遗传不育技术在蚊媒疾病防控中的应用
2015-12-17王玉生李建伟张桂芬李昕玥万方浩
王玉生, 李建伟, 张桂芬, 严 盈,2,3, 李昕玥, 万方浩,4*
1中国农业科学院植物保护研究所,植物病虫害生物学国家重点实验室,北京 100193; 2Department of Entomology,
North Carolina State University, Campus Box 7613, Raleigh, NC 27695-7613, USA; 3Genetic Engineering
and Society Center and W. M. Keck Center for Behavioral Biology, North Carolina State University,
Raleigh, NC 27695-7613, USA; 4青岛农业大学农学与植物保护学院,山东 青岛 266109
遗传不育技术在蚊媒疾病防控中的应用
王玉生1, 李建伟1, 张桂芬1, 严盈1,2,3, 李昕玥1, 万方浩1,4*
1中国农业科学院植物保护研究所,植物病虫害生物学国家重点实验室,北京 100193;2Department of Entomology,
North Carolina State University, Campus Box 7613, Raleigh, NC 27695-7613, USA;3Genetic Engineering
and Society Center and W. M. Keck Center for Behavioral Biology, North Carolina State University,
Raleigh, NC 27695-7613, USA;4青岛农业大学农学与植物保护学院,山东 青岛 266109
摘要:疟疾、登革热等重大传染性蚊媒疾病严重危害人类健康,且目前缺乏有效的药物和疫苗,防治埃及伊蚊、冈比亚按蚊等媒介昆虫是控制和消除这些疾病的有效手段。化学杀虫剂的大规模使用在一定程度上控制了疾病的传播,但其抗药性和环境污染等问题也随之而来。分子生物学的飞速发展为昆虫不育技术(SIT)的更新及害虫防治提供了新的策略,由此发展起来的以释放携带显性致死基因昆虫(RIDL)为代表的一系列遗传不育技术为蚊虫种群防控提供了更加有效的选择。本文概述了遗传技术在蚊虫防控中的应用进展,包括蚊虫遗传防治的历史和策略,阐述了RIDL技术体系的原理,同时介绍了相关遗传控制品系和已经开展的田间释放研究,展示了遗传修饰不育技术在蚊媒疾病防治中的巨大潜力。
关键词:蚊媒昆虫; 遗传防治; 昆虫不育技术; 释放携带显性致死基因昆虫的技术
Application of genetic pest management in the control
of mosquito-borne diseases
Yu-sheng WANG1, Jian-wei LI1, Gui-fen ZHANG1, Ying YAN1,2,3, Xin-yue LI1, Fang-hao WAN1,4*
疟疾、登革热、丝虫病、黄热病等以蚊虫为媒介的重大传染疾病严重威胁人类的健康,蚊媒防治是控制和消除这些疾病的有效手段。传统的蚊媒防治以化学药剂为主,但抗药性以及化学药剂对环境的污染和生态破坏等问题日益严重。分子生物学的发展为蚊媒的防治提供了新的途径,其中以昆虫遗传修饰技术与昆虫不育技术(Sterile insect technique,SIT)(Knipling,1970)相结合发展起来的昆虫遗传修饰不育技术为害虫防治提供了新的思路。通过在媒介种群中引入携带显性致死基因或病原体抗性基因等害虫控制效应基因的人工品系,能够有效降低目标种群的数量或进行种群替代,从而阻断蚊媒对病原微生物的传播(周秀娟等,2008; Catterucciaetal.,2009; Klassen,2009; Wilke & Marrelli,2012)。近年来,蚊媒昆虫的遗传修饰不育技术研究发展迅速,相关品系在世界各地被广泛使用并取得了较好的效果,表明其在蚊媒疾病的防控中具有巨大的应用潜力(Catterucciaetal.,2009; Wilke & Marrelli,2012)。
1 蚊虫遗传防治的历史
SIT是指通过释放辐照(Irradiation)等处理的雄虫与野生型雌虫交配,使其不育从而降低目标昆虫种群数量的一种害虫控制技术(Knipling,1970),其具有物种特异、环境友好、可工厂化生产、大面积控制等特点(Hendrichsetal.,2002)。SIT在蚊媒防治中的应用已有较长的历史(表1; Benedict & Robinson,2003)。获得不育昆虫的手段除了辐照不育以外,还包括化学不育(Chemosterilization,Ch)、胞质不亲和性(Cytoplasmic incompatibility,CI)、杂交不育(Hybrid male sterility,Hy)、减数分裂驱动(Meiotic drive)、染色体移位和重排(Chromosomal translocation and rearrangements,Tr)等,其中以辐照不育应用最为广泛(Benedict & Robinson,2003)。
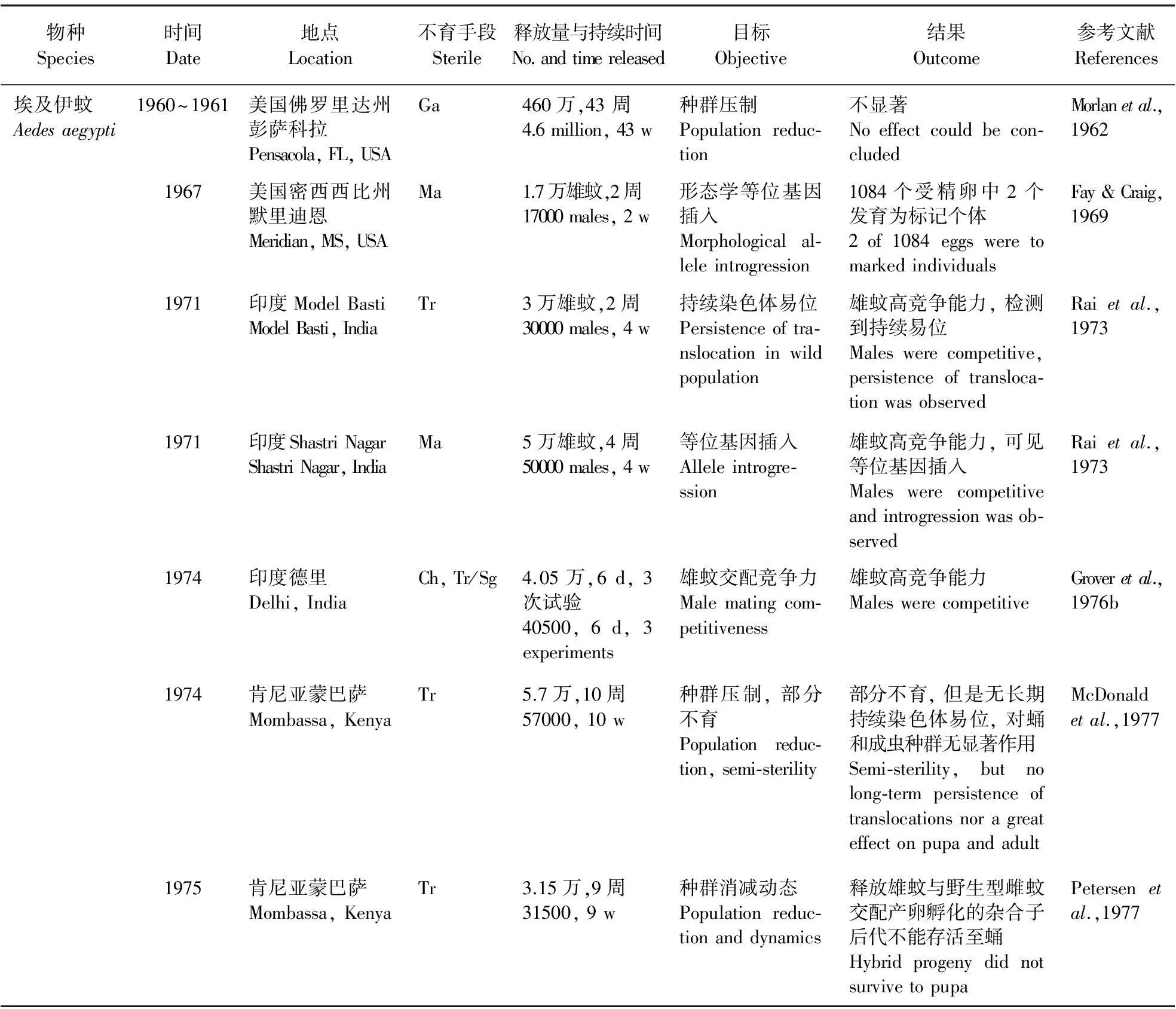
表1 传统SIT技术在蚊媒防治中的应用(Benedict & Robinson,2003)

续表1

续表1
Ch.化学不育法;CI.细胞质不亲和性;Ga.γ射线;Hy.杂交不育;Ma.仅标记(无不育处理);Sg.分离失调;Tr.染色体易位和重排。
Ch. Chemosterilization; CI. Cytoplasmic incompatibility; Ga. Gamma irradiation; Hy. Hyhybrid male sterility; Ma. Marker only; Sg. Segregation distorter; Tr. Translocation and other chromosomal rearrangements.
2 蚊子遗传防治的主要策略
2.1 种群压制
种群压制是通过降低一定区域内目标媒介蚊虫种群数量来控制蚊媒疾病的传播,该策略与化学药剂的目的类似,但是避免了杀虫剂抗性、杀伤非目标昆虫、环境污染等问题,这一策略的主要代表手段包括传统SIT、释放携带显性致死基因昆虫的技术(Release of insects carrying a dominant lethal,RIDL)、X或常染色体连锁的归巢内切酶基因(Homingendonucleasegene,HEG)系统、不相容昆虫技术(Incompatible insect technique,IIT)、致死—拯救(Killer-Rescue)系统、多位点混合(Multi-locus assortment,MLA)等。在种群压制策略中,必须通过周期性释放来保证效应基因在目标种群中的扩散和基因频率的提高。SIT是蚊虫种群压制策略中应用最广泛的是害虫防治手段之一(Alphey,2014),但该技术也存在一些难以克服的缺点,特别是γ射线等在诱导雄蚊不育的同时降低了其野外适合度(Scolarietal.,2008; Thomasetal.,2000)。而基于转座子活性和性别决定系统发展起来的RIDL和fsRIDL(female-specific RIDL)(Alphey,2014; Heinrich & Scott,2000; Thomasetal.,2000),能够释放携带条件致死基因纯合子品系(Homozygous strains)的雄蚊,该纯合子品系与野生型雌蚊交配后,雌性后代在特异致死基因作用下死亡,而雄性后代继续携带致死基因与野生型雌虫交配,引起目标种群数量的减少,连续释放后甚至能根除种群,从而阻断蚊媒对病原微生物的传播(Catterucciaetal.,2009; Klassen,2009; Wilke & Marrelli,2012)。与传统SIT相比,RIDL对蚊虫交配和野外生存适合度的损伤低,且免去了不育处理的环节,fsRIDL甚至不需要性别筛选,这为致死基因作用时间的选择提供了更大的灵活性(Phucetal.,2007; Thomasetal.,2000),大大节省了人力和物力,具有更高的遗传控制效率(Alpheyetal.,2011; Blacketal.,2011)。
HEG驱动系统主要利用HEG酶能够定向识别插入在染色体上特定两段DNA序列之间的特点,当两条同源染色体中一条具有HEG基因时,HEG酶将切割另一条染色体,并以前者为模板进行复制,即homing现象(Sinkins & Gould,2006)。由于归巢内切酶I-PpoI对与X染色体连锁的28S核糖体基因的重复序列高度特异性靶定(Nolanetal.,2011),当其与雄虫X染色体靶定时,可以在精子发生过程中切割X染色体导致后代雌性不存活或不产生X型精子;与常染色体靶定时会导致fsRIDL;而当其与Y连锁则后代所有雄蚊均带有该基因,从而在减数分裂驱动下扩散(Burt,2003; Catterucciaetal.,2005; Deredecetal.,2008; Windbichleretal.,2011)。 而IIT则利用了昆虫内共生菌Wolbachia的胞质不相容性(Cytoplasmic incompatibility,CI),将携带Wolbachia的雄蚊与不携带或携带不同类型Wolbachia的雌蚊交配,诱导产生胞质不相容性,其后代在胚胎期死亡;而含同种类型Wolbachia的雌雄蚊交配产生的后代可正常发育并感染沃尔巴克氏体,从而使携带该Wolbachia的品系在野生种群中迅速扩散,最终降低靶标昆虫的数量(Alphey,2014; Hancock & Godfray,2012; Laven,1967; O′Connoretal.,2012; Werrenetal.,2008; Zabalouetal.,2004、2009)。
2.2 种群替代
种群替代是指将能够传播病原物的蚊虫品系替换为无法致病的品系(Alphey,2014),其主要代表技术包括显性不足(Underdominance,UD)技术、Wolbachia驱动系统、Medea元件驱动系统、转座子(Transposons)转化系统、Y染色体连锁的HEG驱动系统等。在种群替代策略中,效应基因能够在目标种群中自主扩散。蚊媒、病原微生物、抗性基因和基因驱动系统之间复杂的进化关系是种群替代策略研究的核心环节。目前已知的Wolbachia驱动系统、Medea驱动系统、转座子转化系统、显性不足技术、HEG等均能促进蚊媒抗性的产生(Alphey,2014; Chenetal.,2007; Moreiraetal.,2009)。
转座子是基因组中能自主复制和移位的DNA区段,广泛存在于昆虫基因组,不过大多数转座子已发生突变不表现活性。目前,转座子已被广泛应用于分子生物学研究。转座子在染色体不同位点的插入有可能导致外源基因失活或染色体重排,进而使蚊虫的适应性下降。来自粉纹夜蛾Trichoplusiani的piggyBac转座子能特异识别TTAA位点,并准确切除与插入外源基因,可转入的外源基因的大小几乎不受限制,也无物种限制,是遗传修饰系统中应用最广泛的转座子之一(Fraseretal.,1983; Handler,2002),目前应用piggyBac转座子已成功获得了蚊媒的多个遗传转化品系(Fuetal.,2010; Phucetal.,2007; Wiseetal.,2011)。此外,研究者也将Hermes(Jasinskieneetal.,1998; Zhao & Eggleston,1998)、Minos(Catterucciaetal.,2000a、2000b)、Mariner(Coatesetal.,1998)等转座子抗性基因成功转入蚊虫的细胞系或得到遗传修饰品系。由于大部分非蚊虫来源的转座子在蚊虫中的遗传转化成功率较低,给其应用带来了一定的局限性(O′Brochtaetal.,2003),因此需要挖掘蚊媒自身位点特异且非连锁的转座子(Rasgon & Gould,2005)。Arensburgeretal. (2005)已在冈比亚按蚊Anophelesgambiae中发现了Herves转座子,但其调控机制尚不明晰。
内生菌Wolbachia能够通过增强蚊虫的自身免疫力或改变蚊虫的代谢通路等方式(Brennanetal.,2008; Panetal.,2012),诱导蚊媒对病原微生物的抗性(Bianetal.,2010、2013; Moreiraetal.,2009),抑制甚至清除病原微生物的感染(Walkeretal.,2011)。将抗病的蚊虫释放于靶标蚊媒种群中引起胞质不相容性,抗性Wolbachia逐步扩散到靶标种群中,进而取代易感靶标蚊媒,从根源上控制了蚊媒病的传播。基于赤拟谷盗Triboliumcastaneum的Medea元件也能辅助蚊媒对病原微生物抗性的产生(Chenetal.,2007), Medea元件由母系特异启动子驱动的对胚胎有毒性的RNA或蛋白和受精卵特异的启动子驱动解毒蛋白组合在一起。由于雌性杂合子后代均具有母系遗传的毒素基因,当后代未遗传到解毒基因时将死亡,当遗传到母/父系来源的解毒基因时将存活(Alphey,2014)。通过染色体易位等遗传操作产生的显性不足(Curtis,1968; Davisetal.,2001; Magori & Gould,2006)和Y染色体连锁的HEG(Alphey,2014; Burt,2003; Catterucciaetal.,2005; Deredecetal.,2008)也能驱动种群替代。不同种群替代技术的驱动效率不同,驱动效率较低的Wolbachia系统和显性不足系统需要更多的初始释放数量,而驱动效率较高的转座子系统和HEG需要的初始释放数量则相对较低(Alphey,2014)。
3 RIDL技术
3.1RIDL技术原理和现有品系
基于tet-off系统调控效应基因的表达是目前RIDL技术实现蚊虫特异性致死的最主要方式。在tet-off系统中,当大肠杆菌Escherichcoli的转座子Tn10的四环素阻遏因子(tetracycline repressor,tetR)与四环素结合时,tetR不能阻抑四环素抗性操纵子(tetracycline-resistance operon,tetO),因此下游转录不受抑制。将tetR的部分序列与单纯疱疹病毒VP16的转录活性区段组合为四环素转录激活因子(tetracycline transcriptional activator,tTA),tTA与性别/组织/发育阶段特异性启动子构建为tet-off驱动载体,tetO与CMV启动子构成四环素响应元件(Tetracycline response element,TRE),TRE与效应基因组合成效应载体,进而组建为完整的tet-off表达系统(Gossen & Bujard,1992)。在缺乏四环素时,tTA与tetO结合引发效应基因表达;但在饲养条件存在四环素时,tTA与四环素结合而不与tetO结合,无法激活下游效应基因的表达。
通过特定遗传标记筛选转化品系是RIDL构建过程中的关键步骤,利用不同启动子驱动荧光标记是目前的常用手段。优良的启动子—荧光基因表达模式能够大大降低转化筛选的难度,在提高RIDL品系构建效率的同时,有助于释放品系的后期监测。组成型启动子通常具有表达强度高、表达周期长和表达面积大的优点,英国Oxitec公司采用组成型启动子Hr5-IE1构建了性能良好的遗传荧光标记,全身表达DsRed2或GFP的埃及伊蚊Aedesaegypti幼虫在荧光滤镜下清晰可见(图1A);而表达Hr5IE1-DsRed2的幼虫肛乳头呈现出斑点荧光模式,主要是来自核位点的荧光信号(图1B)。此外,3xP3启动子也常常用于构建蚊子的遗传荧光标记,以驱动如AmCyan(图1C)或DsRed(图1D)荧光基因在埃及伊蚊光学神经中的表达。
目前开发的蚊子RIDL品系主要来自Oxitec公司,目标物种包括埃及伊蚊、白纹伊蚊Aedesalbopictus和冈比亚按蚊等,有的品系已经进入田间释放阶段(表2)。Phucetal.(2007)通过模型研究发现,由于存在密度依赖效应,携带晚期表达效应基因的RIDL品系与早期品系相比,不仅能够大大减少释放的初始虫源数量,而且能更快地达到控制种群的目的,具有更好的防治效果;通过构建埃及伊蚊RIDL品系LA513A(OX513A)进行验证,在此系统中tTA通过反复结合tetO而不断积累,最终达到致死剂量;由于tTA既是转录激活因子,也是效应基因,该体系被称作单元件系统(Gongetal.,2005)。此外,Fuetal.(2010)将雌蚊飞行肌特异性启动子AeAct-4(Muozetal.,2004)与tTA元件连接构建tet-off驱动载体,将细胞凋亡基因Nipp1Dm和michelob_x与TRE连接构建效应载体,将驱动品系OX3545分别与效应品系 OX3547和OX3582杂交,获得的雌性后代在四环素缺乏时引发效应基因在飞行肌的特异性表达,从而丧失了飞行能力成为无翅型,比例高达65.8%~98.3%,而雄蚊则不受影响;该体系既需要表达tTA的驱动载体,也需要表达致死基因的效应载体,因此又称作双元件系统(Alpheyetal.,2008)。同时,Fuetal.(2010)利用AeAct-4启动子构建了单元件系统并得到了OX3604C品系,当不存在四环素时,过量表达的tTA导致飞行肌细胞凋亡,后代雌蚊几乎全部无翅,而雄蚊则不受影响。飞行能力对蚊子营养获取、交配及逃生等至关重要(Labbéetal.,2012),因此OX3604C实质上等同于基于tet-off调控下的雌性特异致死品系。Wiseetal.(2011)调查了埃及伊蚊OX3604C品系雄蚊用于SIT的潜力,实验室条件下当以遗传修饰雄蚊∶野生型雄蚊=(8.5~10)∶1,每周释放1次时,10~20周便可压制野生型蚊虫。Labbéetal.(2012)采用白纹伊蚊的AealbAct-4启动子构建了RIDL品系OX4358并取得了与OX3604C近似的结果,表明Actin-4启动子的雌性飞行肌特异活性可能在不同蚊子种类中保守。
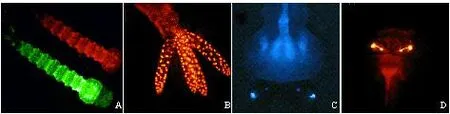
图1 埃及伊蚊遗传修饰品系中不同遗传标记的荧光模式(图片由英国Oxitec公司的Luke Alphey博士提供)
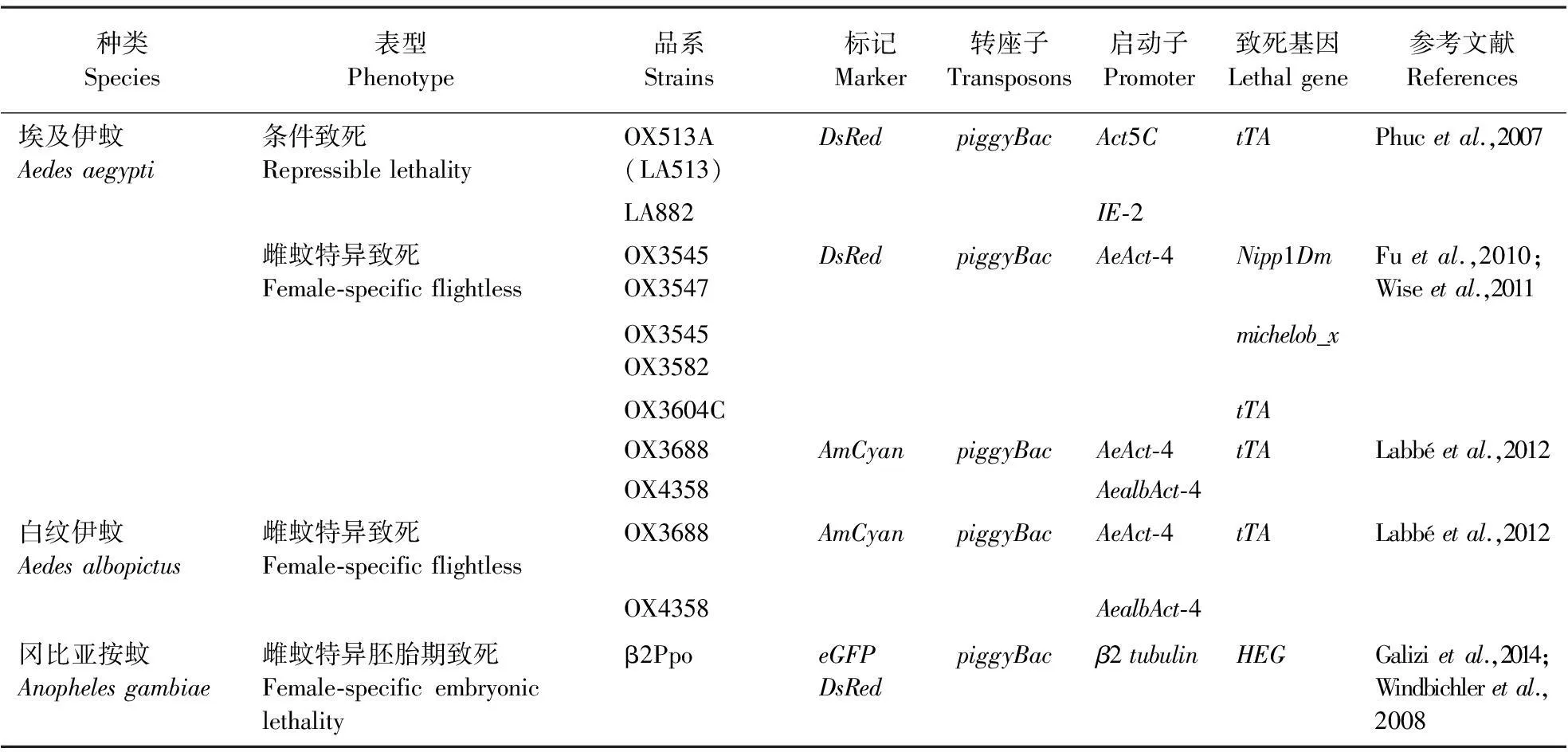
种类Species表型Phenotype品系Strains标记Marker转座子Transposons启动子Promoter致死基因Lethalgene参考文献References埃及伊蚊Aedesaegypti条件致死RepressiblelethalityOX513A(LA513)DsRedpiggyBacAct5CtTAPhucetal.,2007LA882IE-2雌蚊特异致死Female-specificflightlessOX3545OX3547DsRedpiggyBacAeAct-4Nipp1DmFuetal.,2010;Wiseetal.,2011OX3545OX3582michelob_xOX3604CtTAOX3688AmCyanpiggyBacAeAct-4tTALabbéetal.,2012OX4358AealbAct-4白纹伊蚊Aedesalbopictus雌蚊特异致死Female-specificflightlessOX3688AmCyanpiggyBacAeAct-4tTALabbéetal.,2012OX4358AealbAct-4冈比亚按蚊Anophelesgambiae雌蚊特异胚胎期致死Female-specificembryoniclethalityβ2PpoeGFPDsRedpiggyBacβ2tubulinHEGGalizietal.,2014;Windbichleretal.,2008
此外,RIDL品系在冈比亚按蚊的种群遗传控制中也取得了理想进展。Windbichleretal.(2008)采用在雄蚊睾丸精子发生时特异表达的β2tubulin(Catterucciaetal.,2005)启动子驱动效应基因HEG的表达,导致后代雌蚊在胚胎期死亡,大幅度改变了后代的性别构成,使目标种群无法继续繁衍。由于HEG蛋白与X染色体连锁的28S核糖体基因的重复序列高度特异靶定,在β2tubulin驱动下在雄蚊精子发生时切割X染色体,当导入胚胎时还能切割母系来源的X染色体,这种雄蚊与野生型雌蚊交配后导致后代雌蚊在胚胎期死亡,产生的后代几乎全为携带效应基因的雄蚊(Windbichleretal.,2008)。Galizietal.(2014)发现应用该技术的雄蚊与野生型雌蚊交配后产生的子代95%以上为雄性,且雄蚊的生育能力未受明显影响,到第6代时蚊虫种群因缺少雌性而无法繁衍,为有效控制疟疾等传染病的传播提供了更为高效、经济的方法。
3.2 田间试验
研究遗传修饰不育技术的最终目标是将培育的RIDL品系释放于野外替代自然界中如埃及伊蚊、冈比亚按蚊等重大传染性疾病的传播媒介,从而从根本上阻断蚊媒疾病的传播,而RIDL品系能否成功压制野外种群的一个重要特征在于其将携带的显性效应基因向目标种群传递扩散的能力及稳定性(Scottetal.,2002)。RIDL品系在自然界中的生存(如存活率、寿命、羽化率等)、繁殖(交配竞争力等)、扩散(飞行能力等)的适应性和竞争力是反映效应基因传递能力(Massonnet-Bruneeletal.,2013)的重要指标。研究表明,遗传操作中转座子的遗传转化、启动子和标记基因的表达以及在饲养时为获得纯合品系采用的近亲交配等带来的自然选择压力,有可能对遗传修饰品系的适应性产生影响(Catterucciaetal.,2003; Irvinetal.,2004; Marrellietal.,2006; Massonnet-Bruneeletal.,2013)。目前应用最多的OX513A品系,采用的是组成型启动子Act5C,其对适合度的影响可能比组织特异性启动子更大(Massonnet-Bruneeletal.,2013)。因此,必须在实验室和田间开放条件下对RIDL品系的适合度及其释放策略进行详细调查。已有多个国家围绕该方面开展了广泛研究,并提出了RIDL品系的释放前标准(Benedict & Robinson,2003; Yakobetal.,2008)。已经报道的室内研究结果并不完全一致,但基本可以得出如下结论:RIDL品系在生活史特性、繁殖和扩散能力等方面有一定的下降,但是与野生型相比不存在较大的差异;这可以通过进一步优化饲养体系和方法得到改善,最优释放策略的制定也有助于获得理想的控制效果(表3)。
目前田间开放条件的释放研究主要集中于埃及伊蚊的OX513A品系。Harrisetal.(2011)于2009年在Grand Cayman地区首次进行了OX513A雄蚊的田间释放,结果表明,释放的雄蚊能成功与野外雌蚊交配并使其受精,具有与野生雄蚊相当的繁殖能力,释放后收集的卵孵化出的幼虫能检测到荧光,且随时间推移比率逐渐提高,反映了RIDL在野外逐步降低野生型种群的过程。此外,模型分析发现,当田间野生型和释放RIDL品系交配比率达13%~57%时才能成功抑制目标种群。因此,Harrisetal.(2012)于2010进行了第2次释放研究,释放4~6 周后野生型种群受到抑制,11 周时RIDL∶野生雄蚊高达25.2∶1,卵带荧光比率为88%,表明OX513A品系成功实现了对野生型的压制。Lacroixetal.(2012)在马来西亚Pahang地区也进行了OX513A和野生型实验室品系的释放,结果表明,RIDL品系不会对人类健康和环境产生不利影响,OX513A和野生型寿命相当,虽然飞行能力有一定减弱但释放措施的改善能提高其应用效果。此外,Alphey (2014)在巴西应用OX513A品系也成功地实现了对2个目标种群的压制,并且后续的大规模释放研究还在继续开展。
4 总结
遗传不育技术成功地将昆虫不育技术与新兴的遗传修饰技术相结合,成为物种特异、环境安全、科学高效的有害生物治理手段,具有传统防治方法难以比拟的优势。目前,已经开发了包括埃及伊蚊、白纹伊蚊、冈比亚按蚊在内的众多主要媒介蚊虫的特异性致死或无翅型RIDL品系,并进行了一系列适应性测试和安全性评估,针对埃及伊蚊的OX513A品系已经实现了田间释放,验证了RIDL技术在防控蚊媒疾病中的可行性。种群的释放策略对蚊媒种群的遗传控制效果具有重要的影响,合理评估遗传修饰品系的适应性、扩散能力和生殖能力,构建基于昆虫学、流行病学、生物经济学的数学模型,进而优化释放比例,合理布局释放点、释放频率、持续时间等是达到最优释放策略的必要程序(Alpheyetal.,2011; Atkinsonetal.,2007)。此外,遗传控制技术与化学防治、生物防治等多种控制措施联合应用,将能更加有效地控制和阻断疟疾、登革热等重大蚊媒疾病的发生和传播。
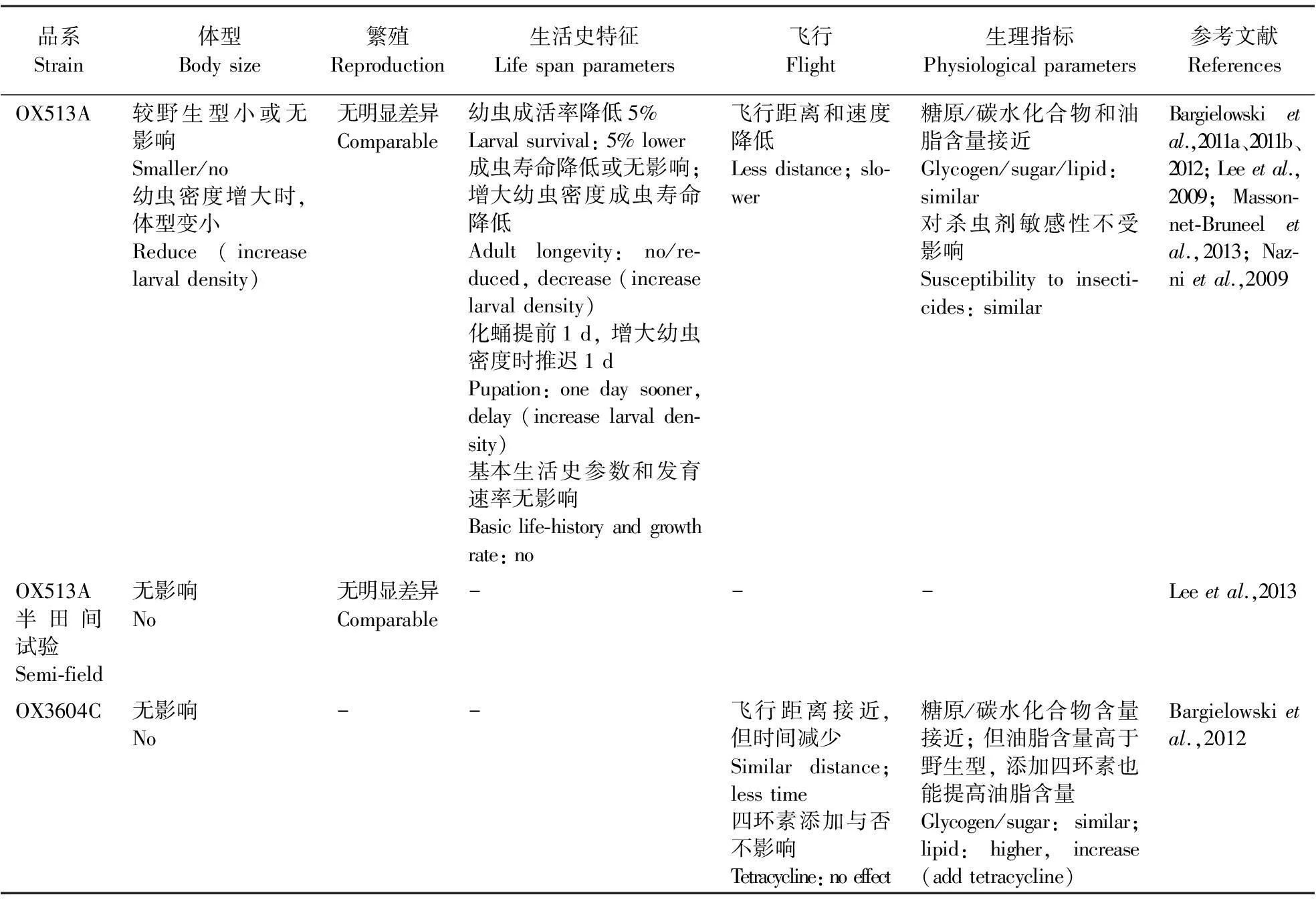
表3 RIDL品系适应性的室内研究结果
参考文献
Alphey L. 2014. Genetic control of mosquitoes.AnnualReviewofEntomology, 59: 205-224.
Alphey L, Benedict M, Bellini R, Clark G G, Dame D A, Service M W and Dobson S L. 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis.Vector-BorneandZoonoticDiseases, 10: 295-311.
Alphey L, Nimmo D, O′Connell S and Alphey N. 2008. Insect population suppression using engineered insects∥Aksoy S.TransgenesisandtheManagementofVector-BorneDisease. Austin, Texas: Landes Bioscience, 93-103.
Alphey N, Alphey L and Bonsall M B. 2011. A model framework to estimate impact and cost of genetics-based sterile insect methods for dengue vector control.PLoSONE, 6: e25384.
Arensburger P, Kim Y J, Orsetti J, Aluvihare C, O′Brochta D A and Atkinson P W. 2005. An active transposable element, Herves, from the African malaria mosquitoAnophelesgambiae.Genetics, 169: 697-708.
Asman S M, Nelson R L and McDonald P T. 1979. Pilot release of sex-linked multiple translocation into aCulextarsalisfield population in Kern County, California.MosquitoSystematics, 39: 248-258.
Asman S M, Zalom F G and Meyer R P. 1980. A field release of irradiated maleCulextarsalisin California∥Grant C D.ProceedingsandPapersofthe48thAnnualConferenceoftheCaliforniaMosquitoandVectorControlAssociation,Inc. Anaheim, California: CMVCA Press, 64.
Atkinson M P, Su Z, Alphey N, Alphey L S, Coleman P G and Wein L M. 2007. Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 104: 9540-9545.
Baker R H, Reisen W K, Sakai R K, Hayes C G, Aslamkhan M, Saifuddin U T, Mahmood F, Perveen A and Javed S. 1979. Field assessment of mating competitiveness of maleCulextritaeniorhynchuscarrying a complex chromosomal aberration.AnnalsoftheEntomologicalSocietyofAmerica, 72: 751-758.
Baker R H, Reisen W K, Sakai R K, Rathor H R, Raana K, Azra K and Niaz S. 1980. Anopheles culicifacies: mating behavior and competitiveness in nature of males carrying a complex chromosomal aberration.AnnalsoftheEntomologicalSocietyofAmerica, 73: 581-588.
Bargielowski I, Alphey L and Koella J C. 2011a. Cost of mating and insemination capacity of a genetically modified mosquitoAedesaegyptiOX513A compared to its wild type counterpart.PLoSONE, 6: e26086.
Bargielowski I, Kaufmann C, Alphey L, Reiter P and Koella J. 2012. Flight performance and teneral energy reserves of two genetically-modified and one wild-type strain of the yellow fever mosquitoAedesaegypti.Vector-BorneandZoonoticDiseases, 12: 1053-1058.
Bargielowski I, Nimmo D, Alphey L and Koella J C. 2011b. Comparison of life history characteristics of the genetically modified OX513A line and a wild type strain ofAedesaegypti.PLoSONE, 6: e20699.
Benedict M Q and Robinson A S. 2003. The first releases of transgenic mosquitoes: an argument for the sterile insect technique.TrendsinParasitology, 19: 349-355.
Bian G, Joshi D, Dong Y, Lu P, Zhou G L, Pan X L, Xu Y, Dimopoulos G and Xi Z Y. 2013.WolbachiainvadesAnophelesstephensipopulations and induces refractoriness to Plasmodium infection.Science, 340: 748-751.
Bian G, Xu Y, Lu P, Xie Y and Xi Z Y. 2010. The endosymbiotic bacteriumWolbachiainduces resistance to Dengue virus inAedesaegypti.PLoSPathogens, 6: e1000833.
Black W C, Alphey L and James A A. 2011. Why RIDL is not SIT.TrendsinParasitology, 27: 362-370.
Breeland S G, Jeffery G M, Lofgren C S and Weidhaas D E. 1974. Release of chemosterilized males for the control ofAnophelesalbimanusin El Salvador I. Characteristics of the test site and the natural population.TheAmericanJournalofTropicalMedicineandHygiene, 23: 274-281.
Brennan L J, Keddie B A, Braig H R and Harris H L. 2008. The endosymbiontWolbachiapipientis induces the expression of host antioxidant proteins in anAedesalbopictuscell line.PLoSONE, 3: e2083.
Burt A. 2003. Site-specific selfish genes as tools for the control and genetic engineering of natural populations.ProceedingsoftheRoyalSocietyofLondon.SeriesB:BiologicalSciences, 270: 921-928.
Catteruccia F, Benton J P and Crisanti A. 2005. AnAnophelestransgenic sexing strain for vector control.NatureBiotechnology, 23: 1414-1417.
Catteruccia F, Crisanti A and Wimmer E A. 2009. Transgenic technologies to induce sterility.MalariaJournal, 8(Suppl 2): S7.
Catteruccia F, Godfray H C J and Crisanti A. 2003. Impact of genetic manipulation on the fitness ofAnophelesstephensimosquitoes.Science, 299: 1225-1227.
Catteruccia F, Nolan T, Blass C, Müller H M, Crisanti A, Kafatos F C and Loukeris T G. 2000a. TowardAnophelestransformation:Minoselement activity in anopheline cells and embryos.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 97: 2157-2162.
Catteruccia F, Nolan T, Loukeris T G, Blass C, Savakis C, Kafatos F C and Crisanti A. 2000b. Stable germline transformation of the malaria mosquitoAnophelesstephensi.Nature, 405: 959-962.
Chen C H, Huang H X, Ward C M, Su J T, Schaeffer L V, Guo M and Hay B A. 2007. A synthetic maternal-effect selfish genetic element drives population replacement inDrosophila.Science, 316: 597-600.
Coates C J, Jasinskiene N, Miyashiro L and James A A. 1998.Marinertransposition and transformation of the yellow fever mosquito,Aedesaegypti.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 95: 3748-3751.
Cousserans J and Guille G. 1974. Expérience de lutte genetique contreCulexpipiensdans la région deMontpellier. Synthèse de quatre annèes de′observations.BulletinBiologique, 108: 253-257.
Curtis C F. 1968. Possible use of translocations to fix desirable genes in insect pest populations.Nature, 218: 368-369.
Curtis C F, Brooks G D, Ansari M A, Grover K K, Krishnamurthy B S, Rajagopalan P K, Sharma L S, Sharma V P, Singh D, Singh K R P and Yasuno M. 1982. A field trial on control ofCulexquinquefasciatusby release of males of a strain integrating cytoplasmic incompatibility and a translocation.EntomologiaExperimentalisetApplicata, 31(2-3): 181-190.
Dame D A, Lowe R E and Williamson D L. 1981. Assessment of released sterileAnophelesalbimanusandGlossinamorsitansmorsitans∥Pal R, Kitzmiller J B and Kanda T.CytogeneticsandGeneticsofVectors:ProceedingsofASymposiumofthe16thInternationalCongressofEntomology. Amsterdam, Netherlands: Elsevier Biomedical Press, 231-248.
Dame D A, Woodard D B, Ford H R and Weidhaas D E. 1964. Field behavior of sexually sterileAnophelesquadrimaculatusmales.MosquitoNews, 24: 6-14.
Davidson G, Odetoyinbo J A, Colussa B and Coz J. 1970. A field attempt to assess the mating competitiveness of sterile males produced by crossing 2 members of theAnophelesgambiaecomplex.BulletinoftheWorldHealthOrganization, 42: 55-67.
Davis S, Bax N and Grewe P. 2001. Engineered underdominance allows efficient and economic introgression of traits into pest populations.JournalofTheoreticalBiology, 212: 83-98.
Deredec A, Burt A and Godfray H C J. 2008. Population genetics of using homing endonuclease genes in vector and pest management.Genetics, 179: 2013-2026.
Fay R W and Craig G B. 1969. Genetically markedAedesaegyptiin studies of field populations.MosquitoNews, 29: 121-127.
Fraser M J, Smith G E and Summers M D. 1983. Acquisition of host cell DNA sequences by Baculoviruses: relationship between host DNA insertions and FP mutants ofAutographacalifornicaandGalleriamellonellanuclear polyhedrosis viruses.JournalofVirology, 47: 287-300.
Fu G L, Lees R S, Nimmo D, Aw D, Jin L, Gray P, Berendonkb T U, White-Cooper H, Scaifea S, Phuc H K, Marinotti O, Jasinskiene N, James A A and Alphey L. 2010. Female-specific flightless phenotype for mosquito control.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 107: 4550-4554.
Galizi R, Doyle L A, Menichelli M, Bernardini F, Deredec A, Burt A, Stoddard B L, Windbichler N and Crisanti A. 2014. A synthetic sex ratio distortion system for the control of the human malaria mosquito.NatureCommunications, 5: 3977, doi: 10.1038/ncomms4977.
Gong P, Epton M J, Fu G L, Scaife S, Hiscox A, Condon K C, Condon G C , Morrison N I, Kelly D W, Dafa′alla T, Coleman P G and Alphey L. 2005. A dominant lethal genetic system for autocidal control of the Mediterranean fruit fly.NatureBiotechnology, 23: 453-456.
Grover K K, Curtis C F, Sharma V P, Singh K R P, Dietz K, Agarwal H V, Razdan R K and Vaidyanathan V. 1976a. Competitiveness of chemosterilised males and cytoplasmically incompatible translocated males ofCulexpipiensfatigans Wiedemann (Diptera, Culicidae) in the field.BulletinofEntomologicalResearch, 66: 469-480.
Grover K K, Suguna S G, Uppal D K, Singh K R P, Ansari M A, Curtis C F, Singh D, Sharma V P and Panicker K N.1976b. Field experiments on the competitiveness of males carrying genetic control systems forAedesaegypti.EntomologiaExperimentalisetApplicata, 20: 8-18.
Hancock P A and Godfray H C J. 2012. Modelling the spread ofWolbachiain spatially heterogeneous environments.JournaloftheRoyalSocietyInterface, 9: 3045-3054.
Handler A M. 2002. Use of thepiggyBactransposon for germ-line transformation of insects.InsectBiochemistryandMolecularBiology, 32: 1211-1220.
Hanson S M, Mutebi J P, Craig G B J and Novak R J. 1993. Reducing the overwintering ability ofAedesalbopictusby male release.JournaloftheAmericanMosquitoControlAssociation, 9: 78-83.
Harris A F, McKemey A R, Nimmo D, Curtis Z, Black I, Morgan S A, Oviedo M N, Lacroix R,etal. 2012. Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes.NatureBiotechnology, 30: 828-830.
Harris A F, Nimmo D, McKemey A R, Kelly N, Scaife S, Donnelly C A, Beech C, Petrie W D and Alphey L. 2011. Field performance of engineered male mosquitoes.NatureBiotechnology, 29: 1034-1037.
Hendrichs J, Robinson A S, Cayol J P and Enkerlin W. 2002. Medfly areawide sterrile insect technique programmes for prevention, suppression or eradication: the importance of mating behavior studies.FloridaEntomologist, 85: 1-13.
Irvin N, Hoddle M S, O′Brochta D A, Carey B and Atkinson P W. 2004. Assessing fitness costs for transgenicAedesaegyptiexpressing the GFP marker and transposase genes.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 101: 891-896.
Jasinskiene N, Coates C J, Benedict M Q, Cornel A J, Rafferty C S, James A A and Collins F H. 1998. Stable transformation of the yellow fever mosquito,Aedesaegypti, with theHermeselement from the housefly.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 95: 3743-3747.
Klassen W. 2009. Introduction: development of the sterile insect technique for African malaria vectors.MalariaJournal, 8(Suppl 2): 11.
Knipling E F. 1970. Suppression of pest Lepidoptera by releasing partially sterile males: a theoretical appraisal.Bioscience, 20: 465-470.
Labbé G M C, Scaife S, Morgan S A, Curtis Z H and Alphey L. 2012. Female-specific flightless (fsRIDL) phenotype for control ofAedesalbopictus.PLoSNeglectedTropicalDiseases, 6: e1724.
Lacroix R, McKemey A R, Raduan N, Wee L K, Ming W H, Ney T G, Rahidah A A S, Salman S,etal. 2012. Open field release of genetically engineered sterile maleAedesaegyptiin Malaysia.PLoSONE, 7: e42771.
Laven H. 1967. Eradication ofCulexpipiensfatigans through cytoplasmic incompatibility.Nature, 216: 383-384.
Laven H, Cousserans J and Guille G. 1972. Eradicating mosquitoes using translocations: a first field experiment.Nature, 236: 456-457.
Lee H L, Joko H, Nazni W A and Vasan S S. 2009. Comparative life parameters of transgenic and wild strain ofAedesaegyptiin the laboratory.DengueBulletin, 33: 103-114.
Lee H L, Vasan S, Ahmad N W, Idris I, Hanum N, Selvi S, Alphey L and Murad S. 2013. Mating compatibility and competitiveness of transgenic and wild typeAedesaegypti(L.) under contained semi-field conditions.TransgenicResearch, 22: 47-57.
Magori K and Gould F. 2006. Genetically engineered underdominance for manipulation of pest populations: a deterministic model.Genetics, 172: 2613-2620.
Marrelli M T, Moreira C K, Kelly D and Jacobs-Lorena M. 2006. Mosquito transgenesis: what is the fitness cost?TrendsinParasitology, 22: 197-202.
Massonnet-Bruneel B, Corre-Catelin N, Lacroix R, Lees R S, Hoang K P, Nimmo D, Alphey L and Reiter P. 2013. Fitness of transgenic mosquitoAedesaegyptimales carrying a dominant lethal genetic system.PLoSONE, 8: e62711.
McDonald P T, Hausermann W and Lorimer N. 1977. Sterility introduced by release of genetically altered males to a domestic population ofAedesaegyptiat the Kenya coast.TheAmericanJournalofTropicalMedicineandHygiene, 26: 553-561.
Milby M M. 1980. Release of heterozygous translocated adult ales for genetic control ofCulextarsalisat an isolated site.MosquitoSystematics, 40: 83-90.
Moreira L A, Iturbe-Ormaetxe I, Jeffery J A, Lu G, Pyke A T, Hedges L M, Rocha B C, Hall-Mendelin S,etal. 2009. AWolbachiasymbiont inAedesaegyptilimits infection with dengue, Chikungunya, and Plasmodium.Cell, 139: 1268-1278.
Morlan H B, McCray E M J and Kilpatrick J W. 1962. Field tests with sexually sterile males for control ofAedesaegypti.MosquitoNews, 22: 295-300.
Nazni W A, Selvi S, Lee H L, Sadiyah I, Azahari H, Derric N and Vasan S S. 2009. Susceptibility status of transgenicAedesaegypti(L.) against insecticides.DengueBulletin, 33: 124-129.
Nimmo D D, Alphey L, Meredith J M and Eggleston P. 2006. High efficiency site-specific genetic engineering of the mosquito genome.InsectMolecularBiology, 15: 129-136.
Nolan T, Papathanos P, Windbichler N, Magnusson K, Benton J, Catteruccia F and Crisanti A. 2011. Developing transgenicAnophelesmosquitoesfor the sterile insect technique.Genetica, 139: 33-39.
O′Brochta D A, Sethuraman N, Wilson R, Hice R H, Pinkerton A C, Levesque C S, Bideshi D K, Jasinskiene N, Coates C J, James A A, Lehane M J and Atkinson P W. 2003. Gene vector and transposable element behavior in mosquitoes.JournalofExperimentalBiology, 206: 3823-3834.
O′Connor L, Plichart C, Sang A C, Brelsfoard C L, Bossin H C and Dobson S L. 2012. Open release of male mosquitoes infected with aWolbachiabiopesticide: field performance and infection containment.PLoSNeglectedTropicalDiseases, 6: e1797.
Pan X, Zhou G, Wu J, Bian G, Lu P, Bian G, Lu P, Raikheld A S and Xi Z. 2012.Wolbachiainduces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquitoAedesaegypti.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 109(1): E23-E31.
Patterson R S, Ford H R, Lofgren C S and Weidhaas D E. 1970a. Sterile males: their effect on an isolated population of mosquitoes.MosquitoNews, 30: 23-27.
Patterson R S, Weidhaas D E, Ford H R and Lofgren C S. 1970b. Suppression and elimination of an island population ofCulexpipiensquinquefasciatuswith sterile males.Science, 168: 1368-1369.
Petersen J L, Lounibos L P and Lorimer N. 1977. Field trials of double translocation heterozygote males for genetic control ofAedesaegypti(L.)(Diptera: Culicidae).BulletinofEntomologicalResearch, 67: 313-324.
Phuc H K, Andreasen M H, Burton R S, Vass C, Epton M J, Pape G, Fu G L, Condon K C, Scaife S, Donnelly C A, Coleman P G, White-Cooper H and Alphey L. 2007. Late-acting dominant lethal genetic systems and mosquito control.BMCBiology, 5: 11.
Rai K S, Grover K K and Suguna S G. 1973. Genetic manipulation ofAedesaegypti: incorporation and maintenance of a genetic marker and a chromosomal translocation in natural populations.BulletinoftheWorldHealthOrganization, 48: 49-56.
Rasgon J L and Gould F. 2005. Transposable element insertion location bias and the dynamics of gene drive in mosquito populations.InsectMolecularBiology, 14: 493-500.
Reisen W K, Baker R H, Sakai R K, Mahmood F, Rathor H R, Raana K and Toqir G. 1981.AnophelesculicifaciesGiles: mating behavior and competitiveness in nature of chemosterilized males carrying a genetic sexing system.AnnalsoftheEntomologicalSocietyofAmerica, 74: 395-401.
Reisen W K, Bock M E, Milby M M and Reeves W C. 1985. Attempted insertion of a recessive autosomalgene into a semi-isolated population ofCulextarsalis(Diptera: Culicidae).JournalofMedicalEntomology, 22: 250-260.
Reisen W K, Milby M M and Asman S M. 1982. Attempted suppression of a semi-isolatedCulextarsalispopulation by the release of irradiated males: a second experiment using males from a recently colonized strain.MosquitoNews, 2: 565-575.
Scolari F, Schetelig M F, Gabrieli P, Siciliano P, GomuLski L M, Karam N, Wimmer E A, Malacrida A R and Gasperi G. 2008. Insect transgenesis applied to tephritid pest control.JournalofAppliedEntomology, 132(9-10): 820-831.
Scott T W, Takken W, Knols B G J and Boёte C. 2002. The ecology of genetically modified mosquitoes.Science, 298: 117-119.
Sinkins S P and Gould F. 2006. Gene drive systems for insect disease vectors.NatureReviewsGenetics, 7: 427-435.
Thomas D D, Donnelly C A, Wood R J and Alphey L S. 2000. Insect population control using a dominant, repressible, lethal genetic system.Science, 287: 2474-2476.
Walker T, Johnson P H, Moreira L A, Iturbe-Ormaetxe I, Frentiu F D, Iturbe-Ormaetxe I, Frentiu F D, McMeniman C J, Leong Y S, Dong Y, Axford J, Kriesner P, Lloyd A L, Ritchie S A, O′Neill S L and Hoffmann A A. 2011. ThewMelWolbachiastrain blocks dengue and invades cagedAedesaegyptipopulations.Nature, 476: 450-453.
Weidhaas D E, Breeland S G, Lofgren C S, Dame D A and Kaiser R. 1974. Release of chemosterilized males for the control ofAnophelesalbimanusin El Salvador IV. Dynamics of the test population.TheAmericanJournalofTropicalMedicineandHygiene, 23: 298-308.
Weidhaas D E and Schmidt C H. 1962. Field studies on the release of sterile males or the control ofAnophelesquadrimaculatus.MosquitoNews, 22: 283-291.
Werren J H, Baldo L and Clark M E. 2008.Wolbachia: master manipulators of invertebrate biology.NatureReviewsMicrobiology, 6: 741-751.
Wilke A B B and Marrelli M T. 2012. Genetic control of mosquitoes: population suppression strategies.RevistadoInstitutodeMedicinaTropicaldeSãoPaulo, 54: 287-292.
Windbichler N, Menichelli M, Papathanos P A, Thyme S B, Li H, Ulge U Y, Hovde B T, Baker D, Monnat R J Jr, Burt A and Crisanti A. 2011. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito.Nature, 473: 212-215.
Windbichler N, Papathanos P A and Crisanti A. 2008. Targeting the X chromosome during spermatogenesis induces Y chromosome transmission ratio distortion and early dominant embryo lethality inAnophelesgambiae.PLoSGenetics, 4: e1000291.
Wise de Valdez M R, Nimmo D, Betz J, Gong H F and James A A. 2011. Genetic elimination of dengue vector mosquitoes.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 108: 4772-4775.
Yakob L, Alphey L and Bonsall M B. 2008.Aedesaegypticontrol: the concomitant role of competition, space and transgenic technologies.JournalofAppliedEcology, 45: 1258-1265.
Yasuno M, Macdonald W W and Curtis C F. 1978. Control experiment with chemosterilized maleCulexpipiensfatigansWied in a village near Delhi surrounded by a breeding-free zone.JapaneseJournalofSanitaryZoology, 29: 325-343.
Zabalou S, Apostolaki A, Livadaras I, Franz G, Robinson A S, Savakis C and Bourtzis K. 2009. Incompatible insect technique: incompatible males from aCeratitiscapitata(Diptera: Tephritidae) genetic sexing strain.EntomologiaExperimentalisetApplicata, 132: 232-240.
Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C and Bourtzis K. 2004.Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control.ProceedingsoftheNationalAcademyofSciencesoftheUnitedStatesofAmerica, 101: 15042-15045.
Zhao Y G and Eggleston P. 1998. Stable transformation of anAnophelesgambiaecell line mediated by theHermesmobile genetic element.InsectBiochemistryandMolecularBiology, 28: 213-219.
(责任编辑:杨郁霞)
1State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, Chinese Academy of Agricultural
Sciences,Beijing100193,China;2Department of Entomology, North Carolina State University, Campus Box 7613, Raleigh,
NC27695-7613,USA;3Genetic Engineering and Society Center and W. M. Keck Center for Behavioral Biology,
NorthCarolinaStateUniversity,Raleigh,NC27695-7613,USA;4College of Agriculture and Plant Protection,
QingdaoAgriculturalUniversity,Qingdao,Shandong266109,China
Abstract:Mosquito-borne diseases, such as dengue fever and malaria, are global problems and pose a serious threat to public health. An estimated 2.5 billion people live in areas at the risk of epidemic transmission. For now, no vaccines are available against the pathogens respnsible for these diseases, and the mosquito control is considered as one of the most effective ways to reduce transmission. Mass application of pesticides could reduce the mosquito population but it also brings problems like insect resistance and environmental pollution. The release of insects with dominant lethality (RIDL) technology and other genetic control systems based on the traditional sterile insect technique (SIT) provide new strategies to control disease vector mosquitos, such as Aedes aegypti and Anopheles gambiae. Those new version of genetic control methods are species-specific and environment-friendly, and now being developed and tested worldwide. Here the principle and recent progress of mosquito genetic control are reviewed. The history of mosquito SIT is introduced, and the genetic control strategies including self-limiting and self-sustaining populations are also illustrated. The development, as well as laboratory and field trials of RIDL strains are described. It is suggested that genetic control strategies such as RIDL are promising methods to fight against mosquitoes carrying human diseases.
Key words:disease vector mosquito; genetic control; sterile insect technique; release of insects carrying a dominant lethal
通讯作者*(Author for correspondence), E-mail: wanfanghao@caas.cn
作者简介:武强, 男, 博士研究生。 研究方向: 昆虫生物化学与分子生物学。 E-mail: wuqiang8510@163.com
基金项目:科技导报社博士生创新研究资助计划(kjdb201001-3); 中德合作科研项目[PPP项目、留金欧(2012) 6014、留金欧(2014 )6013]; 环保公益性行业科研专项(201409061); 农业部2014年农作物病虫鼠害疫情监测与防治(外来入侵生物防治)项目; 人力资源社会保障部2014年度留学人员科技活动择优资助项目
收稿日期(Received): 2014-12-20接受日期(Accepted): 2015-01-17
DOI:10. 3969/j.issn.2095-1787.2015.02.008
