Treatment of major depressive disorders with generic duloxetine and paroxetine: a multi-centered, double-blind,double-dummy, randomized controlled clinical trial
2015-12-08ZhiyangWANGXiufengXUQingrongTANKeqingLICuiMAShipingXIEChenggeGAOGangWANGHuafangLI
Zhiyang WANG, Xiufeng XU, Qingrong TAN, Keqing LI, Cui MA, Shiping XIE, Chengge GAO,Gang WANG, Huafang LI*
•Original research article•
Treatment of major depressive disorders with generic duloxetine and paroxetine: a multi-centered, double-blind,double-dummy, randomized controlled clinical trial
Zhiyang WANG1,2, Xiufeng XU3, Qingrong TAN4, Keqing LI5, Cui MA6, Shiping XIE7, Chengge GAO8,Gang WANG9, Huafang LI1,*
duloxetine; paroxetine; efficacy; safety; major depressive disorder; randomized controlled trial;China
1. Introduction
Depression is a highly prevalent disorder that is a common cause of suicide.[1,2]According to data from the 2010 Global Burden of Disease (GBD) study,[3]mental and substance use disorders were the leading cause of years lived with disability (YLDs) and depressive disorders were the most important type of mental disorder, accounting for 40.5% (confidence interval,31.7-49.2%) of the disability-adjusted life years (DALYs)caused by mental and substance use disorders. The World Health Osrganization predicts that depressive disorders will soon account for 15% of worldwide disease burden, making it the second most important cause of ill health after cardiac diseases.[4]Depressive disorders are important in both high-income countries and in low- and middle-income countries; in China the prevalence of MDD among adults 18 and older is 6%.[5]
The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study[6]reported that MDD is often chronic, severe, and associated with substantial general medical and psychiatric comorbidity; moreover, the remission rate is low – only 33% – when treated with a single antidepressant medication. Pharmacotherapy can, nevertheless, relieve the symptoms of depression and improve the quality of life of depressed patients.Timely and appropriate therapy may increase the clinical cure rate and reduce the disease burden.
Duloxetine is a new anti-depressant drug that selectively inhibits the uptake of serotonin (5-HT) and norepinephrine (NE) by neurons from the synaptic gap, thereby increasing the synaptic pool of available neurotransmitters and, thus, relieving depressive symptoms.[7]In vivoandin vitrostudies have shown that duloxetine is an effective and balanced inhibitor of 5-HT and NE uptake that has little effect on other neurotransmitter receptors (such as M, a1, a2, dopamine D2, and histamine H1 and H2 receptors).[8]Clinically it has been shown to be effective in both the short-term and long-term treatment of adults with major depressive disorder (MDD),[9]comparable in efficacy to selective 5-HT reuptake inhibitors such as fl uoxetine, paroxetine,citalopram, and sertraline.[10,11]Duloxetine has also been reported to be effective in the treatment of generalized anxiety disorder[12]and fi bromyalgia.[13]
This paper reports on a pre-registration trial of generic duloxetine (manufactured by Jiangsu Nhwa Pharmaceutical Co., Ltd. in China) which was approved by the China Food and Drug Administration (approval number: 2006L01603). In this trial we use paroxetine as the active comparator drug because it is a widely used first-line treatment for MDD with proven efficacy and safety that is often used as a positive control drug in Phase 2 and Phase 3 clinical trials.
2. Methods
2.1 Sample
The enrollment process is shown in Figure 1. Psychiatric outpatients with MDD treated at eight outpatient psychiatric centers in China from September 2009 to September 2010 were enrolled in the study. The study sites included the Shanghai Mental Health Center (which coordinated the study), the Affiliated Beijing Anding Hospital of the Capital Medical University, the Nanjing Brain Hospital, Guangzhou Brain Hospital, the Affiliated Xijing Hospital of the Fourth Military Medical University,the First Affiliated Hospital of Xi’an Jiaotong University,the First Affiliated Hospital of Kunming Medical College,and the Mental Health Center of Hebei Province. In total 299 patients met the inclusion criteria for the study:current MDD as diagnosed using the Statistical Manual of Mental Disorders IV (DSM-IV) criteria;[14]aged 18–65 years; 17-item Hamilton Depression rating scale (HAMD-17)[15]score of 20 or above with the depressed mood item scored 2 or more (range 0-4); the severity item of the Clinical Global Impression scale (CGI-S)[15]scored 4 or above (range 1-7); and provided written informed consent. The number of enrollees from the 8 centers ranged from 19 to 55.
Patients who met any of the following exclusion criteria were not considered for the study: any comorbid Axis 1 mental disorder; reports of suicidal ideation or prior suicide attempt; chronic physical disease;history of epilepsy; increased intraocular pressure or angle-closure glaucoma; abuse of psychoactive substance in the prior year; rapid cycling episodes of depression; allergic to duloxetine or paroxetine; history of serious drug allergy; pregnant, breast-feeding, or of childbearing age and not taking effective contraceptive measures; liver enzymes elevated more than twofold the upper limit of normal; participated in a clinical trial within the past 30 days; treated with monoamine oxidase inhibitors four weeks before randomization;discontinued treatment with psychotropic drugs (except hypnotics) less than fi ve half-lives before randomization;previously non-responsive to therapy with duloxetine hydrochloride or paroxetine hydrochloride; received electroconvulsive therapy (ECT) within the past six months; unable to be compliant with the therapy; or had other factors leading investigators to believe the individual inappropriate for recruitment.
2.2 Study design
This is an eight-week randomized, double-blind, doubledummy, parallel-group, positive drug (paroxetine),multi-centered clinical trial. The goal was to determine whether or not generic duloxetine was similar in effectiveness and safety to paroxetine in the treatment of MDD—that is, it is a ‘non-inferiority’ trial. The required sample size was estimated using standard methods for test of non-inferiority. Based on published data,[16]the total HAMD-17 score in patients with MDD drop an average of 13.5 points after 8 eight weeks of treatment with paroxetine. Based on a δ of 2.2 (the standard for non-inferiority trials), a type I error (α)of 0.025, a type II error (β) of 0.20, and a combined standard deviation (S) of 6, the estimated sample size required for each group was 117. Taking intoconsideration potential dropouts during the study, we set the target sample size at 300 subjects, 150 in each group.

Figure 1. Flowchart of the study
Recruited outpatients were randomized to treatment with duloxetine or paroxetine using a computerized random number generator. The Jiangsu Nhwa Pharmaceutical Co., Ltd. provided the medications used in the study: enteric-coated duloxetine hydrochloride tablets(20 mg/tablet; 20090401; Jiangsu Nhwa Pharmaceutical Co., Ltd.), and paroxetine hydrochloride tablets (Seroxat;20 mg/tablet; Glaxo SmithKline Pharmaceutical Co.,Ltd.). In the duloxetine group, patients were treated each morning with duloxetine (40 mg/d; 2 tablets) and paroxetine placebo (1 tablet) during week one, and with duloxetine (60 mg/d; 3 tablets) and paroxetine placebo (1 tablet) from week two to week eight. In the paroxetine (control) group, patients were treated each morning with paroxetine (20 mg/d; 1 tablet) and duloxetine placebo (2 tablets) during week one, and with paroxetine (20 mg/d; 1 tablet) and duloxetine placebo (3 tablets) from week two to week eight.
During the study, drugs used at conventional doses for the treatment of insomnia (zolpidem, zopiclone,zaleplon) and drugs used for controlling physical diseases were allowed. The dose of each allowed drug and the duration of treatment with these drugs were recorded in detail. However, antipsychotics,antidepressants, anxiolytics, anti-manic drugs, systemic psychotherapy (such as cognitive and behavioral therapy), and modified electroconvulsive therapy were not allowed.
Patients were withdrawn or dropped out of the trial if they experienced intolerable side effects, violated the study protocol (i.e., met any of the exclusion criteria listed above), withdrew consent, or were lost to followup.
The investigators were all experienced clinicians;3-5 clinical psychiatrists and one medication coordinator(who maintained the randomization results and distributed medications) participated at each of the 8 study sites. Before the trial, participating clinicians were trained in the diagnosis of MDD using DSM-IV criteria and in the methods of rating the HAMD-17,the Montgomery and Asberg Depression Rating Scale(MADRS), the severity measure of the Clinical Global Impression Scale (CGI-S), the Hamilton Anxiety Scale(HAMA), the Visual Analog Scale of Pain Intensity (VASPI), and the Sheehan Disability Scale (SDS).
2.3 Evaluation of treatment efficacy and safety
The HAMD-17 was assessed at baseline and at the end of the 1st, 2nd, 4th, 6th, and 8thweek of treatment. At each of these clinical visits the treating clinician also asked patients about the occurrence of any adverse events.The total HAMD-17 score after eight weeks of treatment and the difference between the HAMD-17 score at baseline and after eight weeks of treatment were used as the primary measures of therapeutic efficacy. Clinical remission was defined as a HAMD-17 score of ≤7 by the end of the trial. Effectiveness was defined as a decrease of at least 50% in the baseline HAMD-17 score at the end of the trial. The total scores, differences in scores, effectiveness, and clinical remission rates were compared between the two groups.
A variety of secondary measures of efficacy were also assessed at baseline and at the end of the 1st, 2nd,4th, 6th, and 8thweek of treatment: the Montgomery and Asberg Depression Rating Scale (MADRS), the severity measure of the Clinical Global Impression scale(CGI-S), the Hamilton Anxiety scale (HAMA), the Visual Analog Scale of Pain Intensity (VAS-PI), and the Sheehan Disability Scale (SDS).[15]
The following examinations were completed at baseline, at the end of the 8-week trial, and,additionally, at any time during the trial the treating clinician considered such tests necessary: routine blood tests; blood biochemistry to determine liver function,kidney function, and fasting blood glucose; routine urinalysis; a urine pregnancy test (women only); and a 12-lead electrocardiographic examination.
Treatment adherence was assessed by determining the proportion of prescribed medication that was actually taken (as reported by the patient); if 80% or greater of the prescribed medication was taken, adherence was considered ‘good’.
2.4 Statistical analysis
An electronic data management system was used for data management; data entry clerks input trial data into an electronic case report form (eCRF) specifically designed for this study. SAS version 9.1.3 was used for statistical analysis. The primary analysis was a modified intention-to-treat (ITT) analysis, which excluded three patients that were randomized to the duloxetine group but never started medication and, thus, included 146 patients in the duloxetine group and 150 patients in the paroxetine group. In both groups, 29 patients who started medications dropped out during the 8-week trial (see Figure 1); a ‘last observation carried forward’(LOCF) method was used to include their results in the ITT analysis. A secondary ‘per protocol set’ (PPS)analysis of the 117 duloxetine group patients and 121 paroxetine group patients who completed the 8-week trial was also conducted.
Differences between groups in baseline and final HAMD-17 scores and in the magnitude of the change in HAMD-17 scores over the trial were assessed using t-tests. Between group comparison of baseline, final,and change scores for the other five clinical measures(MADRS, HAMA, VAS-PI, CGI-S, SDS) also used t-tests.Within group changes over time were assessed using paired t-tests. Differences between groups in the proportion of individuals that experienced remission,effective treatment, and side-effects were compared using Chi-square tests and Fisher’s exact tests. The trend of change over the 8-week trial in the HAMD-17 score between the groups was assessed using a repeated measures analysis of variance. All tests were two-tailed and statistical significance was set atp<0.05.
The protocol for this study was approved by the Ethics Committee of the Shanghai Mental Health Center.
3. Results
The baseline characteristics of enrolled patients who started the trial medications are shown in Table 1.About half of the participants were experiencing their first episode of depression. On average the current episode of depression had lasted more than 6 months.Compared to the patients in the paroxetine group,those in the duloxetine group were more likely to be female, were somewhat shorter in stature, and had a significantly higher pain index score on the VAS-PI.
As shown in Figure 1, 80.1% (117/146) of patients enrolled in the duloxetine group who started medication and 80.7% (121/150) of patients enrolled in the paroxetine group who started medication completed the 8-week trial. A total of 29 patients dropped out of each arm of the trial. In the duloxetine group,clinicians withdrew 6 patients because of troubling side effects (somnolence, nausea and vomiting, fatigue,abnormal transaminase, psychotic symptoms, and numbness in the occiput) and 2 patients due to lack of effectiveness. In the paroxetine group clinicians withdrew 6 patients due to troubling side effects(fidgeting, agitation, allergy, abnormal liver function,nausea, and hyperhidrosis) and 3 due to lack of effectiveness.
3.1 Treatment efficacy
As shown in Table 2 and Figure 2, based on the modified ITT analysis the HAMD-17 scores decreased significantly from baseline over the course of therapy in both groups.There were no significant differences in the magnitude of the change in HAMD-17 from baseline between the two groups at any point over the 8-week treatment trial. The repeated measures ANOVA assessing the trend in HAMD-17 scores over time supported this finding:Fgroup=2.41,p=0.122;Ftime=782.10,p<0.001); andFgroup*timeinteraction=1.24,p=0.290. The results for the PPS analysis were essentially identical: the mean (sd) drop in the HAMD-17 score in the duloxetine group was 16.0(6.1) versus 16.5 (6.3) in the paroxetine group (t=0.64,p=0.523) and the repeated measures ANOVA confirmed that there were no differences in the rate of change in the HAMD-17 score between groups (Fgroup=0.88,p=0.349;Ftime=591.42,p<0.001); andFgroup*timeinteraction=1.24,p=0.290).
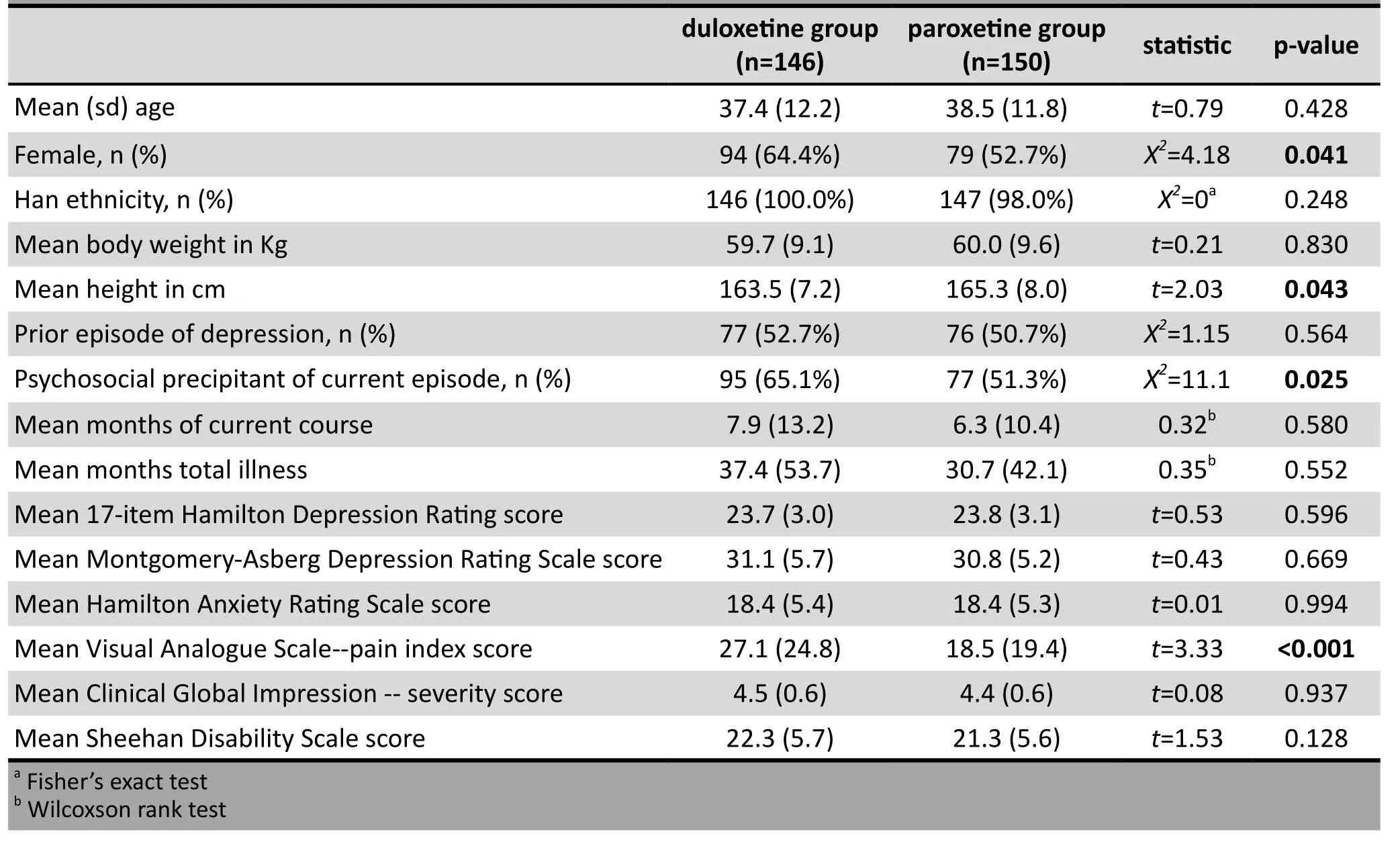
Table 1. Baseline characteristics of patients in the two groups
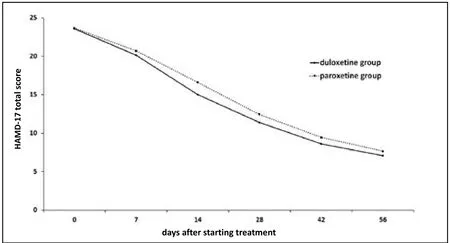
Figure 2. Mean (sd) score of 17-item Hamilton Depression rating scale (HAMD-17) over the course of the 8-week trial in 146 patients with major depressive disorder taking duloxetine and 150 taking paroxetine (using intention-to-treat analysis)
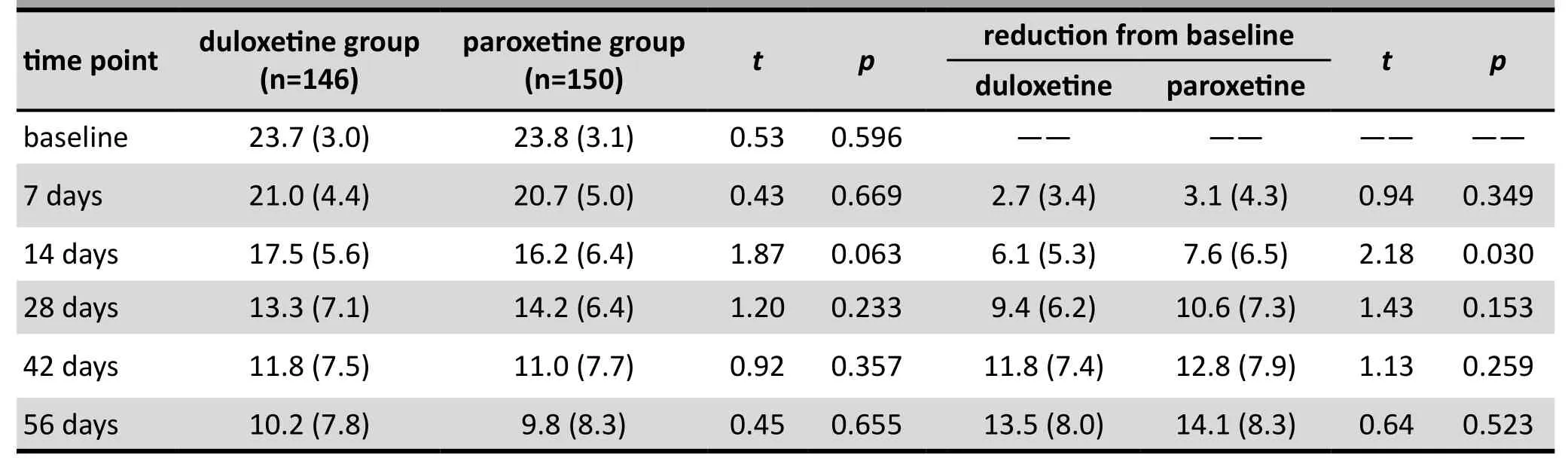
Table 2. Intention-to-treat analysis (last observed value carried forward) of 17-item Hamilton Depression rating scale (HAMD-17) scores at different time points during the trial in the two groups of patients with major depressive disorder
Based on the ITT analysis, 67.1% (98/146) of the patients in the duloxetine group were effectively treated(i.e., drop in baseline HAMD-17 of >50%) compared to 71.3% (107/150) in the paroxetine group (X2=0.62,p=0.433). Using a final HAMD-17 score of 7 or lower as the criteria for ‘remission’, 41.1% (60/146) of the patients in the duloxetine group and 51.3% (77/150) of the patients in the paroxetine group achieved remission(X2=3.12,p=0.077). Using the per protocol set (PPS)analysis, the corresponding rates of effective treatment were 80.3% (94/117) versus 84.3% (102/121) (X2=0.64,p=0.424), and the corresponding rates of remission were 49.6% (58/117) versus 61.2% (74/121) (X2=3.23,p=0.072).
Based on the ITT analysis, the secondary clinical measures all showed significant improvement over the 8-week trial and, as was the case for the HAMD-17 score, there was no significant difference in the magnitude of the change in scores between the two treatment groups. For the duloxetine and paroxetine groups, the mean (sd) decreases in baseline scores after 8 weeks of treatment were as follows: MADRS,21.8 (7.8) versus 20.7 (8.6),t=1.02,p=0.311; HAMA,12.5 (5.7) versus 12.2 (6.2),t=0.32,p=0.749; CGI-S, 2.49(1.00) versus 2.34 (1.14),t=1.02,p=0.309; VAS-PI, 15.4(18.6) versus 13.3 (19.0),t=0.83,p=0.407; SDS, 14.1(6.5) versus 13.5 (7.1),t=0.65,p=0.515.
There was no significant between-group difference in the number patients using other drugs (mainly hypnotics such as zolpidem and drugs used to treat other diseases): 36 patients in the duloxetine group(24.7%] and 41 patients (27.3%]) in the paroxetine group used non-study medications (X2=0.23,p=0.600).The proportion of patients in each group with good adherence (i.e., took 80% or more of prescribed medication) was 77.4% in the duloxetine group and 79.3% in the paroxetine group (X2=0.16,p=0.686).
3.2 Safety
The incidence of the common adverse effects that occurred in 1% or more of the patients is listed in Table 3. The most common adverse events were nausea, dry month, constipation, loss of appetite, and dizziness.With few exceptions (that, as described above, resulted in withdrawal from the study) these adverse events were mild to moderate, of short duration, and resolved without any specific management. Overall, 83 of the 146 patients in the duloxetine group (56.8%) and 82 of the 150 patients in the paroxetine group (54.7%)experienced 1 or more adverse events at some point over the 8-week trial (X2=0.14,p=0.705). None in the paroxetine group had severe adverse events; one patient in the duloxetine group withdrew from the study due to cervical vertebral illness that required hospitalization in a general medical hospital.
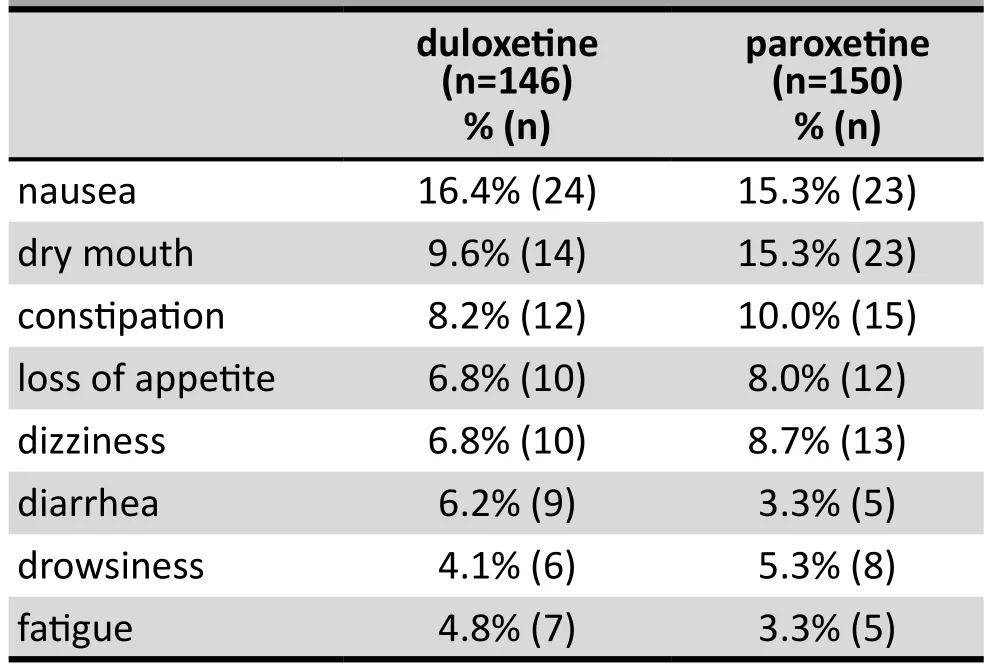
Table 3. Adverse events that occurred in >1% of patients with major depressive disorder at any time during 8 weeks of treatment with duloxetine or paroxetine
4. Discussion
4.1 Main findings
The present study is a registered, multi-centered,randomized, double-blind, double-dummy, positive drug, parallel, and controlled clinical trial. This study aimed to evaluate the efficacy and safety of entericcoated generic duloxetine produced by Jiangsu Nhwa Pharmaceutical Co., Ltd in China for the acute treatment of major depressive disorders in Chinese patients. Noninferiority analysis was used to compare the mean change from baseline to week eight of therapy between duloxetine and paroxetine. Using a modified intentionto-treat analysis, the HAMD-17 score reduction was found to be 13.5 (8.0) and 14.1 (8.3) in the duloxetine and paroxetine groups, respectively. The repeated measures ANOVA indicated that both the rate and magnitude of change in the two groups were similar.Over the 8 weeks of treatment with 40-60 mg of duloxetine per day, 67% of the patients were effectively treated (i.e., >50% drop in baseline HAMD-17 score) and 41% achieved the criteria for remission (final HAMD-17 score <7); these results were not significantly different from those for the paroxetine group. Our findings are consistent with those reported in an open label 12-week trial by Martinez and colleagues[17]and with those of a placebo-controlled study of duloxetine (60 mg/d) as treatment for acute MDD by Detke and colleagues.[18]
In the United States, duloxetine is also approved for the management of generalized anxiety disorder.[19]In the current study we found significant improvement in the HAMA scale scores after 8 weeks of treatment with both duloxetine and paroxetine and no significant difference in the magnitude of the improvement between the two groups. This suggests that duloxetine is equally effective as paroxetine (which is commonly used to treat anxiety) in improving concurrent symptoms of anxiety in depressed patients.
Physical pain is a common somatic symptom of depression. Prior studies have shown that duloxetine is more effective in reducing reported pain in depressed patients than placebo and other selective serotonin reuptake inhibitors (SSRIs).[20]However, in this study duloxetine and paroxetine treatment were both associated with similar significant decreases in the level of pain reported on the VAS-PI scale. The reason for this difference from prior findings may be that in our study, despite the random group assignment, the baseline score of VAS-PI in the duloxetine group was more than 30% higher than that in the paroxetine group(27.1 v. 18.5) – a highly significant difference (p<0.001).Future studies will need to evaluate the therapeutic effect of duloxetine on the somatic symptoms of pain in depressed patients.
The common side effects for duloxetine reported in this study (nausea, dry mouth, constipation, loss of appetite, dizziness, diarrhea, drowsiness and fatigue)were similar to those reported in prior studies.[21]These side effects were largely mild to moderate in severity and did not require specific therapy or management. In the present study duloxetine had no detrimental effect on kidney and liver function, heart rate, blood pressure,or body weight.
4.2 Limitations
The patients entered in this study were those attending outpatient departments at specialized psychiatric hospitals in China, did not have any comorbid disorders,and had moderate to severe symptoms. Moreover,the number of patients screened for the study prior to enrollment but excluded for different reasons(including refusal to participate) were not recorded at all eight centers. Thus it is not known the extent to which the included sample is representative of all depressed individuals in China. The sample size, though large enough to assess the main hypothesis about the comparable effectiveness of the two drugs, was not large enough to conduct stratified analysis (e.g.,by gender, age group, number of previous depressive episodes, and so forth) to determine whether or not there are differences in outcome in different subgroups of patients. Adverse events were assessed by clinicians at each clinical visit during the trial without the use of a standardized instrument, so some less serious adverse events may have been missed. The duration of treatment of 8 weeks was sufficient to assess the acute response of MDD to duloxetine, but longer lasting studies will be needed to determine its effectiveness in chronic depression and its effectiveness in preventing relapses in depressed patients.
4.3 Significance
This study is a multi-centered, double-blind, doubledummy randomized controlled clinical trial conducted in Chinese psychiatric outpatients with MDD. Based on a modified intention-to-treat analysis, the results show that eight weeks of treatment with 40-60mg/d of generic duloxetine in these patients was equally effective, safe, and well-tolerated as treatment with 20mg/d of paroxetine.
Conflict of interest
The authors declare no conflicts of interest.
Funding
This study was supported by Jiangsu Nhwa Pharmaceu-tical Co., Ltd. The Jiangsu Nhwa Pharmaceutical Co. did not participate in the design, implementation, or data analysis for this study.
Ethics approval
The ethics committee of the Shanghai Mental Health Centre approved the study on August 24, 2009.
Informed consent
Every patient who participated in this study signed a consent form at the beginning of the study.
1. Hirschfeld RM. The epidemiology of depression and the evolution of treatment.J Clin Psychiatry. 2012; 73(Suppl 1):5-9. doi: http://dx.doi.org/10.4088/JCP.11096su1c.01
2. Gonda X, Fountoulakis KN, Kaprinis G, Rihmer Z. Prediction and prevention of suicide in patients with unipolar depression and anxiety.Ann Gen Psychiatry. 2007; 6: 23.doi: http://dx.doi.org/10.1186/1744-859X-6-23
3. Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010.Lancet. 2013; 382(9904):1575-1586. doi: http://dx.doi.org/10.1016/S0140-6736(13)61611-6
4. Lecrubier Y. The burden of depression and anxiety in general medicine.J Clin Psychiatry.2001; 62(Suppl 8): 4-9
5. Phillips MR, Zhang J, Shi Q, Song Z, Ding Z, Pang S.Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001-05: An epidemiological survey.Lancet. 2009; 373(9680):2041-2053. doi: http://dx.doi.org/10.1016/S0140-6736(09)60660-7
6. Rush AJ. STAR*D: what have we learned?Am J Psychiatry.2007; 164(2): 201-204
7. Kirwin JL, Gören JL. Duloxetine: a dual serotoninnorepinephrine reuptake inhibitor for treatment of major depressive disorder.Pharmacotherapy. 2005; 25(3): 396-410. doi: http://dx.doi.org/10.1592/phco.25.3.396.61600
8. Westanmo AD, Gayken J, Haight R. Duloxetine: a balanced and selective norepinephrine- and serotonin-reuptake inhibitor.Am J Health Syst Pharm. 2005; 62(23): 2481-2490
9. Ball SG, Desaiah D, Zhang Q, Thase ME, Perahia DG.Efficacy and safety of duloxetine 60 mg once daily in major depressive disorder: a review with expert commentary.Drugs Context. 2013; 2013: 212245. doi: http://dx.doi.org/10.7573/dic.212245
10. Thase ME, Pritchett YL, Ossanna MJ, Swindle RW, Xu J,Detke MJ. Efficacy of duloxetine and selective serotonin reuptake inhibitors: comparisons as assessed by remission rates in patients with major depressive disorder.J Clin Psychopharmacol. 2007; 27(6): 672-676. doi: http://dx.doi.org/10.1097/jcp.0b013e31815a4412
11. Goldstein DJ, Lu Y, Detke MJ, Wiltse C, Mallinckrodt C,Demitrack MA. Duloxetine in the treatment of depression:A double blind placebo controlled comparison with paroxetine.J Clin Psychopharmacol. 2004; 24(4): 389-399
12. Carter NJ, McCormack PL. Duloxetine: a review of its use in the treatment of generalized anxiety disorder.CNS Drugs. 2009; 23(6): 523-541. doi: http://dx.doi.org/10.2165/00023210-200923060-00006
13. Arnold LM, Zhang S, Pangallo BA. Efficacy and safety of duloxetine 30 mg/d in patients with fibromyalgia: a randomized, double-blind, placebo-controlled study.Clin J Pain. 2012; 28(9): 775-781.doi: http://dx.doi.org/10.1097/AJP.0b013e3182510295
14. American Psychiatric Association.DSM IV Sourcebook.Virginia: Am Psychiatric Press Inc; 1994
15. Zhang MY. [Handbook of Rating Scale in Psychiatry. 2 nded].Changsha: Hunan Science and Technology Press; 1998.Chinese
16. Lee P, Shu L, Xu X, Wang CY, Lee MS, Liu CY, et al. Once-daily duloxetine 60 mg in the treatment of major depressive disorder: multicenter, double-blind, randomized,paroxetine-controlled, non-inferiority trial in China, Korea,Taiwan and Brazil.Psychiatry Clin Neurosci.2007; 62(3):295-307
17. Martinez JM, Katon W, Greist JH, Kroenke K, Thase ME,Meyers AL, et al. A pragmatic 12-week, randomized trial of duloxetine versus generic selective serotoninreuptake inhibitors in the treatment of adult outpatients in a moderate-to-severe depressive episode.Int Clin Psychopharmcol. 2012; 27(1): 17-26. doi: http://dx.doi.org/10.1097/YIC.0b013e32834ce11b
18. Detke MJ, Lu Y, Goldstein DJ, Hayes JR, Demitrack MA.Duloxetine, 60 mg once daily for major depressive disorder:A randomized double blind placebo controlled trial.J Clin Psychiatry. 2002; 63(4): 308-315
19. Kornstein SG, Russell JM, Spann ME, Crits-Christoph P, Ball SG. Duloxetine in the treatment of generalized anxiety disorder.Expert Rev Neurother.2009; 9(2): 155-165
20. Gaynor PJ, Gopal M, Zheng W, Martinez JM, Robinson MJ,Marangell LB . A randomized placebo-controlled trial of duloxetine in patients with major depressive disorder and associated painful physical symptoms.Curr Med Res Opin.2011; 27(10): 1849-1858. doi: http://dx.doi.org/10.1185/0 3007995.2011.609539
21. Brunton S, Wang F, Edwards SB, Crucitti AS, Ossanna MJ,Walker DJ, et al. Profile of adverse events with duloxetine treatment: a pooled analysis of placebo-controlled studies.Drug Saf. 2010; 33(5): 393-407. doi: http://dx.doi.org/10.2165/11319200-000000000-00000
, 2015-06-12; accepted, 2015-08-22)

Dr. Wang Zhiyang received a bachelor’s degree from the Department of Medicine at Shanghai Medical University in 1992 and a master’s degree from the department of Psychiatry and Mental Health at the School of Medicine of Fudan University in 2009. Since 1992 she has worked as a clinician, teacher, researcher, and forensic psychiatrist at the Shanghai Mental Health Center. She is currently an attending physician in the Clinical Trials Center of the Department of Psychiatry at Huashan Hospital in Shanghai, where her research focuses on clinical psychopharmacology.
使用仿制度洛西汀或帕罗西汀治疗抑郁症:一项多中心、双盲、双安慰剂、随机对照临床试验
王志阳,许秀峰, 谭庆荣,栗克清, 马崔, 谢世平,高成阁,王刚,李华芳
度洛西汀;帕罗西汀;疗效;安全性;抑郁症;随机对照试验;中国
Background:This study is a pre-registration trial of generic duloxetine that was approved by the China Food and Drug Administration (approval number: 2006L01603).Aim:Compare the treatment efficacy and safety of generic duloxetine to that of paroxetine in patients with major depressive disorders (MDD).Methods:This was a double-dummy, double-blind, multicenter, positive drug (paroxetine), parallel randomized controlled clinical trial. The 299 patients with MDD recruited for the study were randomly assigned to use duloxetine (n=149; 40–60 mg/d) or paroxetine (n=150; 20 mg/d) for 8 weeks. The Hamilton Depression rating scale (HAMD-17) was administered at baseline and 1, 2, 4, 6, and 8 weeks after starting treatment. Remission was defined as a HAMD-17 score below 8 at the end of the trial, and treatment effectiveness was defined as a decrease in baseline HAMD-17 score of at least 50% by the end of the trial.Safety was assessed based on the reported prevalence and severity of side effects and changes in laboratory and electrocardiographic findings. Three patients in the duloxetine group dropped out before starting medication, so results were analyzed using a modified intention-to-treat (ITT) method with 146 in the experimental group and 150 in the control group.Results:Both groups experienced 29 dropouts during the 8-week trial. HAMD-17 scores decreased significantly from baseline throughout the trial in both groups. Based on the ITT analysis, at the end of the trial there was no significant difference between the duloxetine group and the paroxetine group in effectiveness (67.1% v. 71.3%,X2=0.62p=0.433), remission rate (41.1% v. 51.3%,X2=3.12,p=0.077), or in the incidence of side effects (56.8% v. 54.7%,X2=0.14,p=0.705).Conclusion:Generic duloxetine is as effective and safe as paroxetine in the acute treatment of patients with MDD who seek care at psychiatric outpatient departments in China.
[Shanghai Arch Psychiatry. 2015, 27(4): 228-236.
http://dx.doi.org/10.11919/j.issn.1002-0829.215064]
1Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
2Department of Psychiatry, Huashan Hospital, Fudan University, Shanghai, China
3Department of Psychiatry, First Affiliated Hospital, Kunming Medical College, Kunming, Yunnan Province, China
4Xijing Hospital, Fourth Military Medical University, Xi’an, Shaanxi Province, China
5Hebei Mental Health Center, Baoding, Hebei Province, China
6Guangzhou Brain Hospital, Guangzhou, Guangdong Province, China
7Affiliated Brain Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China
8First Affiliated Hospital of Xi’an, Jiao Tong University Medical College, Xi’an, Shaanxi Province, China
9Beijing Anding Hospital, Capital Medical University, Beijing, China
*correspondence: lhlh_5@163.com
A full-text Chinese translation of this article will be available at http://dx.doi.org/10.11919/j.issn.1002-0829.215064 on Oct 26, 2015.
背景:本研究是经国家食品药品监督管理总局批准的仿制度洛西汀注册前试验(批准号:2006L01603)。目的:比较仿制度洛西汀和帕罗西汀治疗抑郁症患者的疗效和安全性。方法:这是一项双盲双安慰剂 (double dummy)、多中心、有效药物(帕罗西汀)平行随机对照临床试验。将纳入的299 例抑郁症患者随机分组,使用度洛西汀(n=149; 40-60 mg/d) 或帕罗西汀 (n=150; 20 mg/d) 连续治疗8周。在基线和开始治疗后的第1、2、4、6和8周使用汉密尔顿抑郁量表(Hamilton Depression rating scale, HAMD-17) 评估。缓解的定义为研究终点HAMD-17评分低于8分,治疗有效的定义为研究终点HAMD-17得分较基线至少降低了50%。根据报告的不良反应的发生率、严重程度以及实验室检查结果、心电图结果的变化来评估安全性。度洛西汀组中有三例患者在开始用药前退出,采用修正的意向治疗分析 (intention-to-treat, ITT) 方法以比较研究组146例患者和对照组150例患者的研究结果。结果:在8周的研究期间两组有均29例患者脱落。与基线比,两组HAMD-17评分在整个试验过程中均显著降低。根据ITT分析,研究终点时度洛西汀组和帕罗西汀组在疗效方面差异无统计学意义 (67.1% v. 71.3%,X2=0.62,p=0.433), 缓 解 率 (41.1% v. 51.3%,X2=3.12,p=0.077) 及不良作用发生率56.8% v. 54.7%,X2=0.14,p=0.705) 等方面的差异也无统计学意义。结论:对于在国内精神科门诊就医的抑郁症患者而言,急性期使用仿制度洛西汀与使用帕罗西汀同样安全有效。
本文全文中文版从2015年10月26日起在
http://dx.doi.org/10.11919/j.issn.1002-0829.215064可供免费阅览下载
猜你喜欢
杂志排行
上海精神医学的其它文章
- Introduction to longitudinal data analysis in psychiatric research
- Case report of comorbid schizophrenia and obsessivecompulsive disorder in a patient who was tube-fed for four years by family members because of his refusal to eat
- Obsessive compulsive symptoms in bipolar disorder patients:a comorbid disorder or a subtype of bipolar disorder?
- Comorbid bipolar disorder and obsessive-compulsive disorder
- Comparison of the effectiveness of duloxetine in depressed patients with and without a family history of affective disorders in first-degree relatives
- Single-blind, randomized controlled trial of effectiveness of Naikan therapy as an adjunctive treatment for schizophrenia over a one-year follow-up period
