each well and incubated for 1 hour. Cell proliferation was assessed using an ELISA plate reader at 450 nm.
Enzyme linked immunosorbent assay
A total of 1×105RP-2 cells were placed in each well of a 12-well plate and cultured in DMEM containing 1% FBS for 6 hours. The cells were cultured for another 24 hours in the absence or presence of 1 μg/mL LPS. The supernatants were measured with ELISA kits for IL-1, IL-6, TNF-α, MCP-1, Rantes, or MIP-1α (Uscn Life Science Inc, TX, USA) according to the manufacturer's protocols. Briefly, microtiter wells were pre-coated with100 μL of each standard or supernatants for 2 hours at 37 ℃. The plates were then developed by sequential addition of biotinylated primary antibody, avidin-conjugated horseradish peroxidase and tetramethylbenzidine substrate solution, and the color reaction was measured at 450 nm.Statistical analysis
The values we obtained represent mean±SD of the measurements from at least 4 separate RP-2 cell cultures. Statistical analysis of the data was performed using SPSS 11.5 for Windows. Student'sttest was used for paired data.Pvalues <0.05 were considered statistically significant.
Since desmin is expressed at variable levels by activated PSCs,[5,7,13]we examined its production in RP-2 cells as compared with activated PSCs. The levels of desmin mRNA or protein in RP-2 cells were comparable between passage 35 versus passage 120 and were obviously elevated compared to activated PSCs on day 14 after isolation (P<0.01) (Fig. 2A, C). In contrast, expression of collagen I was similar in RP-2 cells at passage 35 or 120 and in primary activated PSCs on day 14 (Fig. 2B).
Results
Characteristics of rat PSC cell line
Three days after seeding, cytoplasmic fat droplets were detected in PSCs by oil red O staining or their translucence under phase contrast (Fig. 1A, B). An immortalized PSC cell line, RP-2, was successfully generated by transfection of primary rat PSC with RSV promoter/enhancer-driven SV40 large T antigen. RP-2 cells exhibited a myofibroblast-like shape, and were morphologically very similar to primary, culture-activated PSCs. The cells expressed SV40 large T antigen in their nuclei and also formed a pattern of staining of GFAP, desmin, vimentin, or α-SMA that is typical of activated PSCs (Fig. 1C-G).
TGF-β dependency of collagen I or matrix remodeling proteins in RP-2 cells
As assessed by Western blotting, RP-2 cells treated with TGF-β1exhibited higher levels of collagen I protein than control non-treated cells (Fig. 2D). Furthermore, TGF-β1-treated cells showed a decreased ability to produce MMP-1 or MMP-2 and an enhanced production of TIMP-2 (Fig. 2E). In contrast, PDGF-treated RP-2 cells demonstrated unchanged production of collagen I, MMP-1, or MMP-2 but somewhat diminished levels of TIMP-2 (Fig. 2D, E).
Adhesion of RP-2 cells to FN is mediated by integrin α5β1

Fig. 1. Key characteristics of immortalized rat PSC cell line, RP-2. Micrographs of cytoplasmic oil droplets detected by (A) oil red O staining or (B) phase contrast microscopy in PSCs 3 days after seeding; RP-2 cells were detected by immunocytochemistry to show the presence of (C) SV40 large T antigen, (D) GFAP, (E) desmin, (F) vimentin, or (G) α-SMA (A, B: original magnification ×400; C-G: original magnification ×200).
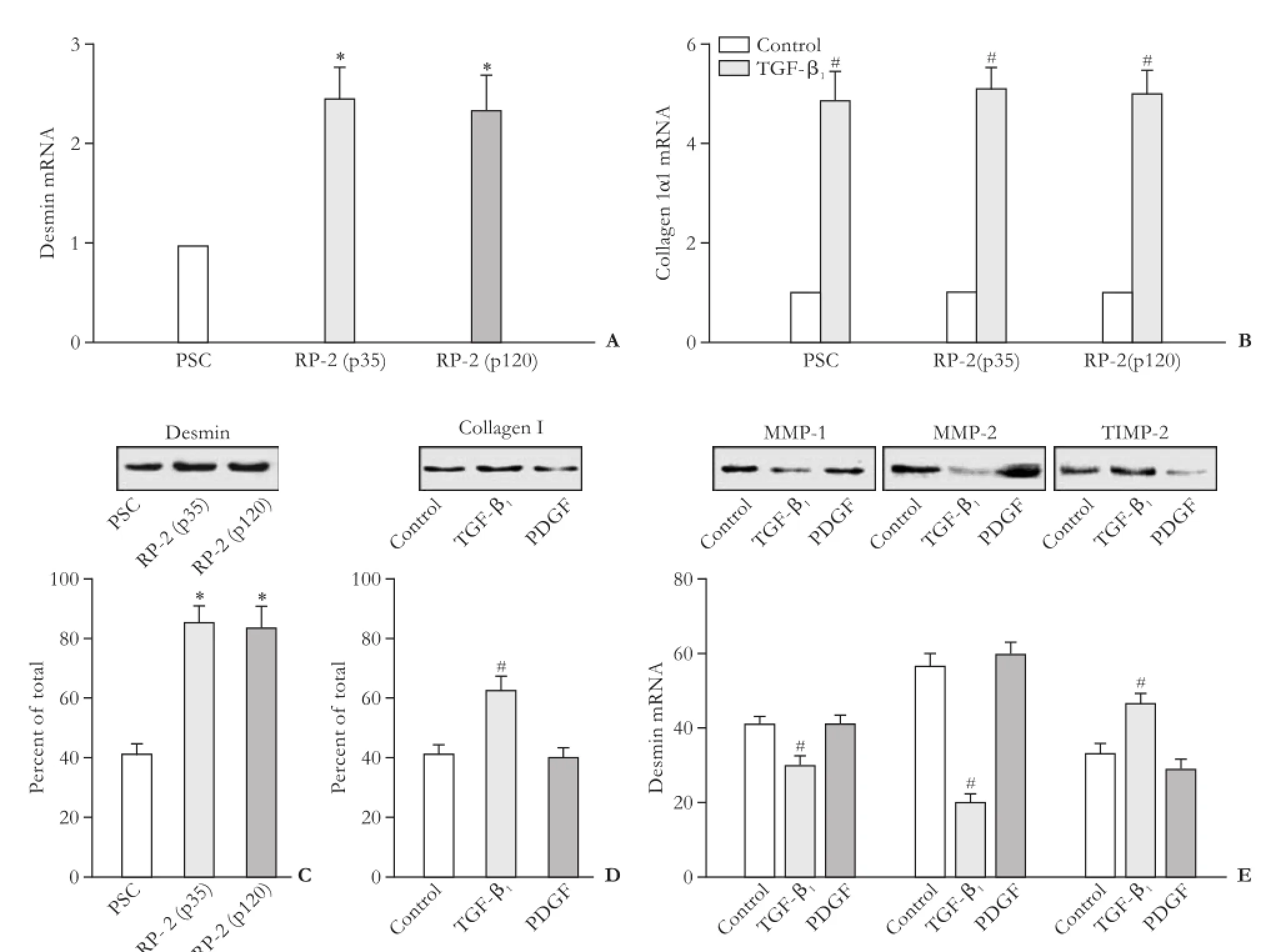
Fig. 2. Expression of profibrotic genes in RP-2 cells (*:P<0.01, vs PSC; #:P<0.01, vs control). Real-time qPCR analysis showing actions of expression of (A) desmin or (B) collagen I mRNA between primary PSC (passage 4), RP-2 cells (passage 35) and RP-2 cells (passage 120). Normalized with β-actin mRNA is shown as mean±SD of triplicate determinations. Western blotting showing the levels of desmin in different cell groups (C) or the effect of TGF-β1or PDGF treatment on (D) collagen I levels or (E) MMP-1, MMP-2, or TIMP-2 levels. Densitometry of the bands is shown as the mean±SD of triplicate determinations.
Expression of integrin α5β1in RP-2 cells was detected by an indirect immunofluorescence procedure and a dramatic morphological transformation of the cells was seen by addition of 2 μg/mL FN to the medium (Fig. 3A). As shown in Fig. 3B, FN significantly promoted RP-2 cell adhesion (P<0.01 vs control), an effect that was blocked by EDTA but supported by Mn2+. However, there was no effect on FN-dependent RP-2 cell adhesion in the presence of both EDTA and Mn2+or in the presence of heparin or heparinase I (Fig. 3B). These data suggested that cell surface integrins but not heparan sulfate proteoglycan contributed the regulation of cell adhesion by FN. FN-mediated cell adhesion was fully blocked by anti-α5β1, but not by normal mouse IgG (Fig. 3C), indicating that cell surface integrin α5β1was a principal FN receptor in RP-2 cells, consistent with our previous data for primary rat PSC.[7]cell migration with similar efficiency. The cell migration in response to FN was fully blocked when the cells were treated with an anti-integrin α5β1monoclonal antibody, but not with mouse IgG (Fig. 3E). Treatment of RP-2 cells with PDGF, but not TGF-β1or FN, significantly enhanced cell proliferation (Fig. 3F).
Migration or proliferation of RP-2 cells
Previously we found that FN, TGF-β1or PDGF is chemotactic for activated PSC,[7]and then we determined whether these proteins were involved in stimulating RP-2 cell migration. As shown in Fig. 3D, 100 ng/mL FN, 20 ng/mL TGF-β1, or 10 ng/mL PDGF stimulated
LPS activates the TLR4 pathway
In view of emerging role of PSC in innate immunity, we determined the expression of TLR4 and its response genes in RP-2 cells. TLR4 and its co-receptor CD14 were detected in RP-2 cells and their respective levels were enhanced by the exposure of cells to LPS (Fig. 4). We examined the role of the LPS-TLR4 pathway in modulating the secretion of proinflammatory and chemotactic cytokines from RP-2 cells after stimulation with LPS. The secretion of IL-1, IL-6, TNF-α, MCP-1, Rantes and MIP-1α was elevated respectively 7.3-fold, 2.9-fold, 2.1-fold, 2.0-fold, 4.5-fold and 2.5-fold in LPS-treated cellscompared with controls (eachP<0.01, Table 2). To gain further insight into LPS-TLR4 signaling, RP-2 cells were treated with MyD88 inhibitors (ST2825) in the presence or absence of LPS. ST2825 fully blocked basal or LPS-stimulated IL-1 or IL-6 mRNA transcription in the cells (Fig. 4C, D).
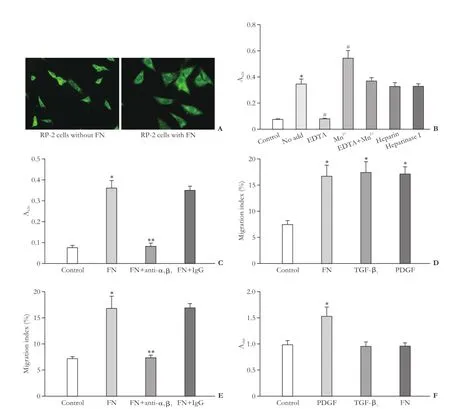
Fig. 3. RP-2 cell adhesion, migration and proliferation (*:P<0.01, vs control; #:P<0.01, vs no add; **:P<0.01, vs FN). A: Immunostaining for integrin α5β1showing morphology of control cells (left) as compared to that of the cells adhered to FN (10 μg/mL) (right) in 4-well Lab-Tek® chamber slides (original magnification ×400). B: RP-2 cell adhesion assays were performed on microtiter wells that had been precoated with FN following preincubation of the cells for 30 minutes in vehicle buffer (no add) or EDTA (5 mmol/L) or Mn2+(10 mmol/L) or in combination with both or heparin (2 μg/mL) or heparinase I (2 U/mL). C: RP-2 cells were preincubated with anti-integrin α5β1(25 μg/mL) or mouse IgG (25 μg/mL) for 30 minutes before adding the cells to the wells that had been precoated with FN. D: RP-2 cell migration assays were performed in the absence or presence of FN or TGF-β1or PDGF. E: RP-2 cells were preincubated with anti-integrin α5β1or mouse IgG for 30 minutes. Cell migration assays were performed in the absence or presence of FN. F: RP-2 cell proliferation was evaluated in the absence or presence of PDGF, TGF-β1or FN.

Table 2. The levels of cytokines in the supernatant of RP-2 cells
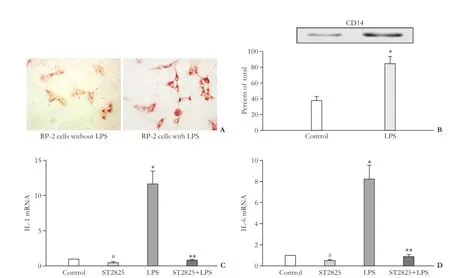
Fig. 4. Expression of TLR4 or its response genes in RP-2 cells (*:P<0.01, vs control; #:P<0.05, vs control; **:P<0.01, vs LPS). A: TLR4 protein detected by immunocytochemistry (original magnification ×400) or B: TLR4 co-receptor CD14 protein detected by Western blotting after RP-2 cells were treated with or without LPS (1 μg/mL). Densitometry of the bands is shown as the mean±SD of triplicate determinations. Real-time qPCR analysis showing that ST2825 could fully block expression of (C) IL-1 or (D) IL-6 mRNA in LPS-treated cells or partially block in LPS-untreated cells. Normalized with β-actin mRNA is shown as mean±SD of triplicate determinations.
Discussion
By establishing RSV promoter/enhancer-driven SV40 large T antigen gene expression in primary rat PSCs, we have established an immortalized PSC cell line, RP-2, that is stable for >120 passages. Many features of RP-2 cells confirm their similarity to activated PSCsin vivoorin vitro. These include RP-2 cell expression of α-SMA which is a typical marker of a myofibroblastic cells, including activated PSCs in chronic pancreatic diseases and fibrosis.[1,20-22]Additionally, RP-2 cells also express some selective markers of activated PSC, including vimentin and GFAP.[1,7,21]This cell line is smaller in size of myofibroblast-like phenotype than primary activated PSCs and distinguishable from other rat PSC cell lines (LTC-14, SAM-K) by its persistent expression of desmin.[16,17]Therefore, we speculate that RP-2 cells may be a subtype derived from activated PSCs.
Previous studies[7,13]reported that the activated myofibroblast phenotype of PSCs was associated with overexpression of collagen I mRNA. This finding is confirmed and extended by our data showing that RP-2 cells synthesize collagen I mRNA and protein, the levels of which are enhanced in response to TGF-β1. Increasing evidence has shown that activated PSCs (and also activated HSCs) have the capacity to synthesize and secrete matrix degrading enzymes (MMP-1, MMP-2) and their inhibitors (TIMP-2), suggesting that these cells may also play a role in the regulation of ECM degradation.[9,23,24]The present study showed that in RP-2 cells, MMPs or TIMPs are decreased or increased after exposure of the cells to TGF-β1, showing that ECM deposition in RP-2 cells is principally regulated by TGF-β1, as is also the case in primary PSC (or HSC) cultures.[9,23,24]
In addition to the production of ECM proteins, matrix degrading enzymes and their inhibitors, activated PSCs also have the capacity to adhere, migrate, or proliferate.[1,7,14]Each of these functions is stimulated during progressive activation by diverse extracellular stimuli such as growth factors, cytokines, and ECM proteins.[7,14]The present study showed that RP-2 cells proliferate in response to PDGF, adhere to FN, and migrate in response to TGF-β1, PDGF, or FN. Using blocking antibodies to integrin α5β1, we found that FN-mediated RP-2 cell adhesion and migration involve the binding of FN to integrin α5β1. Furthermore, immunocytochemistry confirmed the presence of integrin α5β1in RP-2 cells and that its staining intensity was enhanced when the cells adhered to FN, which appears to be strongly correlatedwith an accelerated morphological transformation. The above studies pave a way for studying the relationship of integrins with some matrix protein ligands using this cell line. Future studies may give interesting insight into the physiological role and mechanisms of FN in regulating PSC function.
TLR4 together with its two co-receptors (CD14 and myeloid differentiation protein 2, MD2) comprise the LPS receptor complex.[25]CD14 is a glycophophatidylinositol-linked protein expressed on the surface of LPS-responsive cells such as macrophage or monocytes.[26,27]Over the last decade, activated human HSCs have been found to express LPS-recognizing receptors such as TLR4, CD14 and MD2 consistent with the identification of intact TLR4 signaling in the cells.[26,27]Upon LPS stimulation, Kupffer cells or HSCs produce proinflammatory cytokines and chemotactic cytokines that recruit each of these cell types to participate in liver inflammation and fibrosis.[26-29]In terms of human chronic pancreatitis, our previous studies showed that expression of TLR4 mRNA in PSCs or macrophages was increased in patients with chronic pancreatitis and there was a significant positive correlation between the levels of plasma LPS and concentrations of serum proinflammatory cytokines (TNF-α, IL-6 and IL-12) in patients with chronic pancreatits.[30]In vitrostudies confirmed that TLR4 as well as the associated molecules CD14 and MD2 were expressed in rat PSCs. Upon activation of TLR4 by LPS, PSCs produce chemokines MCP-1.[11]This finding is confirmed and extended by our data showing that expression of TLR4 or co-receptor CD14 as well as their response genes (IL-1, IL-6, TNF-α, MCP-1, Rantes, MIP-1α) were detected in normal RP-2 cells and enhanced after LPS treatment. Using ST2825, a heptapeptide inhibitor of MyD88 dimerization that controls TLR mediated inflammatory responses,[31]we found that LPS enhances IL-1 and IL-6 mRNA production in RP-2 cells through a MyD88-dependent pathway. Apart from its natural exogenous ligand LPS, we speculate that other endogenous substrates for TLR4 probably bind and activate TLR4 in normal RP-2 cells, including hyaluronic acid, free fatty acid, FN and heat shock proteins,[26,32-36]because basal levels of proinflamatory and chemostatic cytokines decrease in the supernatant of ST2825-treated RP-2 cells. Our results demonstrated that RP-2 cells provide a new tool in the study of LPS/TLR4 signal transduction and the immune response. Our data also suggested that PSCs, in addition to macrophages, may be a target for LPS-induced pancreas injury and provide a direct link between inflammatory and fibrotic pancreas injury.
In conclusion, a stable activated rat PSC line RP-2 has been established by RSV promoter/enhancer-driven SV40 large T antigen expression. This cell line retains typical PSC properties, and is a desmin-positive cell with a myofibroblast-like phenotype. RP-2 cell not only exhibits a useful model for understanding of the pathogenesis of pancreatic fibrosis as other PSC lines, but also provides a novel tool in the study of pancreatic inflammation and immunity.
Contributors:PRL and XM performed the experiments and analyzed data. GRP designed the study and wrote the manuscript. BDR coordinated the study and edited the manuscript. All authors contributed to the design and interpretation of the study and to further drafts. GRP is the guarantor.
Funding:This study was support by grants from the National Natural Science Foundation of China (81070370 and 81270544).Ethical approval:The study was approved by the Ethics Committee on Animal Experiments of the First Hospital of Jilin University (Permit Number: 2013-207).
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Apte MV, Pirola RC, Wilson JS. Pancreatic stellate cells: a starring role in normal and diseased pancreas. Front Physiol 2012;3:344.
2 Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology 2013;144:1210-1219.
3 Lunardi S, Muschel RJ, Brunner TB. The stromal compartments in pancreatic cancer: are there any therapeutic targets? Cancer Lett 2014;343:147-155.
4 Shi C, Washington MK, Chaturvedi R, Drosos Y, Revetta FL, Weaver CJ, et al. Fibrogenesis in pancreatic cancer is a dynamic process regulated by macrophage-stellate cell interaction. Lab Invest 2014;94:409-421.
5 Erkan M, Adler G, Apte MV, Bachem MG, Buchholz M, Detlefsen S, et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 2012;61:172-178.
6 Marzoq AJ, Giese N, Hoheisel JD, Alhamdani MS. Proteome variations in pancreatic stellate cells upon stimulation with proinflammatory factors. J Biol Chem 2013;288:32517-32527.
7 Gao R, Brigstock DR. Connective tissue growth factor (CCN2) in rat pancreatic stellate cell function: integrin alpha5beta1 as a novel CCN2 receptor. Gastroenterology 2005;129:1019-1030.
8 He FH, Gao RP, Piao RL, Tian ZZ, Zhang KY. Detection and clinical significance of pancreatic and serum connective tissue growth factor in the assessment of pancreatic fibrosis. Am J Gastroenterol 2012;107:S88.
9 Phillips PA, McCarroll JA, Park S, Wu MJ, Pirola R, Korsten M, et al. Rat pancreatic stellate cells secrete matrix metalloproteinases: implications for extracellular matrix turnover. Gut 2003;52:275-282.
10 Vonlaufen A, Xu Z, Daniel B, Kumar RK, Pirola R, Wilson J, et al. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology 2007;133:1293-1303.
11 Masamune A, Kikuta K, Watanabe T, Satoh K, Satoh A, Shimosegawa T. Pancreatic stellate cells express Toll-like receptors. J Gastroenterol 2008;43:352-362.
12 Apte MV, Haber PS, Applegate TL, Norton ID, McCaughan GW, Korsten MA, et al. Periacinar stellate shaped cells in rat pancreas: identification, isolation, and culture. Gut 1998;43: 128-133.
13 Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, et al. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998;115:421-432.
14 Gao R, Brigstock DR. A novel integrin alpha5beta1 binding domain in module 4 of connective tissue growth factor (CCN2/ CTGF) promotes adhesion and migration of activated pancreatic stellate cells. Gut 2006;55:856-862.
15 Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J Gastroenterol 2003;9:2751-2758.
16 Satoh M, Masamune A, Sakai Y, Kikuta K, Hamada H, Shimosegawa T. Establishment and characterization of a simian virus 40-immortalized rat pancreatic stellate cell line. Tohoku J Exp Med 2002;198:55-69.
17 Sparmann G, Hohenadl C, Tornøe J, Jaster R, Fitzner B, Koczan D, et al. Generation and characterization of immortalized rat pancreatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2004;287:G211-219.
18 Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, et al. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 2005;54:142-151.
19 Zarrin AA, Malkin L, Fong I, Luk KD, Ghose A, Berinstein NL. Comparison of CMV, RSV, SV40 viral and Vlambda1 cellular promoters in B and T lymphoid and non-lymphoid cell lines. Biochim Biophys Acta 1999;1446:135-139.
20 Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, et al. Senescence of activated stellate cells limits liver fibrosis. Cell 2008;134:657-667.
21 Puche JE, Saiman Y, Friedman SL. Hepatic stellate cells and liver fibrosis. Compr Physiol 2013;3:1473-1492.
22 Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol 2010;7:425-436.
23 Cao Q, Mak KM, Lieber CS. Leptin represses matrix metalloproteinase-1 gene expression in LX2 human hepatic stellate cells. J Hepatol 2007;46:124-133.
24 Hartland SN, Murphy F, Aucott RL, Abergel A, Zhou X, Waung J, et al. Active matrix metalloproteinase-2 promotes apoptosis of hepatic stellate cells via the cleavage of cellular N-cadherin. Liver Int 2009;29:966-978.
25 Kunda PE, Cavicchia JC, Acosta CG. Lipopolysaccharides and trophic factors regulate the LPS receptor complex in nodose and trigeminal neurons. Neuroscience 2014;280:60-72.
26 Guo J, Friedman SL. Toll-like receptor 4 signaling in liver injury and hepatic fibrogenesis. Fibrogenesis Tissue Repair 2010;3: 21.
27 Perugorria MJ, Murphy LB, Fullard N, Chakraborty JB, Vyrla D, Wilson CL, et al. Tumor progression locus 2/Cot is required for activation of extracellular regulated kinase in liver injury and toll-like receptor-induced TIMP-1 gene transcription in hepatic stellate cells in mice. Hepatology 2013;57:1238-1249.
28 Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol 2012;590:447-458.
29 Inokuchi S, Tsukamoto H, Park E, Liu ZX, Brenner DA, Seki E. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin Exp Res 2011;35:1509-1518.
30 Tian ZZ, Gao RP, Li YJ, He FH, Zhang KY. Bacterial endotoxin: a trigger factor for human chronic pancreatitis. Am J Gastroenterol 2012;107:S88.
31 Zhu J, Mohan C. Toll-like receptor signaling pathways--therapeutic opportunities. Mediators Inflamm 2010;2010:781235.
32 Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis 2007;27:413-426.
33 Zhang J, Wang H, Xiao Q, Liang H, Li Z, Jiang C, et al. Hyaluronic acid fragments evoke Kupffer cells via TLR4 signaling pathway. Sci China C Life Sci 2009;52:147-154.
34 Gupta RA, Motiwala MN, Dumore NG, Danao KR, Ganjare AB5. Effect of piperine on inhibition of FFA induced TLR4 mediated inflammation and amelioration of acetic acid induced ulcerative colitis in mice. J Ethnopharmacol 2015;164: 239-246.
35 Kelsh R, You R, Horzempa C, Zheng M, McKeown-Longo PJ. Regulation of the innate immune response by fibronectin: synergism between the III-1 and EDA domains. PLoS One 2014;9: e102974.
36 Imamura Y, Wang PL. Salivary histatin 3 inhibits heat shock cognate protein 70-mediated inflammatory cytokine production through toll-like receptors in human gingival fibroblasts. J Inflamm (Lond) 2014;11:4.
Received January 30, 2015
Accepted after revision June 3, 2015
Author Affiliations:Department of Hepatic-biliary-pancreatic Medicine, the First Hospital of Jilin University, Changchun 130021, China (Piao RL, Xiu M and Gao RP); The Research Institute at Nationwide Children's Hospital, Columbus, OH 43205, United States; Division of Pediatric Surgery, Department of Surgery, the Ohio State University, Columbus, OH 43205, United States (Brigstock DR)
Run-Ping Gao, MD, PhD, Department of Hepatic-biliary-pancreatic Medicine, the First Hospital of Jilin University, 71 Xinmin Avenue, Changchun 130021, China (Tel: +86-431-88715110; Fax: +86-431-85612468; Email: gao_runping@yahoo.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(15)60415-5
Published online September 9, 2015.
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Primary hepatic solitary fibrous tumor with histologically benign and malignant areas
- Pigmented well-differentiated hepatocellular neoplasm with β-catenin mutation
- Trametinib and dactolisib but not regorafenib exert antiproliferative effects on rat pancreatic stellate cells
- Coagulopathy and the prognostic potential of D-dimer in hyperlipidemia-induced acute pancreatitis
- Combined vascular resection and analysis of prognostic factors for hilar cholangiocarcinoma
- Ankaflavin ameliorates steatotic liver ischemiareperfusion injury in mice
