Application and Characterization of Cellulose Acetate Membrane Produced from Herbaceous Plant:Solidago Canadensis L.
2015-12-20YANGZaifu杨再福XULianlian徐莲莲ZHAOXiaomin赵晓敏ZHUDandan朱丹丹
YANG Zai-fu (杨再福) ,XU Lian-lian (徐莲莲),ZHAO Xiao-min (赵晓敏),ZHU Dan-dan (朱丹丹)
1 College of Environmental Science and Engineering,Donghua University,Shanghai 200051,China
2 Department of Civil and Environmental Engineering,Virginia Tech,Blacksburg,VA 20640,USA
Introduction
With the exhaustion of fossil energy, the abundant renewable energy has become new available energy.Cellulose is the most abundant natural polymer on earth whose capacity can reach 1.5 ×1012t by photosynthesis every year[1].Nowadays,cellulose is considered as the main raw material in the fields of energy and chemical industry[2].Herbaceous plant which is the cellulose rich biomass resource has become the preferred plant in Europe and United States[3].Energy grass is a usually perennial plant belonging to gramineous grass.Energy grass is rich in cellulose and hemicellulose and produces little ash,the research on the recycling of energy grass therefore has become a hot spot,for example, preparation of cellulose acetate from cornstalk,rice husk,wheat straw,and bagasse[4-5],and preparation of biochar from peanut shell,straw,and tea fruit peel[6-8].
Solidago Canadensis L.native to North America was introduced to China as an ornamental plant in 1930s.Then it became a dominant specie quickly,and was listed as one of world 100 invasive plants[9].According to statistics,the area of its intrusion was up to 30 thousands hectares at China costal region.At present,the main application of Solidago Canadensis L.is preparation of medicine and essence from its flower and leaf[10].The application of its stem was also relatively infrequent,for example the stem could be used to make solidago cleanser and solidago bath.Solidago Canadensis L.'s stem is rich in cellulose as its holocellulose content is 81.86% and ash content is 1.7% which is lower than the other gramineous plants whose ash content is generally more than 2%[11].
Therefore,in this study cellulose was separated and extracted from Solidago Canadensis L.'s stem,and the cellulose diacetate (CDA)and the membrane were prepared under mild conditions.Additionally,each production was characterized and the pure water flux and rejection rate of membrane was tested.
1 Experimental
1.1 Delignification of Solidago Canadensis L.
Solidago Canadensis L.was collected from the farmland(Songjiang district, Shanghai, China) and immersed in distilled water for 24 h to remove dust.The residues were dried at 110℃for 12 h,ground and sieved to a particle size smaller than 80 mesh.The delignification was carried out by organic solvents (formic acid/hydrochloric acid)[12]:5 g of raw material was mixed with 60 mL mixture of formic acid and hydrochloric acid in a volume ratio of 59:1.The mixture was filtered after reaction for 2 h at 80℃.Then NaOH solution(17.5%)was added into the filter residue to adjust pH to 11.The filter residues were bleached by 9% H2O2,washed and dried to get cellulose pulp.
1.2 Synthesis of cellulose diacetate
Cellulose diacetate was produced from a homogeneous acetylation and the reaction equation was shown in Scheme:5 g of cellulose produced from Solidago Canadensis L.was mixed with glacial acetic acid to be activated for a period of time until the cellulose was evenly distributed in the solution in an ice water bath[13-15].Then the mixture of sulfuric acid 9%-11%bymass.of pulp and 55 mL acetic anhydride was added into the mixture.After 5 min,the mixture was continually stirred under 55℃ as soon as the dope was precipitated out.Then,the distilled water added into the mixture and the cellulose triacetate was generated.The mixture was filtered,and the residue was washed by the distilled water until the pH is 7.The 70% acetic acid and 98% sulfuric acid working as catalyst were added into the residues and heated slowly to 60℃ for 2 h.Finally,the distilled water was added into the mixture and the CDA was generated.

Scheme 1 Reaction of acetylation
1.3 Membrane production
The membranes were produced by solution casting:casting solution of different concentrations was prepared by CDA of different concentrations[16].The solvent is the mixture of DMF and acetone(volume ratio is 2 ∶1).The casting solution was stirred at 55 ℃for 4 h,stilled for 24 h and cast on a glass plate using a cast knife open at 200 μm.The plate was then dipped into a distilled water bath until the membrane was detached from the plate at room temperature.
1.4 Characterization of productions
Surface functional group of the materials of delignified cellulose and CDA was examined using Fourier transform infrared spectroscopy (FT-IR)(NEXUS-670,Thermo Fisher,USA)analysis with scanning range of 400 to 4 000 cm-1.The membrane was examined using attenuated total reflectance Fourier transform infrared spectroscopy (ATR-IR)(NEXUS-670,Thermo Fisher,USA).The crystallinity and crystalline of cellulose pulp and CDA were tested by X-radial diffractometer(XRD)(D/Max-2550 PC,RIGAKU,Japan).The surface and section of membrane were detected by a scanning electron microscopy (SEM) (Quanta-250, FEI, Czech).The whiteness of the pulp and CDA was tested by whiteness meter(SBD-1,Hangzhou Daji Photoelectric instrucnent Co.,Ltd.,China).
1.5 Determination of degree of substitution (DS)
DS is the average value of acetyl groups which replace the hydroxyl groups in the glucosidic units[17].DS of the material was determined to characterize CDA.DS was determined through a saponification reaction:5 mL NaOH (0.25 mol/L)and 5 mL dehydrated ethanol were added to about 0.1 g CDA.Then,this mixture was left to stand for 24 h.After that,10.00 mL HCl (0.25 mol/L)was added to the system,and was left to stand for 30 min.Next,the mixture was titrated using a standard 0.25 mol/L NaOH solution,using phenolphthalein as indicator.This procedure was repeated in triplicate.
Equation (1)was used to determine the DS.

where X means DS;Vbimeans NaOH volume added to the system;Vbtmeans NaOH volume spent in titration;μbmeans NaOH concentration;Vameans HCl volume added to the system;μameans HCl concentration;M means molar weight of the acetyl group and mcameans weight of cellulose acetate sample.
1.6 Determination of pure water flux
The pure water flux was measured by putting the membrane in ultrafiltration cup with an effective diameter of 40 mm to record the volume of water passing through the membrane within a certain time.
Equation (2)was used to determine the pure water flux.

where Jwmeans the pure water flux;Vwmeans the water volume through the membrane;Δt means the ultrafiltration time;and A means the effective area of the membrane.
1.7 Determination of rejection rate
The rejection rate of membrane was measu red by bull serum albumin (BSA)solution.
Equation (3)was used to determine the reject rate.
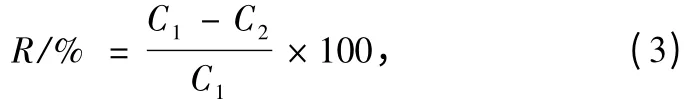
where C1means the dope concentration of BSA;C2means the solution concentration through the membrane;and R means the rejection rate.
2 Results and Discussion
2.1 Characterization of cellulose pulp and CDA
Table 1 summarizes some property parameters of cellulose pulp,CDA produced from Solidago Canadensis L.stem and commercial CDA.For cellulous pulp, the degree of polymerization (DP)is 713,the whiteness is 87.3,and the content of α-cellulous is 97.23%,while the acetification of cellulous requires that the content of α-cellulous should be higher than 95%,the DP should be in the range within 1 000 to 1 200,and the whiteness should be higher than 90.Result shows that the properties of pulp generally fulfill the requirements of acetification.DS of cellulose diacetate produced from the pulp is 2.31.DP decreases by 30.58% while whiteness increases slightly.Compared with the commercial CDA,which is produced by senior wood pulp,DP and whiteness of CDA produced from Solidago Canadensis L.is similar to those of the commercial CDA.DS of CDA produced from Solidago Canadensis L.is within the range from 2.22 to 2.76.Compared with the CDA produced by bamboo,CDA produced from Solidago Canadensis L.has higher value of DP.As a result the CDA produced from Solidago Canadensis L.meets the requirements of membrane preparation.

Table 1 Properties of cellulose pulp,CDA,and commercial CDA
Figure 1 shows the XRD patterns of cellulose pulp and CDA produced from Solidago Canadensis L..Cellulose pulp's diffraction peaks are at 12.19°,16.28°,and 22.22°;while CDA's diffraction peaks are at 8.82° and 20.75°.Crystalline region gradually transits to amorphous area,and no obvious boundaries appear.Comparison of XRD data shows that the crystallinity of cellulose is higher than that of CDA.The cellulose pulp crystallinity is 46.14%,while CDA crystallinity is 29.88%.After acetylation, hydrogen-bond interaction weakens the allomorph of cellulous changes and the crystallinity decreases[20].
Figure 2 is the FT-IR spectra of cellulose pulp and CDA.Analyses results are:in comparison,absorption peak of CDA at 1 754 cm-1is stronger than that of cellulose,which indicates that ester group exists in CDA;absorption peak of CDA at 1 238 cm-1indicates the existence of C—O group of ester group;the absorption peak at 1 376 cm-1shows the existence of C—H group at CH3— =C O group,and the absorption peak of CDA is stronger than that of cellulose pulp,indicating the generation of acetyl group.Additionally,the absorption peak at 3 484 cm-1of CDA is weaker than that of the cellulose pulp,indicating that the hydroxyl of cellulose has been partly replaced by acetyl group.
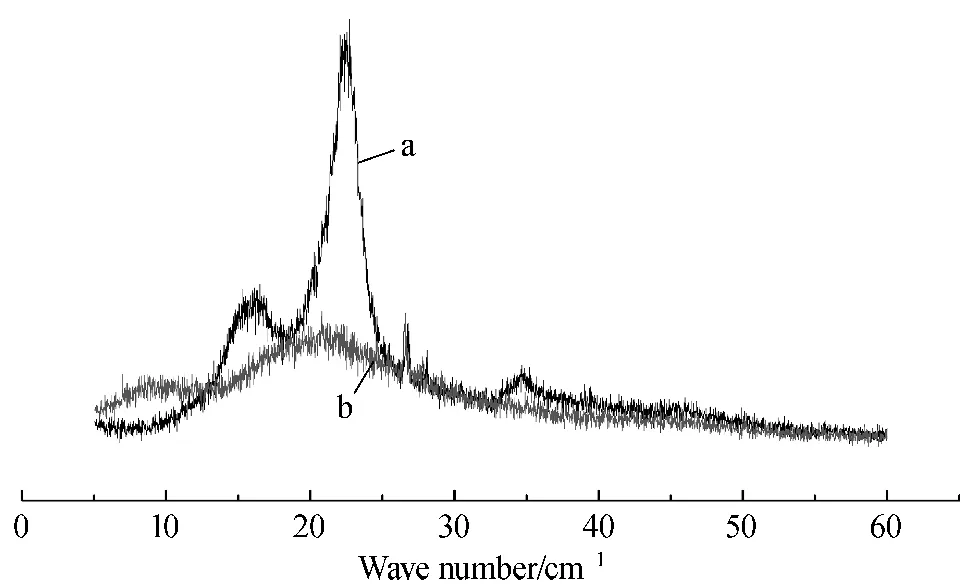
Fig.1 XRD patterns of cellulose pulp (a)and CDA (b)

Fig.2 FT-IR spectra of cellulose pulp (a)and CDA (b)
2.2 Characterization of membrane

Fig.3 SEM photographs of cellulose acetate membrane (a)surface and (b)section
SEM photographs of membranes are presented in Fig.3:(a)for surface morphology of membrane and (b)for section morphology.The surface feature shows a large number of micropore,as the aperture is about one-tenth micrometer.The section morphology shows that the section of membrane is layered and porous.

Fig.4 ATR-IR spectrum of membrane

Fig.5 Effect of CDA concentrations on pure water flux and reject rate
ATR-IR spectrum of membrane are presented in Fig.4.The spectrum is similar to the one in Fig.2 (b).The important bands are:the bands locates at 1 745 cm-1=indicating C O groups;the bands locates at 3 469 cm-1indicating —OH groups;the bands locates at 1 370 cm-1indicating acetoxy methyl groups;the bands locates at 1 230 cm-1indicating ester groups;and the bands locates at 1 040 cm-1indicating C—O—C groups[21-22].
2.3 Assessment on membrane properties
Figure 5 summarizes the effect of CDA concentrations on pure water flux and reject rate.When the CDA concentration of casting solution is between 8% and 12%,the membrane pure water flux is between 10-30 mL/ (cm2·h).With the increase of CDA concentration,the water flux decreases,while the rate of decay becomes small.In addition,with the increase of CDA concentration,the rejection rate increases.According to Fig.5,the membrane has best combination properties when the CDA concentration is 10%.Therefore,the CDA concentration is determined at 10%.
The porosity is used to characterize the membrane flux,and the contact angle is used to characterize the hydrophilic and hydrophobic of the material surface.Table 2 shows that the porosity decreases with the increase of CDA concentration,which is consistent with the result of membrane flux.The contact angle of membrane is 22°-60°,indicating the membrane has good hydrophilic performance.The contact angle decreases with the increase of CDA concentration, indicating the hydrophilic performance improves.

Table 2 Effect of CDA concentration on porosity and contact angle
Figure 6 shows the changes of pure water flux and rejection rate with time.Within the experiment time of 100 min,the pure water flux decreases while the rejection rate increases over time.The attenuation rate of purewater flux is about 40%.The membrane with good hydrophilic performance and good glaze surface has small rejection rate scope,showing the membrane has fine antifouling properties.
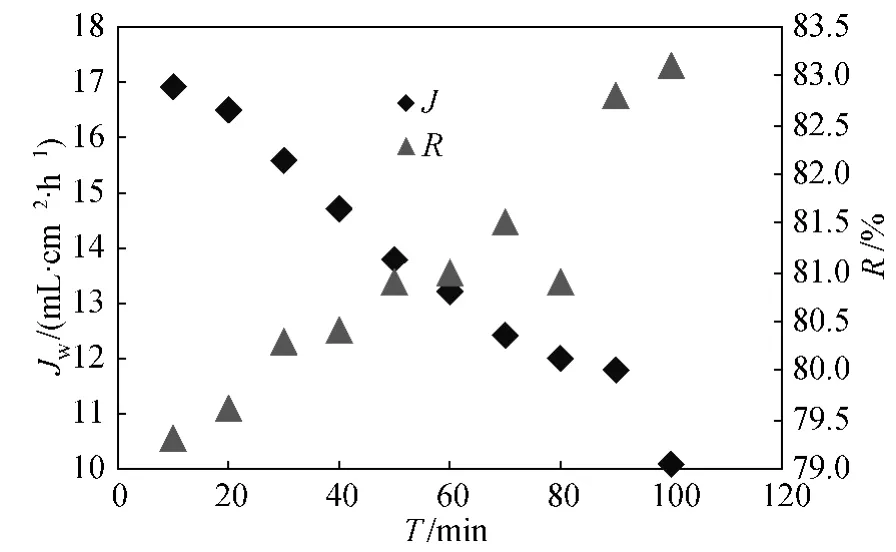
Fig.6 Changes of pure water flux and rejection rate over time
3 Conclusions
(1)It was demonstrated the capability of producing CDA from chemical recycling of herbaceous plant: Solidago Canadensis L.When acetylation is made with received material,the properties of self produced CDA are similar to the commercial CDA.
(2)It was possible to produce membranes from homemade CDA.Meanwhile,the result of test on membrane properties shows that the membrane has high pure water flux,rejection rate and hydrophillic nature.
[1]Klemm D,Heublein B,Fink H P,et al.Cellulose:Fascinating Biopolymer and Sustainable Raw Material[J].Angewandte Chemie-Intemational Edition,2005,44(22):3358-3393.
[2]Gandini A.Polymers from Renewable Resources:a Challenge for the Future of Macromolecular Materials[J].Macromolecules,2008,41(22):9491-9504.
[3]Jasinskasa A,Zaltauskasa A,Kryzeviciene A.The Investigation of Growing and Using of Tall Perennial Grasses as Energy Crops[J].Biomass and Bioenergy,2008,32(11):981-987.
[4]Biswas A,Saha B C,Lawton J W,et al.Process for Obtaining Cellulose Acetate from Agricultural By-products [J].Carbohydrate Polymer,2006,64(1):134-137.
[5]Shaikh H M,Pandare K V,Nair G,et al.Utilization of Sugarcane Bagasse Cellulose for Producing Cellulose Acetates:Novel Use of Residual Hemicellulose as Plasticizer [J].Carbohydrate Polymers,2009,76(1):23-29.
[6]Ahmada M,Leea S S,Doub X M,et al.Effects of Pyrolysis Temperature on Soybean Stover-and Peanut Shell-Derived Biochar Properties and TCE Adsorption in Water[J].Bioresource Technology,2012,118(2):536-544.
[7]Wua W X,Yang M,Feng Q B,et al.Chemical Characterization of Rice Straw-Derived Biochar for Soil Amendment[J].Journal of Applied Polymer Seience,2012,27(4):268-276.
[8]Gao J J,Kong D D,Wang Y F,et al.Production of Mesoporous Activated Carbon from Tea Fruit Peel Residues and Its Evaluation of Methylene Blue Removal from Aqueous Solutions[J].BioReources,2012,8(2):2145-2160.
[9]Lu Q G,Gan Z H.100 Invasive Plants on Earth[J].Word Environment,2001,32(4):42-43.
[10]Apati P,Szentmihalyi K,Balazs A,et al.HPLC Analysis of Flavonoids in Pharmaceutical Preparations from Canadian Goldenrod[J].Chromatographia,2002,56(1):S65-S68.
[11]Šutovskáa M,Capekb P,Kocmálováa M,et al.Characterization and Biological Activity of Solidago Canadensis Complex[J].International Journal of Biological Macromolecules,2013,52(2):192-197.
[12]Lam H Q.Formic Acid Pulping of Rice Straw[J].Industrial Crops and Products,2001,78(14):65-71.
[13]Saka S,Ohlnae K.Thermal Properties of Cellulose Triaeetate as Prepared from Low-Grade Dissolving Pulp[J].Journal of Applied Polymer Science,1996,62(3):1003-1010.
[14]Saka S,Takanashi K,Matsumara H.Effeets of Solvent Addition to Aeetylation Medium on Cellulose Triacetate Prepared from Low-Grade Hardwood Dissolving Pulp[J].Journal of Appliedn Polymer Seience,1998,69(5):1445-1449.
[15]Shashidhara G M,Guruprasad K H.Effect of Concentrated Sulfuric Acid and Nitroethane on Insoluble Residues in Aeetylating Medium of Cellulose Aeetate Prepared from Low-Grade Pulps[J].Journal of Applied Polymer Seience,2005,98(4):1765-1771.
[16]van de Witte P,Dijkstra P J.Phase Separation Process in Polymer Solutions in Relation to Membrane Formation[J].Journal of Membrane Science,1996,117(1):1-31.
[17]Puleo A C,Paul D R,Kelley S S.The Effect of Degree of Acetylation on Gas Sorption and Transport Behavior in Cellulose Acetate[J].Journal of Membrane Science,1989,47(3):301-332.
[18]He J X,Cui S Z,Wang S Y.Preparation and Crystalline Analysis of High-Grade Bamboo Dissolving Pulp for Cellulose Acetate[J].Journal of Applied Polymer Science,2007,107(2):1029-1038.
[19]Samios E,Dart R K,Dawkins J V.Preparation,Characterization and Biodegradation Studies on Cellulose Acetates with Varying Degrees of Substitution[J].Polymer,1997,38(12):3045-3054.
[20]Zhang J,Wang S Y.Structural Characteristics of Cellulose Diacetate[J].Journal of Donghua University:Natural Science,2005,31(3):94-96,122.
[21]Adebajo M O,Frost R L,Kloprogge J T.Raman Spectroscopic Investigation of Acetylation of Raw Cotton[J].Spectrochimica Acta,Part A,2006,64(2):448-453.
[22]da Silva Meirelesa C,Filhoa G R,Ferreira M F Jr.,et al.Characterization of Asymmetric Membranes of Cellulose Acetate from Biomass:Newspaper and Mango Seed[J].Carbohydrate Polymers,2010,80(3):954-961.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Group Performance Evaluation in Universities with Entropy Method
- Synthesis and Application of Polyurethane Modified Organic Silicone Wet Rubbing Fastness Improver
- Inactivation of Giardia Intestinalis by Peroxone Process (H2 O2 /O3)and Its Disinfection Mechanisms
- Optical Measurements to Reveal Roles of Slightly Crosslinked Poly(dimethyldiallylammonium chloride)s in Fixing Anionic Dyes on Cotton Fabric
- Portfolio Choice under the Mean-Variance Model with Parameter Uncertainty
- Behavior of Benzene Decomposition by Using Pulse Modulated Power Supply
