基于三羧酸构筑的两个金属-有机骨架(MOFs):合成,晶体结构和性质
2015-12-15谢梦淋吴梦芹温一航
赵 灿 张 优 谢梦淋 吴梦芹 温一航
(浙江师范大学物理化学研究所,浙江省固体表面反应化学重点实验室,金华321004)
基于三羧酸构筑的两个金属-有机骨架(MOFs):合成,晶体结构和性质
赵 灿 张 优 谢梦淋 吴梦芹 温一航*
(浙江师范大学物理化学研究所,浙江省固体表面反应化学重点实验室,金华321004)
以1,3-二(4′-羧基苯氧基)苯甲酸(H3L)为配体与金属盐反应,在水热条件下成功合成了2个金属-有机骨架(MOFs),分别为[Cd2(CH3COO)(L)(H2O)2]n(1)和[Na(H2L)]n(2)。由于H3L配体配位模式的不同,配合物表现出不同的网络结构。单晶结构分析表明,配合物1属于三斜晶系,P1空间群,配合物2属于单斜晶系,C2/c空间群。配合物1中Cd2+离子的次级构筑单元经由L3-连接形成三维网络结构。配合物2是一个5-连接的拓扑网络结构,其拓扑符号为(46·64)。此外,还对配合物1的荧光性能进行了分析。
金属-有机骨架;1,3-二(4′-羧基苯氧基)苯甲酸;水热合成;拓扑;荧光性能
DO I:10.11862/CJIC.2015.095
0 Introduction
The rational designed synthesis of metal-organic frameworks(MOFs)has become an exciting and expanding approach to novel materials.Such interest is attracted not merely by the fascinating structural architectures of MOFs,but also by their extensive potential applications in the fields of magnetism[1], sensors[2],adsorption[3],catalysis[4],ion-exchange[5]and optical properties[6].In the past decades,many MOFs with excellent properties have been prepared by hydrothermal synthesis,which has been proven to be an available approach to obtain MOFs,though the mechanism about construction and influence ofstructure is ambiguous and unpredictable[7].The resulting structures will be influenced by many factors,such as the coordination geometry of metal center,the structural features of ligand,the solvent system,and so on[8].Thus,it is very important to select suitable organic ligands to modify the building blocks and control the assembled motifs for required products[9]. As we know,compared with the rigid ligands,flexible ligands have diverse coordination modes and can adopt various conformations to meet the coordination requirement of metal ions,which can help construct MOFs with fascinating topology and properties[10]. Among various organic ligands,the aromatic polycarboxylic acid have been extensively applied as linkers because they usually adopt binding modes as diverse as terminal monodentate,chelating to one metal center,bridging bidentate in a syn-syn,synanti,and anti-ant i configuration to two metal centers, and bridging tridentate to two metal centers as well as afford H-bonding and aromatic stacking[11].In addition, the spin communication between paramagnetic metal ions can be enhanced via carboxylate bridges[12].In our work,we choose a flexible ligand,1,3-bis(4′-carboxylphenoxy)benzoic acid(H3L)with the following considerations:First,the H3L ligand contains three carboxylate groups,which makes the ligand have more coordination modes and can construct highdimensional structures and new topology;Second,the conjugated system of H3L ligand may enhance the luminescence properties of metal-organic compounds[13]. Recently,our group has reported serious metalorganic compounds with new topology based on flexible carboxylate ligand[14].And now in this paper, we synthesize and characterize two new metal-organic compounds based on H3L ligand,that is,[Cd2(CH3COO) (L)(H2O)2]n(1)and[Na(H2L)]n(2),in which both of them display 3D structural networks.Furthermore,the luminescence property of compound 1 has also been investigated.
1 Experimental
1.1 M aterials and instrumentations
All of the chemical reagents and solvents are commercially available and used without further purification.The fluorescence spectra were obtained at room temperature on a LS50B luminescence spectrometer(Perkin-Elmer,Inc.,USA).Elemental analyses were carried out on a Perkin-Elmer 2400 Series II analyzer.Single crystal X-ray diffraction data of two complexes were collected on a Bruker APEX-II CCD diffractometer.
1.2 Preparation of com pounds
1.2.1 Synthesis of[Cd2(CH3COO)(L)(H2O)2]n(1)
A mixture of Cd(CH3COO)2·2H2O(0.266 g,1.0 mmol),H3L(0.196 g,0.5 mmol),NaOH(0.04 g,1.0 mmol)were dissolved in 18 mL H2O,and it was sealed in a 25 mL stainless steel reactor and heated to 423 K for 3 days.Then the mixture was cooled to temperature at the rate of 5℃·h-1,and the colourless block crystals were obtained(Yield:55%based on Cd).Anal.Calcd.(%)for C23H18Cd2O12(711.17):C 38.81, H 2.53;Found(%):C 39.05,H 2.68.
1.2.2 Synthesis of[Na(H2L)]n(2)
A mixture of H3L(0.197 g,0.5 mmol),CoSO4· 7H2O(0.5 mmol,0.141 g)and NaOH(0.06 g,1.5 mmol)were dissolved in 18 mL H2O.The mixture was sealed in a 25 mL stainless steel reactor and heated to 433 K for 3 days,and was cooled to room temperature at the rate of 5℃·h-1.The colorless block crystals were obtained(Yield:45%based on Na).Anal.Calcd. (%)for C21H13NaO8(416.30):C 60.58,H 3.15;Found (%):C 62.03,H 2.94.
1.3 X-ray crystallography
The single crystal X-ray diffraction data of complexes 1 and 2 were collected on a Bruker SMART APEX-II CCD diffractometer with Mo Kα radiation (λ=0.071 073 nm).All of the structures were solved by direct method and refined by full-matrix leastsquares on F2using SHELX-97 program package[15]. Non-hydrogen atoms were refined anisotropically.The hydrogen atoms of ligands were generated theoretically, then refined isotropically by riding on their parent carbon atoms.Crystal data for complexes 1 and 2 are listed in Table 1.The selected bond distances and bond angles are listed in Table 2.
CCDC:1009539,1;1009540,2.

Table1 C rystal data and structure refinement for com p lexes 1 and 2
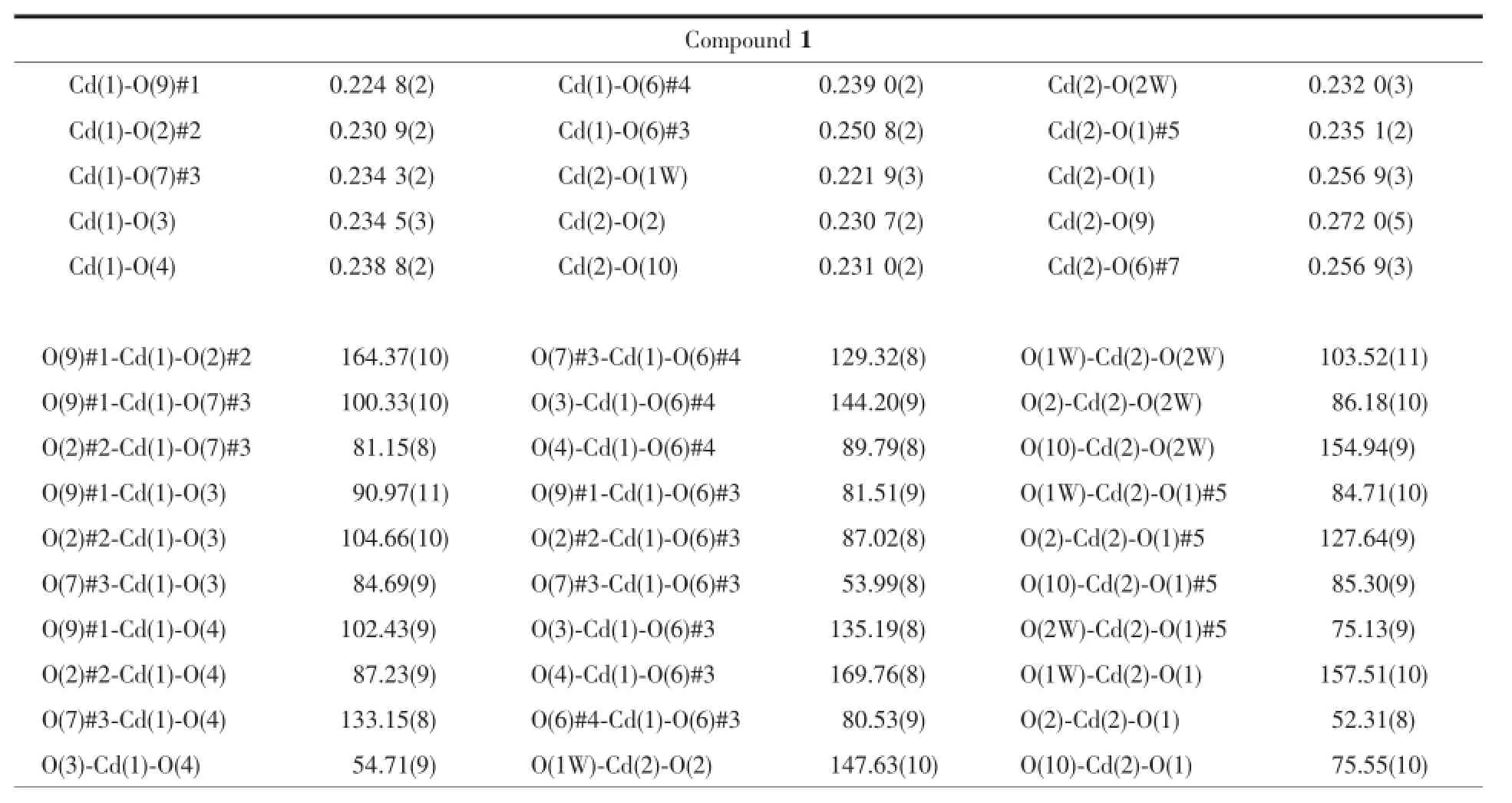
Table2 Selected bond lengths(nm)and angles(°)for tw o com pounds
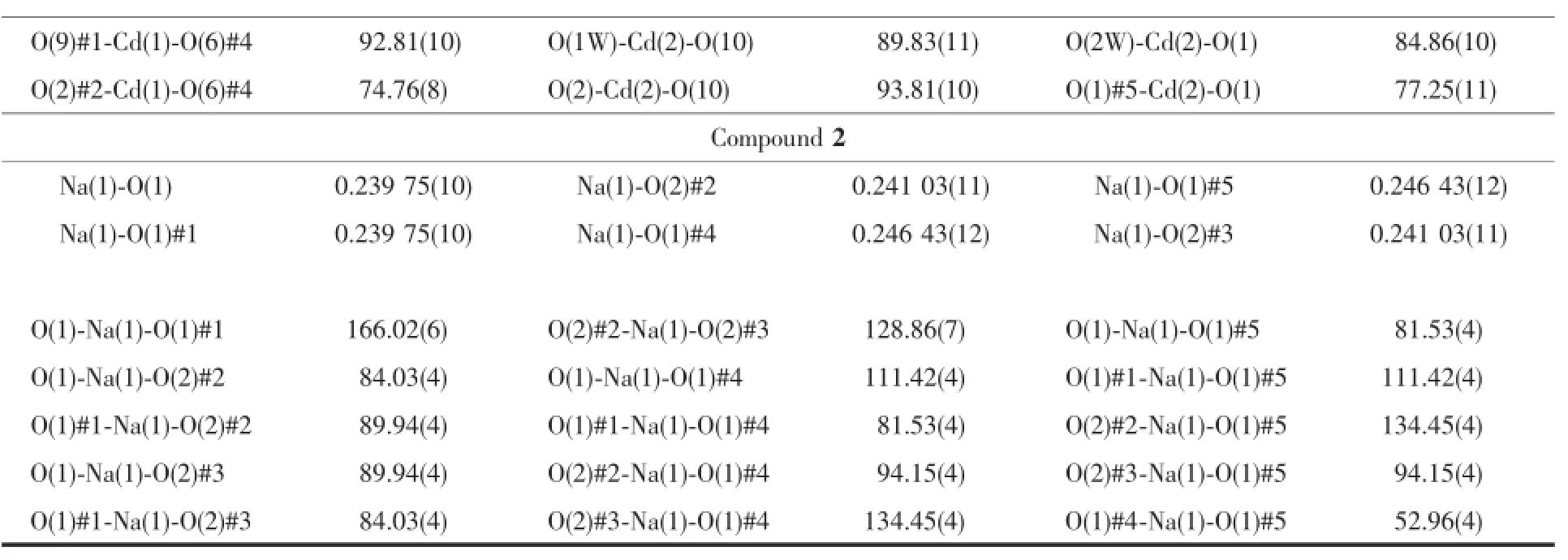
Continued Table 2
2 Results and discussion
2.1 Crystal structure of the compounds
2.1.1 Crystal structure of[Cd2(CH3COO)(L)(H2O)2]n(1) X-ray crystallography shows that compound 1crystallizes in triclinic system with P1 space group. As shown in Fig.1a,the asymmetric unit is composed of two crystallographically independent Cd2+cations, namely Cd(1)and Cd(2),one L3-anion,one Ac-anion and two coordinated water molecules.The Cd(1)ion is in a capped octahedral geometry,coordinated by one O atom from Ac-anion with the Cd(1)-O distances of 0.230 9(2)nm,and six O atoms from four L3-ligands with the Cd(1)-O distances of 0.224 8(2)~0.250 8(2) nm,including one O atom(O6#4)as a cap.The Cd(2) ion is in a dodecahedron geometry,coordinated by three O atoms from two L3-ligands with the Cd(2)-O distances of 0.231 0(2)~0.272 0(5)nm,three O atoms from two Ac-anions with the Cd(2)-O distances of 0.230 7(2)~0.256 9(3)nm,and two O atoms from two coordinated water molecules,with the Cd(2)-O distances of 0.221 9(3)~0.232 0(3)nm.The H3L ligands and acetic groups are deprotonated forming L3-anions and Ac-anions,and the three carboxylate groups of the L3-ligand adopt the different coordination modes (type A in Scheme 1).One adopts bidentated chelating mode;one is tridentated chelating-bridge two metal ions;the last one is quadridentated chelating-bridge three metal ions.Thus,the L3-ligand can be looked as μ6-η1∶η1∶η1∶η1∶η2∶η3-bridged ligand.The Ac-anion adopts quadridentated chelating-bridge three metal ions(type C in Scheme 1),so it could be regarded as μ3-η2∶η2-bridged coordinated group.In this structure, every four neighboring Cd2+ions are connected by two Ac-anions to form secondary building units(SBUs) (Fig.1b).These SBUs are further bridged via L3-ligands into 3D network(Fig.1c).
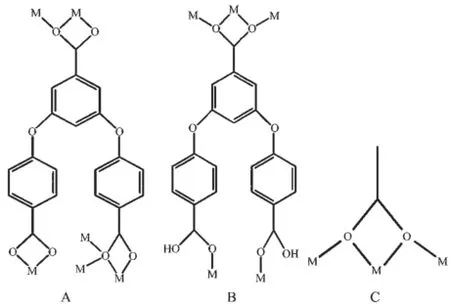
Scheme 1 Coordination modes of the ligand L3-and Acgroup in 1
2.1.2 Crystal structure of[Na(H2L)]n(2)
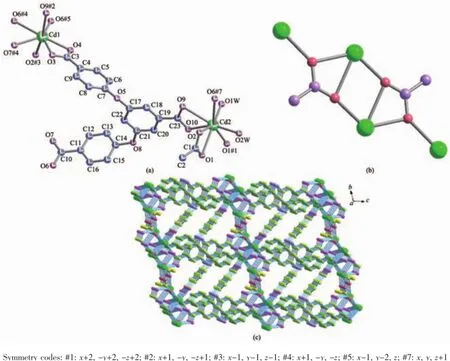
Fig.1(a)Coordination environment of the Cd2+ions in 1 with 50%thermal ellipsoids;(b)A view of the secondary building unit in 1;(c)A view of the 3D structural framework in 1

Fig.2(a)Coordination environment of the Na+ion in 2 with 50%thermal ellipsoids;(b)A view of the 1D zig-zag chain in 2; (c)(5,5)-connected topology of 2
X-ray crystallography displays that compound 2 crystallizes in monoclinic system with C2/c space group.As shown in Fig.2a,the molecular structure of 2 is composed of one crystallographically independent Na+cation and a H2L-ligand.The Na1 ion is sixcoordinated by two O atoms from one chelating carboxylate group of one L3-ligand and four O atoms from four monodentate bridging L3-ligands,whichgenerate a distorted octahedral geometry with the Na1…O distances of 0.239 63(12)~0.246 38(13)nm.The H3L ligands are part deprotonated forming H2L-anions,and the three carboxylate groups of the H2L-ligand adopt the different coordination modes(type B in Scheme 1).Two adopt monodentated bridging one metal ion,and another one is quadridentated chelating-bridge three metal ions.Thus,the L3-ligand can be looked as μ5-η1∶η1∶η2∶η2-bridged ligand.
In the structure,every two Na+ions are connected by the μ2-O atoms from the H2L-ligands to generate a 1D zig-zag chain(Fig.2b).These chains are bridged into 3D networks by H2L-ligands.If the H2L-ligand is regarded as a linker,the Na+centres could be clarified as five-connected nodes.Thus the topology of the structure could be simplified as a(5,5) network with(46·64)topology.
2.2 Fluorescent property
Coordination compounds with d10metal and conjugated organic ligands have latterly been focused due to their excellent photoluminescent properties with potential applications[16].Hence,the emission spectra of compound 1,together with the free ligand have been investigated in the solid state at room temperature(Fig.3).The free H3L ligand exhibits strong photoluminescence with an emission maximum peaks at 352 nm(λex=290 nm).While,the emission spectra shows maximal emission bands at 344 nm (λex=288 nm)for 1.Compared with the free ligand,a slightly blue-shifted of emission by ca.8 nm appears in the spectrum of compound 1,which may be attributed to the coordination environment around the metal ions,since the photoluminescence behaviour is intimately associated with the metal ions and the coordinated ligands around them[16b].
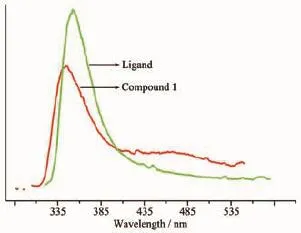
Fig.3 Emission spectra of compound 1 and the free H3L ligand in the solid state at room temperature
Because metal ions with d0and d10configurations are especially difficult to oxidize or reduce,the emission of compound 1 cannot be assigned as metalto-ligand charge transfer(MLCT)or ligand-to-metal charge transfer(LMCT)in nature.It can probably be based on the photoluminescence of intra-ligand and ligand-to-ligand charge transition(LLCT),and appears to be substantially influenced by the conformational and accumulational changes of the ligand[17].Thus, comparing to that of the free ligand,the fluorescence quenching of complex 1 can be attributed to the deprotonated effect of H3L ligand,the pH value,the solvent,and the coordination interaction of the organic ligand to Cd2+ion.
3 Conclusions
Two coordination compounds have been successfully synthesized with a flexible carboxylate connecter 1,3-bis(4′-carboxylphenoxy)benzoic acid (H3L).The ligand L adopts flexible coordination modes to meet various requirements of different metals, building new coordination compounds with intriguing constructions.In terms of the coordination modes of the ligand in 1 all three of the carboxylate groups are deprotonated,participating in the coordination,while in 2 all three of the carboxylate groups also take part in the coordination(Only one of the carboxylates is deprotonated).Both of them display interesting 3D networks.In addition,the luminescence property of 1 has also been investigated.It is believed that the prime research of this work will supply further access to the rational design and synthesis of the novel functional coordination compounds based on flexible carboxylate ligands.
[1](a)Akhtar M N,Zheng Y Z,Lan Y H,et al.Inorg.Chem., 2009,48(8):3502-3504(b)Nippe M,Wang J F,Bill E,et al.J.Am.Chem.Soc., 2010,132(40):14261-14272 (c)Zheng Y Z,Evangelisti M,Winpenny R E P.Chem.Sci., 2011,2:99-102
[2](a)Zhao B,Chen X Y,Chen Z,et al.Chem.Commun.,2009: 3113-3115 (b)Yan C,Li K,Wei S C,et al.J.Mater.Chem.,2012,22: 9846-9852 (c)Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012,112 (2):1126-1162
[3](a)Li C J,Lin Z J,Peng M X,et al.Chem.Commun.,2008: 6348-6350 (b)Mahata P,Ramya K V,Natarajan S.Inorg.Chem.,2009, 48(11):4942-4951
[4](a)Shibasaki M,Yoshikawa N.Chem.Rev.,2002,102(6):2187 -2210 (b)Handa S,Gnanadesikan V,Matsunaga S,et al.J.Am. Chem.Soc.,2010,132(13):4925-4934
[5](a)Wang P,Ma J P,Dong Y B,et al.J.Am.Chem.Soc., 2007,129(35):10620-10621 (b)Li Z Y,Dai J W,Yue S T,et al.CrystEngComm,2010, 12:2014-2017
[6](a)Feng X,Zhao J S,Liu B,et al.Cryst.Growth Des.,2010, 10:1399-1408 (b)Pan J,Jiang F L,Yuan D Q,et al.CrystEngComm,2013, 15:5673-5680 (c)Zhou L,Zhang J,Li Y Z,et al.CrystEngComm,2013,15: 8989-8997 (d)HUANG Yan-Ping(黄燕萍),ZHANG Xiao(张潇),LIU Lei (刘蕾),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(5):1001-1008 (e)Zhang S Q,Jiang F L,Wu M Y,et al.Cryst.Growth Des., 2012,12:1452-1463 (f)YU Mei-Hui(于美慧),HU Ming(胡明),FENG Zhan-Yu(冯占宇),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(6):1261-1266 (g)He X,Lu X P,Tian Y Y,et al.CrystEngComm,2013,15: 9437-9443 (h)Lin Y W,Jian B R,Huang S C,et al.Inorg.Chem.,2010, 49(5):2316-2324
[7]Ma L F,Wang L Y,Hu J L,et al.Cryst.Growth Des.,2009, 9(12):5334-5342
[8](a)Hosseini M W.Acc.Chem.Res.,2005,38(4):313-323 (b)Steel P J.Acc.Chem.Res.,2005,38(4):243-250 (c)Liu J Q,Wang Y Y,Ma L F,et al.Inorg.Chem.Commun., 2007,10(9):979-982
[9](a)Hagrman P J,Hagrman D,Zubieta J.Angew.Chem.Int. Ed.,1999,38(18):2638-2684 (b)Kanoo P,Gurunatha K L,Maji T K.Cryst.Growth Des., 2009,9(9):4147-4156 (c)Wang W H,Xi P H,Su X Y,et al.Cryst.Growth Des., 2007,7(4):741-746
[10](a)Yu Q,Zeng Y F,Zhao J P,et al.Cryst.Growth Des., 2010,10(4):1878-1884 (b)Dai F N,Dou J M,He H Y,et al.Inorg.Chem.,2010,49 (9):4117-4124 (c)Won E,Jin Y,JungDY.Inorg.Chem.,2002,41(3):501-506 (d)Cui J H,Li Y Z,Guo Z J,et al.Cryst.Growth Des., 2012,12:3610-3618 (e)Cui J H,Yang Q X,Li Y Z,et al.Cryst.Growth Des., 2013,13:1694-1702 (f)Wei X H,Yang L Y,Liao S Y,et al.Dalton Trans.,2014, 43:5793-5800 (g)Patra R J,Titi H M,Goldberg I.New J.Chem.,2013,37: 1494-1500
[11](a)Habib H A,Sanchiz J,Janiak C.Dalton Trans.,2008: 4877-4884 (b)Lu W G,Gu J Z,Jiang L,et al.Cryst.Growth Des., 2008,8(1):192-199 (c)O′Keeffe M,Yaghi O M.Chem.Rev.,2012,112(2):675-702
[12]Kurmoo M.Chem.Soc.Rev.,2009,38:1353-1379
[13](a)Jia L N,Hou L,Wei L,et al.Cryst.Growth Des.,2013, 13(4):1570-1576 (b)Chen K,Sun Z G,Zhu Y Y,et al.Cryst.Growth Des., 2011,11(10):4623-4631 (c)Kim P S G,Hu Y F,Brandys M C,et al.Inorg.Chem., 2007,46(3):949-957
[14](a)Ji J,Zhang Y,Yang Y F,et al.Eur.J.Inorg.Chem., 2013:4336-4344 (b)Zhang Y,Ji J,Fu J D,et al.Inorg.Chem.Commun., 2013,35:181-185 (c)Ji J,Zhang Y,Fu J D,et al.J.Inorg.Organomet.Polym., 2013,23:1347-1353 (d)Zhang Y,Ji J,Zhang C Y,et al.Inorg.Chem.Commun., 2014,46:122-126
[15](a)Sheldrick G M.SHELXS-97 and SHELXL-97,Program for the Solution and the Refinement of Crystal Structure, University of Göttingen,Germany,1997. (b)APEX 2 Software Suite(SMART,SAINT,SADABS,etc.), Bruker AXS Inc.,Madison,Wisconsin,USA
[16](a)Zhang X P,Zhou J M,Shi W,et al.CrystEngComm,2013, 15:9738-9744 (b)Roy B,Mukherjee S,Mukherjee P S.CrystEngComm, 2013,15:9596-9602 (c)Huang X F,Ma J X,Liu W S.Inorg.Chem.,2014,53(12):5922-5930 (d)Xu Y Y,Wu X X,Wang Y Y,et al.RSC Adv.,2014,4: 25172-25182 (e)Wang X J,Liu Y H,Xu C Y,et al.Cryst.Growth Des., 2012,12:2435-2444
(f)LI Ke(李可),LI Shu-Jing(李书静),ZHANG Xiao-Peng(张小朋),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(7):1647-1652 (g)Zhang R B,Li Z J,Cheng J K,et al.Cryst.Growth Des., 2008,8:2562-2573
[17](a)Guo X M,Guo H D,Zou H Y,et al.CrystEngComm, 2013,15:9112-9120 (b)Li H M,Zhou J M,Shi W,et al.CrystEngComm,2014, 16:834-841 (c)Cui P,Chen Z,Gao D L,et al.Cryst.Growth Des.,2010, 10:4370-4378 (d)Duan L,Wu Z H,Ma J P,et al.Inorg.Chem.,2010,49: 11164-11173
Two Metal-O rganic Frameworks Based on Tricarboxylic Acid: Syntheses,Structures and Fluorescent Properties
ZHAO Can ZHANG You XIE Meng-Lin WU Meng-Qin WEN Yi-Hang*
(Zhejiang Key Laboratory for Reactive Chemistry on Solid Surfaces, Institute of Physical Chem istry,Zhejiang Normal University,Jinhua,Zhejiang 321004,China)
Two metal-organic frameworks(MOFs),formulated as[Cd2(CH3COO)(L)(H2O)2]n(1)and[Na(H2L)]n(2) (H3L=1,3-bis(4′-carboxylphenoxy)benzoic acid)were successfully prepared by hydrothermal reaction of H3L ligand with metal salts.Because of the various coordination modes of the H3L ligand,these compounds display structural diversity.Compound 1 crystallizes in triclinic system with P1 space group and 2 in monoclinic system with C2/c space group.The single crystal structures show that 1 possesses a 3D structural framework,in which the secondary building units(SBUs)are bridged by the L3-ligands,2 features a 3D 5-connected network with(46·64) topology.Moreover,the luminescent property of compound 1 has been investigated.CCDC:1009539,1;1009540,2.
metal-organic frameworks;1,3-bis(4′-carboxylphenoxy)benzoic acid;hydrothermal reaction;topology;luminescent property
O614.24+2;O614.112
A
1001-4861(2015)04-0781-08
2014-10-13。收修改稿日期:2015-01-01。
浙江省自然科学基金(No.LY12B01002)和浙江师范大学“浙江省省属高校化学重中之重学科”和“先进催化材料教育部重点实验室”开放课题基金项目(No.ZJHX201406)资助项目。
*通讯联系人。E-mail:wyh@zjnu.edu.cn;会员登记号:S060017686M。
