鹅绒藤属植物甾体成分及其药理作用研究进展
2015-12-17何达海罗华秀马雅茹张秀瑛
何达海,杨 帅,罗华秀,秦 茜,马雅茹,张秀瑛
(西南民族大学化学与环境保护工程学院,四川 成都 610041)
鹅绒藤属植物甾体成分及其药理作用研究进展
何达海,杨 帅,罗华秀,秦 茜,马雅茹,张秀瑛
(西南民族大学化学与环境保护工程学院,四川 成都 610041)
鹅绒藤属植物在我国分布广泛,且大部分具有药用价值.甾体皂苷,该属植物的主要成分,表现出极大的结构多样性.化学结构多样性体现在母核(甾体苷元)、母核取代基、糖链长度、单糖种类、绝对构型(D或L)以及糖链中糖苷键的类型(α或β),特别是在同一个糖链中同时存在D-糖和L-糖的情况在其它天然产物中并不多见,糖链长度多达7个单糖的结构在糖苷类化合物中也十分罕见.从母核和其取代基的角度将2007-2014年从该属植物中分离出来的新甾体皂苷共119个,归纳为5种17亚种.同时也对该属植物甾体皂苷的生物活性如抗肿瘤、抗病毒、神经保护和杀虫等进行了综述.
鹅绒藤属;甾体皂苷;糖链;抗肿瘤;抗病毒;神经保护;杀虫
甾体,大量分布于动物、植物和真菌中的一类重要有机化合物,具有举足轻重的生理功能,如生理调节剂、荷尔蒙、维生素原等.现代药理学研究发现,甾体表现出广泛的医学治疗和生物农药应用价值,如抗肿瘤[1]、抗病毒[2]、神经保护[3]、杀虫[4]等.
鹅绒藤属植物富含甾体类化合物,是天然甾体化合物的资源宝库.鹅绒藤属(Cynanchum)植物属于萝藦科(Asclepiadaceae),全世界约200种,我国有53种,12变种,全国各省均有分布.鹅绒藤属植物大部分为药用植物,如徐长卿[5](Cynanchum paniculatum)的干燥根及根茎用于祛风、化湿、止痛、止痒等.青羊参[6](Cynanchum otophyllum)的根用于补肾、祛风除湿、解毒镇痉等.飞来鹤[7]来源牛皮消(Cynanchum auriculatum)的根或全草用于健脾益气、安神补血、收敛精气、养阴补虚等.白首乌[8](Cynanchum bungei)的块根用于安神、补血等.
鹅绒藤属植物次生代谢产物种类齐全,结构多样.迄今为止,从该属植物中分离出来的化合物涵盖了甾体皂苷、生物碱、萜类、黄酮、甾醇等,尤其以表现出多种生物活性的甾体皂苷最为丰富.仅2007-2014年间,从该属植物中分离出的新甾体皂苷就多达119个.皂苷母核为C21甾体和C20、C19降甾体,其结构如图1所示;糖链少的连1个,多则达7个,氧糖苷键均连接与甾体母核的C-3位.糖的种类丰富,目前从该属植物中鉴定出来的糖多达7种,且部分糖基的绝对构型既有D构型,也有L构型;糖的异头碳既有α连接,也有β连接.糖的连接顺序均为1→4直链连接方式.据我们所知,目前关于鹅绒藤属植物化学成分或甾体成分研究进展综述均为2007年以前的研究工作[9-12].药理作用进展综述也有待更新[13].自2007年以来,已有大量有关鹅绒藤属植物的化学成分研究及相关化合物现代药理学研究的报道.因此,本文主要针对2007-2014年分离出来的新甾体皂苷结构分类及从该属植物中分离的甾体皂苷生物活性.
1 结构分类
根据甾体皂苷的骨架类型将它们分为A、B、C、D、E、F总共五大类(详见图1).在本文中除非特别说明,全文糖的连接顺序均为1→4,构型均为β-D,全文用到的缩写:Ac=acetyl,Cin=cinnamoyl,Bz=benzyl,Nic=nicotinoyl,Mebu=2-methylbutyryl,PHB=p-Hydroxybenzoyl,3-demethyl-2-deoxythevetopyranosyl=canaropyranoside=Cana,Cym=cymaropyranosyl,Digit=digitoxopyranosyl,Dig=diginopyranosyl,Glc=glucopyranosyl,Ole=oleandropyranosyl,Thev=thevetopyranosyl.表格中用到的取代基代码为:S1=Cym-α -L-Dig-Cym,S2=α-L-Cym-Cym-α-LCym-Digit-Digit,S3=α-L-Cym-Cym-α-LCym-3-O-AcDigit-Digit,S4=Cym-α-L-Cym -Digit-Digit,S5=Cym-α-L-Cym-3-O-Ac-Digit-Digit,S6=Ole-Cym,S7=Glc-Glc-α-LCym-Ole-Cym,S8=Glc-Glc-Cym-Ole-Digit,S9=α-L-Cym-α-D-Ole-α-L-Cym-Glc-α -D-Ole-Ole-Dig,S10=Cym-α-D-Ole-α-L -Cym-Glc-Ole-Cym-Dig,S11=Thev-Cym-Digit,S12=Thev-Cym-Cym,S13=Glc-Ole-Cym -Digit,S14=Glc-Glc-Ole-Ole-Cym-Cym,S15=Glc-Cym-Ole-Cym-Digit,S16=Glc-Thev-Cym-Digit,S17=Glc-Glc-Cym-Ole-Cym,S18=Glc-Cym-Ole-Cym-Cym,S19=Cym-Ole-Cym -Digit,S20=Cym-Ole-Cym-Cym,S21=Glc-Glc -Ole-Cym-Cym,S22=Glc-Glc-Cym-Ole-Cym -Cym,S23=Glc-Ole-Cym-Cym-α-L-Cym-Ole-Cym,S24=Glc-α-L-Cym-Cym-Cym-α-L-Cym-Ole-Cym,S25=α-L-Dig-Digit,S26=α -L-Cym-Digit-Digit,S27=Digit-α-L-Cym-Digit,S28=α-L-Dig-Cym,S29=α-L-Dig-Cym,S30=Digit-Digit,S31=α-L-Cym-Cym-α -L-Dig-Cym,S32=Glc-Cym-α-L-Dig-Cym,S33=Glc-α-L-Cym-Cym-α-L-Dig-Cym,S34=Glc-Glc-α-L-Cym-Cym-α-LDig-Cym,S35=α-Ole-Digit-Ole,S36=α-LDig-Digit-Cym,S37=α-L-Ole-Digit-Ole,S38=α-Cym-Digit-Ole,S39=α-L-Cym-Cym-Ole,S40=α-L-Cym-Digit-Cana,S41=Digit-β -L-Cym,S42=Glc-Cym,S43=Ole-α-L-Dig-Cym,S44=α-D-Ole-Cana-Cym,S45=α-DOle-Digit-Cym,S46=Glc-α-D-Ole-Digit-Cym,S47=Digit-Cym,S48=4-OCH3-α-L-Dig -Digit-Cym,S49=Glc-Glc-Ole-β-L-Cym-β -L-Cym-Ole,S50=Glc-Glc-Ole-Ole-Ole-Ole,S51=Glc-Glc-Ole-β-L-Cym-Cana-Ole,S52=Glc-Glc-Ole-Ole-β-L-Cym-Cana,S53=Ole-Cana-Thev,S54=Ole-Cana-Ole,S55=Ole -Digit-Ole,S56=Cym-Cana-Ole,S57=α-LOle-Cym-Ole,S58=Glc-Ole-Digit-Ole,S59=Glc-Glc-α-L-Cym-Digit-Ole,S60=Glc-Glc -α-L-Cym-Digit-Cana,S61=Cym-Digit-Thev,S62=α-L-Cym-Cym-Digit-Thev,S63=Digit-Ole,S64=β-L-Dig,S65=α-L-Cym-Dig-it-Ole,S66=β-L-Dig-Cym-Thev,S67=α-LDig-Cym-Cym-Thev,S68=α-L-Cym-Cym-Cym-Thev,S69=Glc-Glc-Ole-β-L-Cym-Ole -Ole,S70=α-L-Ole-Digit-Cym,S71=Glc-α-L-Ole-Digit-Cym

图1 鹅绒藤属植物甾体皂苷母核结构Fig.1 Structures ofsteroidal saponin skeletons isolated from Cynanchum
1 A1类型
A1类型是新近发现数量最多的甾体皂苷类型(见表1).Liu等[14]从牛皮消(Cynanchum auriculatum)根的乙醇提取物中分离并鉴定出具有减弱食欲的甾体皂苷化合物cynauricosides A-I.Yang等[15]则从牛皮消根的乙醇提取物中分离并鉴定出具有抗抑郁作用的在体皂苷cynanauriculosides C-E.Zhao等[16]从青羊参(Cynanchum otophyllum)根茎乙醇提取物中得到2个连接有多达7个单糖的甾体皂苷,糖苷键的构型和糖的绝对构型多样,如β-D、α-L、α-D.这在自然界中十分少见.Shen等[17]从青羊参根乙醇提取物中得到了4个甾体皂苷otophyllosides TW.Shan等[18]从青羊参根乙醇提取物中得到了甾体皂苷cynotophylloside G.刘海港等[19]从白首乌(Cynanchum bungei)地下部分乙醇提取物中分离并鉴定出新甾体皂苷告达亭3-O-β-D-吡喃洋地黄毒糖苷.Ma等[20]对青羊参乙醇提取物进行了研究,从中分离鉴定出6个C-12乙酰化甾体皂苷otophyllosides H-M.Ma等[21]进一步从青羊参根乙醇提取物中分离得出青羊参苷和告达亭苷类化合物otophyllosides N-P,且在同一化合物中同时出现D-和L-加拿大麻糖.Zhao等[3]从青羊参根茎乙醇提取物中分离鉴定出多羟基取代甾体皂苷化合物cynanotosides A、B和E.Ma等[22]从青羊参根乙醇提取物中分离和鉴定出多羟基取代甾体皂苷类化合物cynotophyllosides A-C.Shi等[23]从青羊参根乙醇提取物中分离和鉴定出多羟基取代甾体皂苷类化合物cynotophyllosides I和J.Name 1=告达亭-3-O-β-D-吡喃洋地黄毒糖苷.植物代码(下表同):a.Cynanchum auriculatum牛皮消;b.Cynanchum stauntonii柳叶白前;c.Cynanchum bungei白首乌;d.Cynanchum paniculatum徐长卿;e. Cynanchum chekiangense蔓剪草;f.Cynanchum otophyllum青羊参;g.Cynanchum wilfordii隔山消;h.Cynanchum atratum白薇;i.Cynanchum komarovii牛心朴子;j.Cynanchum forrestii大理白前;k.Cynanchum amplexicaule合掌消;l.Cynanchum versicolor变色白前;m.Cynanchum ascyrifolium潮风草;n.Cynanchum inamoenum竹灵消

表1 鹅绒藤属植物中A1、C1、D4类型甾体皂苷Table 1 Types A1,C1,D4steroidal saponins isolated from Cynanchum
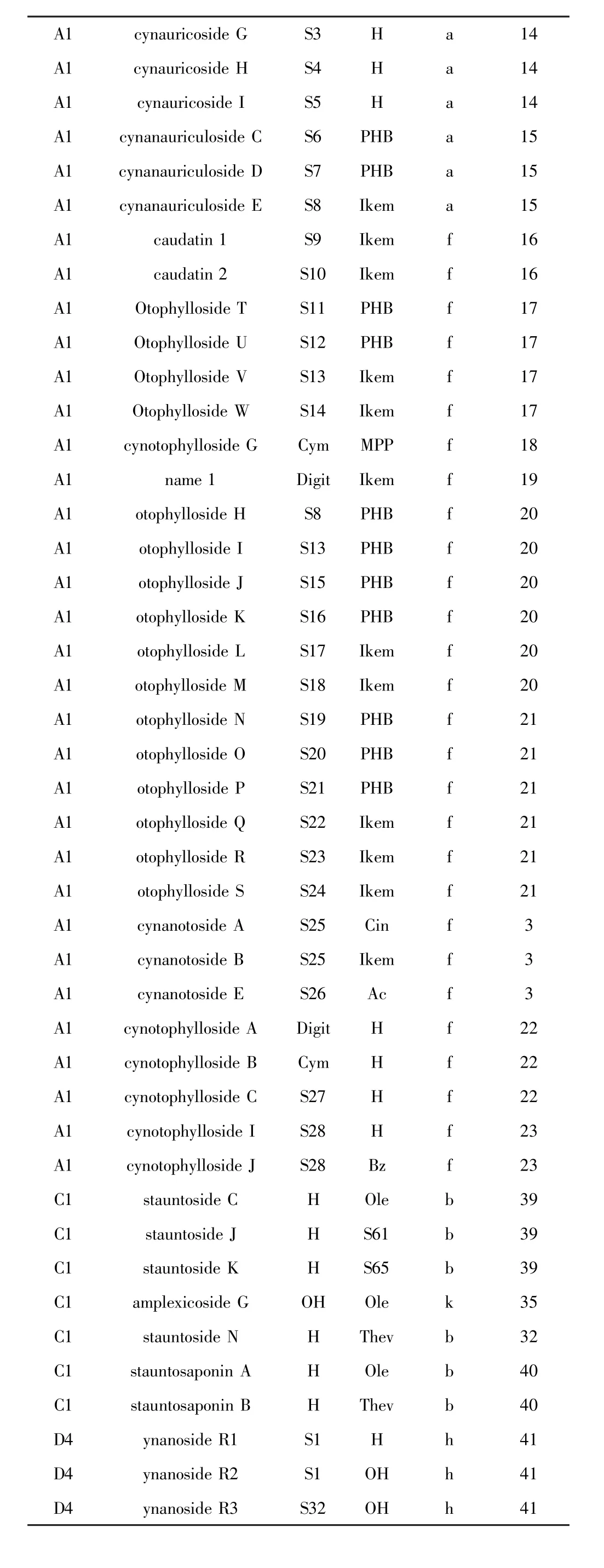
A1cynauricoside GS3Ha14 A1cynauricoside HS4Ha14 A1cynauricoside IS5Ha14 A1cynanauriculoside CS6PHBa15 A1cynanauriculoside DS7PHBa15 A1cynanauriculoside ES8Ikema15 A1caudatin 1S9Ikemf16 A1caudatin 2S10Ikemf16 A1Otophylloside TS11PHBf17 A1Otophylloside US12PHBf17 A1Otophylloside VS13Ikemf17 A1Otophylloside WS14Ikemf17 A1cynotophylloside GCymMPPf18 A1name 1DigitIkemf19 A1otophylloside HS8PHBf20 A1otophylloside IS13PHBf20 A1otophylloside JS15PHBf20 A1otophylloside KS16PHBf20 A1otophylloside LS17Ikemf20 A1otophylloside MS18Ikemf20 A1otophylloside NS19PHBf21 A1otophylloside OS20PHBf21 A1otophylloside PS21PHBf21 A1otophylloside QS22Ikemf21 A1otophylloside RS23Ikemf21 A1otophylloside SS24Ikemf21 A1cynanotoside AS25Cinf3 A1cynanotoside BS25Ikemf3 A1cynanotoside ES26Acf3 A1cynotophylloside ADigitHf22 A1cynotophylloside BCymHf22 A1cynotophylloside CS27Hf22 A1cynotophylloside IS28Hf23 A1cynotophylloside JS28Bzf23 C1stauntoside CHOleb39 C1stauntoside JHS61b39 C1stauntoside KHS65b39 C1amplexicoside GOHOlek35 C1stauntoside NHThevb32 C1stauntosaponin AHOleb40 C1stauntosaponin BHThevb40 D4ynanoside R1S1Hh41 D4ynanoside R2S1OHh41 D4ynanoside R3S32OHh41
1.2 A2、A3类型
A3类型的甾体皂苷主要出自青羊参(详见表2),A3类型相对少见,从牛皮消中分离得到.Zhao等[3]从青羊参根和茎乙醇提取物中分离鉴定出具有神经保护作用的A2类型甾体皂苷cynanotoside C和D.Shan等[18]从青羊参根乙醇提取物中分离鉴定出甾体皂苷cynotophylloside H.Ma等[22]从青羊参根乙醇提取物中分离鉴定出甾体皂苷cynotophyllosides D和E.Shi等[24]从青羊参根乙醇提取物中分离鉴定出C-12位乙酸取代,C-20位2-甲基丁酸取代的sarcostin.Lu等[25]从牛皮消根的乙醇提取物中分离鉴定了化合物cyanoauriculoside G.Lu等[26]从牛皮消根的乙醇提取物中分离鉴定了化合物cyanoauriculosides C-E,它们均在C-12位肉桂酸取代、C-20位2-甲基丁酸取代.Teng等[27]从牛皮消根乙醇提取物中分离鉴定出cyanoauriculosides A和B.
Lu等[25]从牛皮消根中分离鉴定出A3类型甾体皂苷类化合物cyanoauriculoside H.
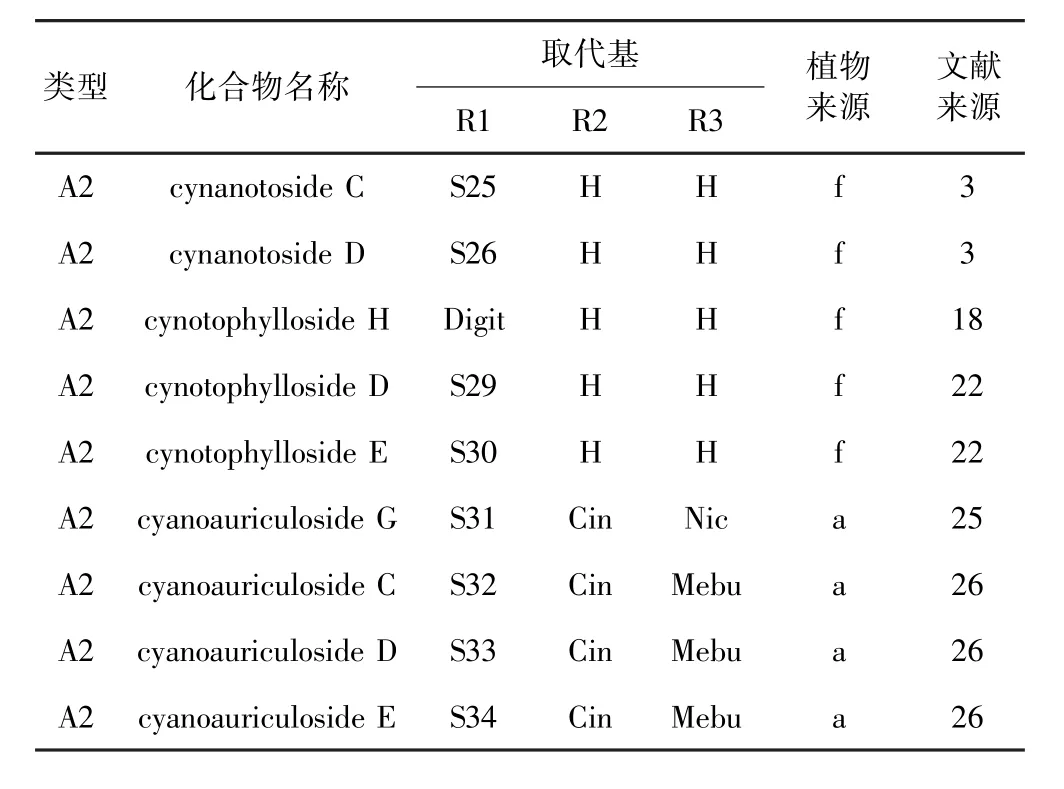
表2 鹅绒藤属植物中A2、A3、D2类型甾体皂苷Table 2 Types A2,A3,D2steroidal saponins isolated from Cynanchum
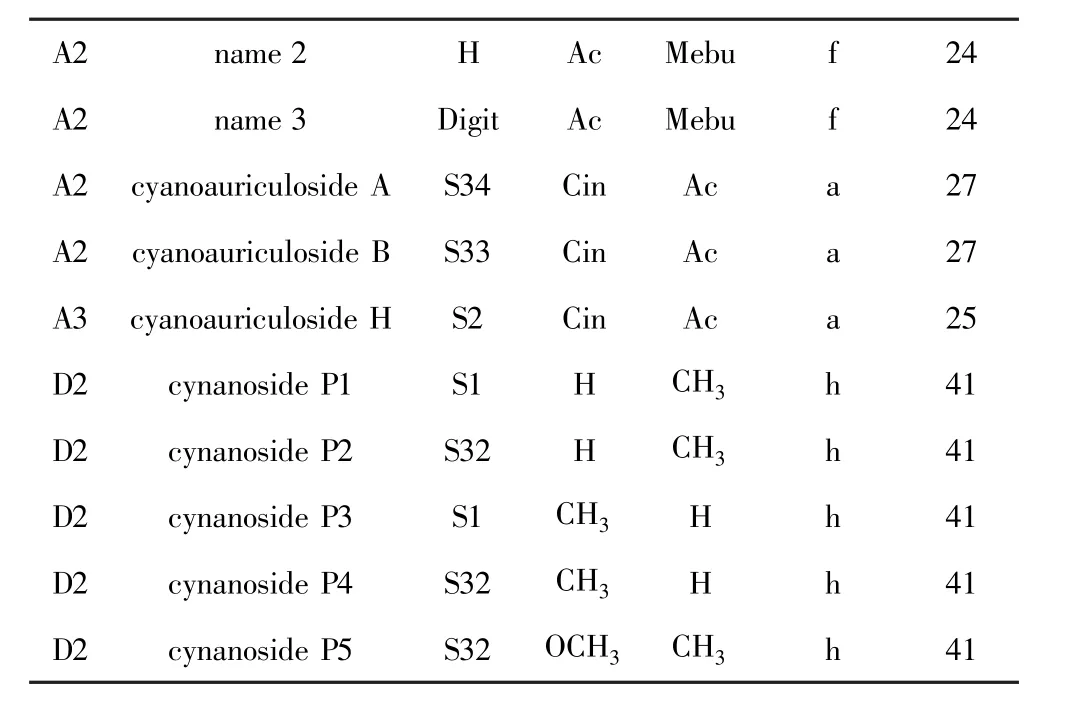
Name 2=12β-O-Ac-20-O-(2-Mebu)-sarcostin,name 3=12β -O-Ac-20-O-(2-Mebu)-sarcostin 3-O-Digit.
1.3 B1、B2、B3类型
B1、B2类型甾体皂苷详细信息请见表3,B3类型甾体皂苷见.Oh等[28]从中药徐长卿(Cynanchum paniculatum)根乙醇提取物中分离鉴定出B1类型甾体皂苷.Yan等[2]从白薇(Cynanchum atratum)根乙醇提取物中分离鉴定出cynanoside N.Chen等[29]从合掌消(Cynanchum amplexicaule)中也分离鉴定出B1类型甾体皂苷类化合物stauntogenin-α-L-Ole-Digit-Ole.
Chen等[29]从合掌消中也分离鉴定出B2类型甾体皂苷类化合物.
Oh等[28]从中药徐长卿根乙醇提取物中分离鉴定出B3类型(详见表4)甾体皂苷.Kim等[30]从徐长卿根中分离鉴定出甾体皂苷cynanside B.Zhang等[31]从柳叶白前(Cynanchum stauntonii)中分离鉴定出glaucogenin E.Yu等[32]从柳叶白前根乙醇提取物中分离鉴定出stauntosides L和M.Fu等[33]从柳叶白前根乙醇提取物中分离鉴定出glaucogenin-C衍生物. Liu等[34]从大理白前(Cynanchum forrestii)根乙醇提取物中分离鉴定出cynaforrosides K-N和Q.Chen等[35]从合掌消根中分离鉴定出amplexicosides A-F. Chen等[36]从合掌消根中分离鉴定出amplexicogenin C的衍生物.Wang等[37]从竹灵消(Cynanchum inamoenum)根甲醇提取物中分离鉴定出inamosides E -G.Yan等[2]从白薇根中分离鉴定出甾体皂苷cynanosides A-M.Wang等[38]从白薇根乙醇提取物分离鉴定出cynatratosides A和B.Yan等[2]从白薇根乙醇提取物中分离鉴定出cynanoside O.
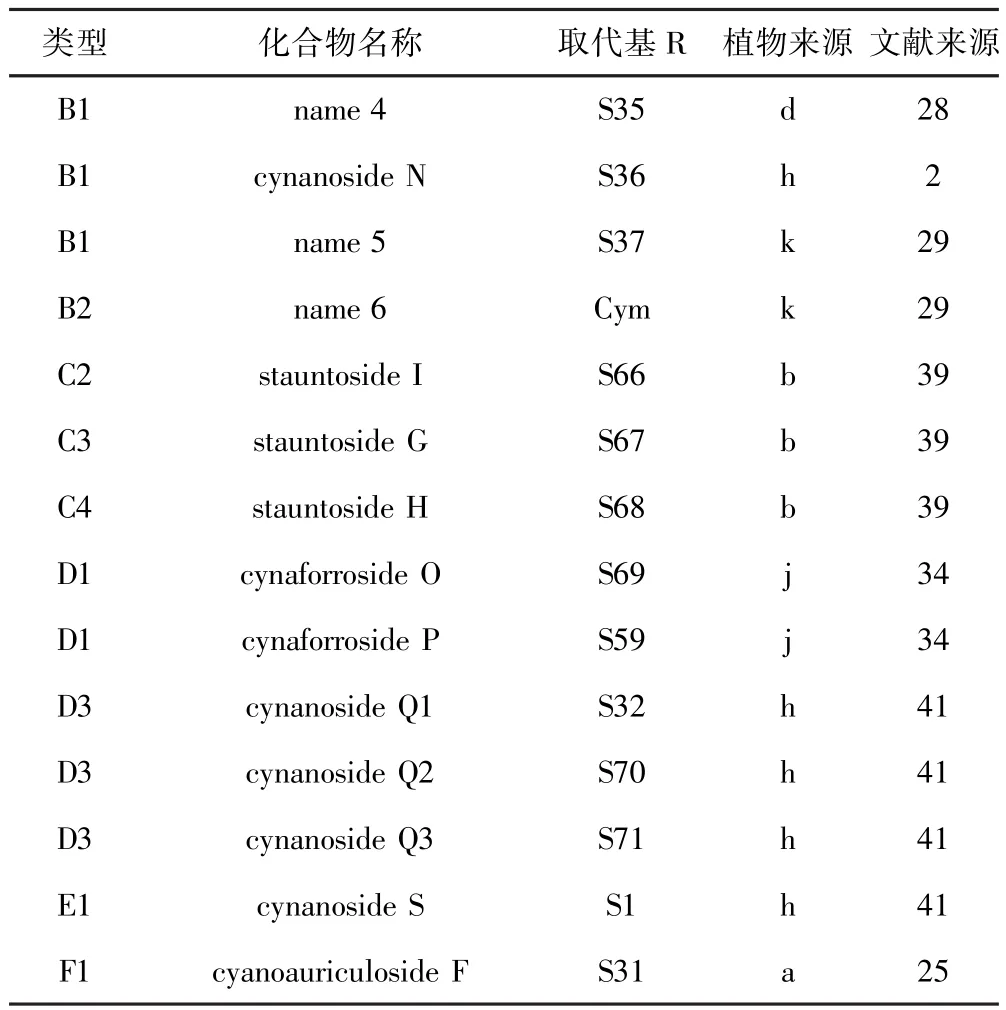
表3 鹅绒藤属植物中B1、B2、C2、C3、C4、D1、D3、E1、F1类型甾体皂苷Table 3 Types B1,B2,C2,C3,D1,D3,E1,F1steroidal saponins isolated from Cynanchum
1.4 C1、C2、C3、C4类型
Yu等[39]从柳叶白前根乙醇提取物中分离鉴定出C1类型(详见表1)甾体皂苷stauntosides C、J和K. Chen等[35]从合掌消根中分离鉴定出amplexicoside G. Yu等[32]从柳叶白前中分离鉴定出stauntoside N.Shibano等[40]从柳叶白前中分离鉴定出stauntosaponins A和B.
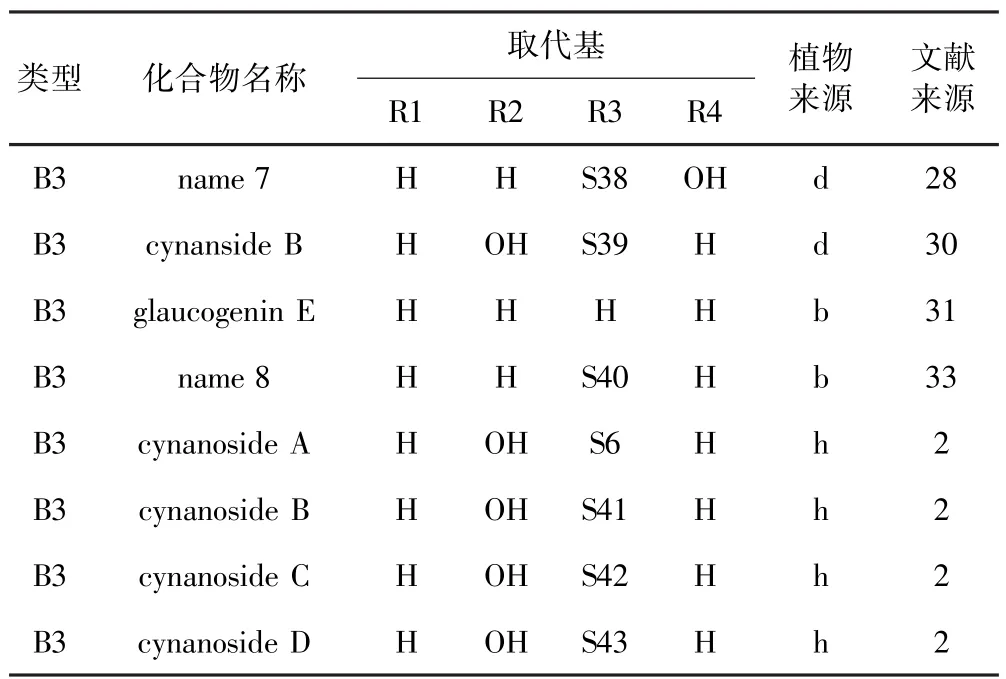
表4 鹅绒藤属植物中B3类型甾体皂苷Table 4 Type B3steroidal saponins isolated from Cynanchum
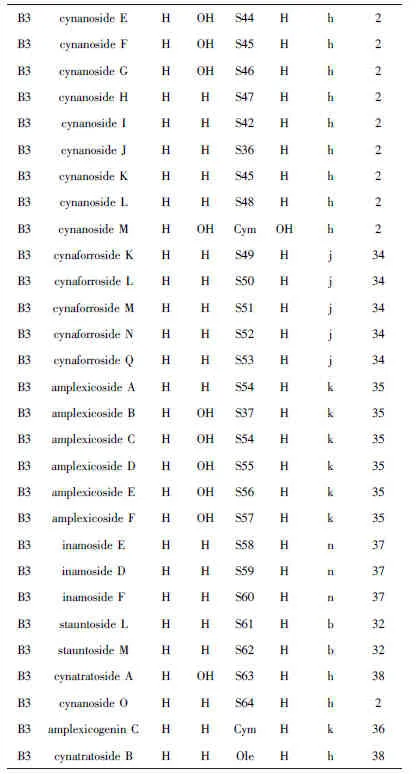
Name 7=(3β,8β,9α.16α,17α)-14,16β:15,20α:18.20β-triepoxy -16β:17α-dihydroxy-14-oxo-13,14:14,15-disecopregna-5,13 (18)-dian-3-ylα-Cym-β-Digit-β-Ole,name 8=glaucogenin-C 3-O-α-L-Cym-Digit-Cana.
Yu等[39]从柳叶白前根乙醇提取物中分离鉴定出C2类型甾体皂苷stauntoside I、C3类型甾体皂苷stauntoside G和C4类型甾体皂苷stauntoside H.C2、C3、C4类型详见表3.
1.5 D1、D2、D3、D4、E1、F1类型
Liu等[34]从大理白前根乙醇提取物中分离鉴定出D1类型(详见表3)甾体皂苷cynaforrosides O和P.
Bai等[41]从白薇根甲醇提取物中分离鉴定出D2类型(详见表2)甾体皂苷cynanosides P1-P5,D3类型(详见表3)甾体皂苷cynanosides Q1-Q3,D4类型(详见表1)cynanosides R1-R3,以及E1类型(详见表3)甾体皂苷cynanoside S.新发现的F1类型(详见表3)甾体皂苷较为少见,仅有1个化合物cyanoauriculoside F,该化合物为Lu等[25]从牛皮消根中分离鉴定出来.
2 药理活性
2.1 抗肿瘤作用
Peng等[42]在小鼠体内实验研究表明,A1类型甾体皂苷caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyranoside和caudatin对可移植H22肿瘤具有明显的抑制作用.Kim等[30]采用SRB法评价了B3类型甾体皂苷cynansides A和B对A549,SK-OV-3,SK-MEL-2和HCT-15肿瘤细胞的细胞毒活性.化合物cynansides A和B对SK-MEL-2的抑制活性IC50分别为26.55和17.36 μM.Zhang等[43]研究发现A1类型甾体皂苷auriculosides A和B对人盲肠未分化腺癌细胞、人前列腺癌细胞、人宫颈癌细胞和人肺腺癌细胞具有明显的细胞毒活性.Ye等[44]究了从白首乌中分离的甾体皂苷化合物A1类型甾体皂苷caudatin 3-O-Ole-Cym-Cym对人体肿瘤细胞(胃癌细胞、结肠癌细胞和肝癌细胞)体外细胞毒活性实验.结果显示,该化合物能有效促进胃癌细胞的凋亡,同时显著提高caspase-3在胃癌细胞中的表达,具有明显的抗肿瘤活性.
2.2 免疫作用
Li等[45]研究表明,D1类型甾体皂苷chekiangensoside A和B3类型甾体皂苷chekiangensoside B能有效缓解由刀豆素A和脂多糖引起的小鼠脾细胞增殖,且呈现出剂量依赖性.Ye等[46]通过体外实验研究表明,stemucronatosides D、E和G同样能剂量依赖性地抑制由刀豆素A和脂多糖引起的小鼠脾细胞增殖.而stemucronatoside F则在适当的浓度条件下明显增强上述细胞增殖作用.
2.3 神经保护作用
Zhao等[3]采用MTT法筛选由高半胱氨酸(HCA)导致的海马神经元细胞系HT22细胞凋亡,结果显示cynanotosides A(A1类型)和B(B3类型),以及cynotophylloside H(A2类型)对由HCA引起的细胞凋亡具有明显的保护作用,且呈现出剂量依赖效应.
2.4 抗病毒作用
Yan等[2]采用蛋白质印迹法研究了Cynanchum atratum中甾体皂苷类化合物对烟草花叶病毒的抗病毒作用,其中的B3类型甾体皂苷cynanosides A、G、M、cynatratoside-F和glaucogenin-A 3-O-Cym-α -L-Dig-Cym(该化合物没有在其结构鉴定的参考文献中找到)对烟草花叶病毒具有明显抗病毒作用. 2.5 杀虫作用
Fu等[4]研究了白薇中甾体皂苷B3类型甾体皂苷cynatratoside-C对鱼皮外寄生虫多毛鱼虱的毒杀活性.结果表明,cynatratoside-C在5 h内对鱼皮外寄生虫多毛鱼虱的未被包裹的分裂前体半数有效浓度(EC50)为0.083 mg/L;此外,浓度为0.125和0.06 mg/L的cynatratoside-C能够完全阻止被包裹的分裂前体复制和分裂;浓度增大至2 mg/L时可以在48 h内治愈被感染的草鱼.
2.6 其它作用
Liu等[14]从Cynanchum auriculatum中寻找食欲抑制剂的研究中,对所得甾体皂苷化合物wilfoside K1N(A1类型)进行大鼠体内实验,结果显示其良好的食欲抑制作用和减轻效果.Shibano等[40]研究发现C1类型甾体皂苷stauntosaponins A和B对钠/钾-ATPase具有中等强度的抑制作用.Yue等[47]运用活性导向的策略从柳叶白前中制备出具有抑制乙酰胆碱和碳酰胆碱引起的气管收缩活性的甾体皂苷化合物cynatratoside B(B3类型),其半数抑制浓度(EC50)分别为0.67和0.38 μg/mL,具有治疗咳嗽的效果.
鹅绒藤属植物资源丰富,很多具有较高的药用价值,且具有长期的临床用药基础.近年来对该属植物的主要成分甾体类化合物研究较为深入,单体化合物的药理活性得到了广泛研究.但是,具有开发价值的新颖结构化合物并不多见,且现代药理学机制研究还很欠缺.从现有文献来看,虽然甾体化合物结构多样,但本属植物中甾体皂苷生物活性与其结构还没有发现明显相关性.因此,根据化学成分和药理作用从鹅绒藤属植物中发掘新药源或拓展药用植物的新适应症,以及构效关系的深入研究具有很重要的现实意义.此外,在全面研究化学成分并加以现代药理学研究的同时应建立药材的质量标准,为以后品种甄别和质量控制提供科学依据.
[1]SALVADOR J A R,CARVALHO J F S,NEVES M A C,et al.Anticancer steroids:linking natural and semi-synthetic compounds[J].Natural Product Reports,2013,30(2):324-374.
[2]YAN Y,ZHANG J X,LIU K X,et al.Seco-pregnane steroidal glycosides from the roots of Cynanchum atratum and their anti-TMV activity [J].Fitoterapia,2014,97:50-63.
[3]ZHAO Z M,SUN Z H,CHEN M H,et al.Neuroprotective polyhydroxypregnane glycosides from Cynanchum otophyllum[J].Steroids,2013,78(10):1015-1020.
[4]FU Y W,ZHANG Q Z,XU D H,et al.Antiparasitic Effect of Cynatratoside-C from Cynanchum atratum against Ichthyophthirius multifiliis on Grass Carp[J].Journal of Agricultural and Food Chemistry,2014,62 (29):7183-7189.
[5]国家药典委员会.中国药典一部2010年版[M].北京:中国医药科技出版社,2010:268-269.
[6]王国强.全国中草药汇编(卷二)[M].北京:人民卫生出版社,2014:584.
[7]王国强.全国中草药汇编(卷二)[M].北京:人民卫生出版社,2014:168-169.
[8]王国强.全国中草药汇编(卷二)[M].北京:人民卫生出版社,2014:382.
[9]吴振洁,丁林生,赵守训.鹅绒藤属植物的化学成分和药理作用[M].国外医药(植物药分册),1991(4):147-154.
[10]刘卫卫,张朝晖,吴立云,等.鹅绒藤属植物化学成分与药理作用研究进展[J].中药材,2003,26(3):216-218.
[11]武毅,周洪雷.鹅绒藤属植物化学成分研究进展[J].中南药学,2006,4(5):371-375.
[12]白虹,王元书,刘爱芹.鹅绒藤属植物C_(21)甾体类化学成分研究进展[J].天然产物研究与开发,2007,19(5):897-904.
[13]刘冠军,王辉,张敏迪.鹅绒藤属植物药理作用研究进展[J].西北药学杂志,2009,24(5):430-432.
[14]LIU S,CHEN Z,WU J,et al.Appetite suppressing pregnane glycosides from the roots of Cynanchum auriculatum[J].Phytochemistry,2013,93:144-153.
[15]YANG Q X,GE Y C,HUANG X Y,et al.Cynanauriculoside C-E,three new antidepressant pregnane glycosides from Cynanchum auriculatum[J].Phytochemistry Letters,2011,4(2):170-175.
[16]ZHAO Y B,HE H P,LU C H,et al.C21 steroidal glycosides of seven sugar residues from Cynanchum otophyllum[J].Steroids,2006,71(11 -12):935-941.
[17]SHEN D Y,WEI J C,WAN J B,et al.Four new C21 steroidal glycosides from Cynanchum otophyllum Schneid[J].Phytochemistry Letters,2014,9:86-91.
[18]SHAN W G,LIU X,MA L F,et al.New polyhydroxypregnane glycosides from Cynanchum otophyllum[J].Journal of Chemical Research,2012,36(1):38-40.
[19]刘海港,黄雄,谢光辉,等.白首乌中一个新的C_(21)甾体苷类化合物[J].中国药科大学学报,2009,40(1):34-36.
[20]MA X X,JIANG F T,YANG Q X,et al.New pregnane glycosides from the roots of Cynanchum otophyllum[J].Steroids,2007,72(11-12): 778-786.
[21]MA X X,WANG D,ZHANG Y J,et al.Identification of new qingyangshengenin and caudatin glycosides from the roots of Cynanchum otophyllum[J].Steroids,2011,76(10-11):1003-1009.
[22]MA L F,SHAN W G,ZHAN Z J.Polyhydroxypregnane Glycosides from the Roots of Cynanchum otophyllum[J].Helvetica Chimica Acta,2011,94(12):2272-2282.
[23]SHI L M,LIU W H,YU Q,et al.Two new polyhydroxypregnane glycosides from the roots of Cynanchum otophyllum[J].Journal of Chemical Research,2013,37(7):404-405.
[24]SHI L M,LIU W H,YU Q,et al.Two new C21steroids from the roots of Cynanchum otophyllum[J].Journal of Chemical Research,2011,35 (2):126-128.
[25]LU Y,TENG H L,YANG G Z,et al.Three New Steroidal Glycosides from the Roots of Cynanchum auriculatum[J].Molecules,2011,16: 1901-1909.
[26]LU Y,XIONG H,TENG H LI,et al.Three New Steroidal Glycosides from the Roots of Cynanchum auriculatum[J].Helvetica Chimica Acta,2011,94(7):1296-1303.
[27]TENG H L,LU Y,LI J,et al.Two new steroidal glycosides from the root of Cynanchum auriculatum[J].Chinese Chemical Letters,2011,22 (1):77-80.
[28]OH J Y,KIM C S,LEE K R.C21 Steroidal Glycosides from the Root of Cynanchum paniculatum[J].Notes,2013,34(2):637.
[29]CHEN G,YI S,HUA H-M,et al.Two new steroidal glycosides from Cynanchum amplexicaule[J].Journal of Asian Natural Products Research,2012,14(6):559-563.
[30]KIM C S,OH J Y,CHOI S U,et al.Chemical constituents from the roots of Cynanchum paniculatum and their cytotoxic activity[J].Carbohydrate Research,2013,381(15):1-5.
[31]ZHANG M,WANG J S,LUO J,et al.Glaucogenin E,a new C21 steroid from Cynanchum stauntonii[J].Natural Product Research,2012,27 (2):176-180.
[32]YU J Q,ZHANG Z H,DENG A J,et al.Three New Steroidal Glycosides from the Roots of Cynanchum stauntonii[J].BioMed Research International 2013,2013:1-7.
[33]FU M H,WANG Z J,YANG H J,et al.A new C21-steroidal glycoside from Cynanchum stauntonii[J].Chinese Chemical Letters,2007,18(4):415-417.
[34]LIU Y,QU J,YU S S,et al.Seven new steroidal glycosides from the roots of Cynanchum forrestii[J].Steroids,2007,72(4):313-322.
[35]CHEN H,XU N,ZHOU Y,et al.Steroidal glycosides from the roots of Cynanchum amplexicaule Sieb.et Zucc[J].Steroids,2008,73(6):629 -636.
[36]CHEN G,CHEN H,LI W,et al.Steroidal glycosides from Cynanchum amplexicaule[J].Chemistry of Natural Compounds,2013,48(6):1021 -1023.
[37]WANG L Q,WANG J H,SHEN Y M,et al.Three new C21 steroidal glycosides from the roots of Cynanchum inamoenum[J].Chinese Chemical Letters,2007,18(10):1235-1238.
[38]WANG S,SHAN W,MA L,et al.Two new pregnane glycosides from the roots of Cynanchum atratum[J].Journal of Chemical Research,2013,37(12):727-729.
[39]YU J Q,DENG A J,QIN H L.Nine new steroidal glycosides from the roots of Cynanchum stauntonii[J].Steroids,2013,78(1):79-90.
[40]SHIBANO M,MISAKA A,SUGIYAMA K,et al.Two secopregnanetype steroidal glycosides from Cynanchum stauntonii(Decne.)Schltr.ex Levl[J].Phytochemistry Letters,2012,5(2):304-308.
[41]BAI H,LI W,ASADA Y,et al.Twelve pregnane glycosides from Cynanchum atratum[J].Steroids,2009,74(2):198-207.
[42]PENG Y R,LI Y B,LIU X D,et al.Antitumor activity of C-21 steroidal glycosides from Cynanchum auriculatum Royle ex Wight[J].Phytomedicine,2008,15(11):1016-1020.
[43]ZHANG R S,YE Y P,SHEN Y M,et al.Two New Cytotoxic C-21 Steroidal Glycosides from the Root of Cynanchum auriculatum[J].Tetrahedron,2000,56(24):3875-3879.
[44]YE L F,WANG Y Q,YANG B,et al.Cytotoxic and apoptosis-inducing properties of a C21-steroidal glycoside isolated from the roots of Cynanchum auriculatum[J].Oncology letters,2013,5(4):1407-1411.
[45]LI X,SUN H,YE Y,et al.C-21 steroidal glycosides from the roots of Cynanchum chekiangense and their immunosuppressive activities[J]. Steroids,2006,71(1):61-66.
[46]YE Y,SUN H,LI X,et al.Four new C-21 steroidal glycosides from the roots of Stephanotis mucronata and their immunological activities [J].Steroids,2005,70(12):791-797.
[47]YUE G G L,CHAN K M,TO M H,et al.Potent Airway Smooth Muscle Relaxant Effect of Cynatratoside B,a Steroidal Glycoside Isolated from Cynanchum stauntonii[J].Journal of Natural Products,2014,77 (4):1074-1077.
(责任编辑:李建忠,付强,张阳,罗敏;英文编辑:周序林,郑玉才)
Progress in steroid constituents of cynanchum and pharmacological effects of the steroids
HE Da-hai,YANG Shuai,LUO Hua-xiu,QIN Xi,MA Ya-ru,ZHANG Xiu-ying
(School of Chemistry&Environmental Protection Engineering,Southwest University for Nationalities,Chengdu 610041,P.R.C.)
Cynanchum species are widely distributed in China,and most of them have medicinal value.Steroidal saponins,the main component of this genus,demonstrate a rich diversity.The chemical diversity includes variable nucleus(steroid aglycone),substituents,length of sugar chain,monosaccharide type,absolute configuration(D or L)of a monosaccharide,type(α or β)of a glycoside bond.Especially,it is rare occurrence of a natural compound that possesses a sugar with both D-and L-monosaccharide,and it is also uncommon for glycosides bearing a sugar chain which is assembled by seven units of monosaccharide.Based on the nucleus and its substituent,a total of 119 steroidal saponins isolated from this genus between 2007 and 2014,were grouped into five kinds of 17 subspecies.In addition,bioactivity of the saponins including antitumor,antiviral,neuroprotective and insecticidal effects are reviewed.
Cynanchum;steroidal saponin;sugar chain;antitumor;antiviral;neuroprotective;insecticidal
R284;R285
A
2095-4271(2015)04-0423-09
10.11920/xnmdzk.2015.04.006
2015-05-25
何达海(1980-),男,汉族,四川金堂人,讲师,博士,研究方向:天然药物化学以及有机质谱分析,Email:dahaihe2007@yeah.net
西南民族大学2015年省级大学生创新创业训练计划项目(NO.S201510656126)
