1H-3-(3-吡啶基)-5-(3′-吡啶基)-1,2,4-三唑的钴配合物的合成、晶体结构、热稳定性及配体的DFT计算
2015-12-05刘怀贤周惠良刘翔宇宋伟明胡奇林
孙 琳 刘怀贤 周惠良 刘翔宇 宋伟明 李 冰 胡奇林
(宁夏大学化学化工学院,银川 750021)
1H-3-(3-吡啶基)-5-(3′-吡啶基)-1,2,4-三唑的钴配合物的合成、晶体结构、热稳定性及配体的DFT计算
孙琳刘怀贤周惠良刘翔宇*宋伟明李冰*胡奇林
(宁夏大学化学化工学院,银川750021)
合成了一个多功能的配体1H-3-(3-吡啶)-5-(3′-吡啶)-1,2,4-三唑(3,3′-Hbpt,1)并得到了配体的晶体结构,运用DFT理论计算了配体的最优构型、优势构象和电荷分布。在此基础上,水热合成了一个配位化合物:[Co(3,3′-Hbpt)2(H2O)4]·(ad)·6H2O(2)(ad=己二酸),结构分析表明配合物2是零维单核化合物,它的三维超分子结构是由分子间氢键连接而成,其中包含着由游离的己二酸分子填充的矩形孔道。值得注意的是,配体在配合物中的几何结构和构象与理论计算的结果一致。另外,利用热重分析研究了配合物2的热稳定性。
1H-3-(3-吡啶基)-5-(3′-吡啶基)-1,2,4-三唑;理论计算;配位化合物;晶体结构;热稳定性
0 Introduction
The design andconstructionof coordination polymers(CPs)are of great interest in the field of crystal engineering,for their intriguing variety of architectures and tremendous potential applications in non-linearoptics,catalysis,gasabsorption, luminescence,magnetism and medicine[1-5].However,itis still a great challenge to rationally predict and control their exact structures of CPs because the structural natures are diverse depending on various factors such as the metal ions,pH values,solvents, the ligands and/or co-ligands,synthetic methods,etc[6-12].Selecting appropriate organic ligands to construct expected CPs is still a committed step with a longterm issue in the field of coordination chemistry[13-20]. As we all known,pyridine-based triazole ligands have been proved as a type of excellent connector to engender a wide range of CPs resulting from the several advantages below:(i)The abundant nitrogen electron-donatingatomsinthemoleculecould selectively coordinate to metal ions,facilitating the generation of CPs[21].(ii)The ligand indicates various coordination fashions,such as multidentate or bridging building modes,offering the possibility to obtain the fascinating CPs[22-25].In this work,we adopt 1H-3-(3-pyridyl)-5-(3′-pyridyl)-1,2,4-triazole(3,3′-Hbpt)as ligandtoassemblethetargetsupramolecular architectures.
One thing to note is that 3,3′-Hbpt has potential tendency to show four typical conformations under different surroundings(Scheme 1).Therefore,it is an efficient strategy to conduct research on the reaction systems in the basic of the combination of theory and experiment,whichisconducivetoformingthe structural model and revealing the preferred geometry, and even estimating the coordination mode of the compounds,especially in the application of non-metal organic compounds[26-27].Whats more,it has long been a target for scientists to control the reaction conditions and use theoretical calculations to guide and verify experimental results[28-32].It is tantalizing to introduce the theoretical calculation into the investigation of CPs.
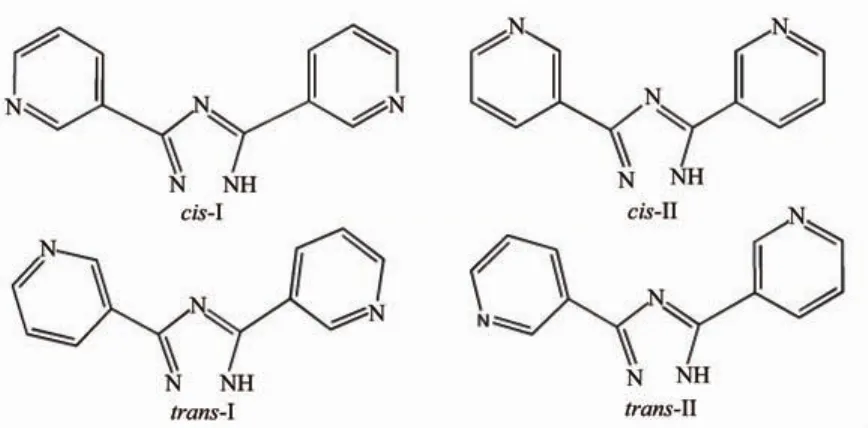
Scheme 1 Four typical conformations of 3,3′-Hbpt
Based on the considerations above,we have synthesized the 3,3-Hbpt·2H2O(1)ligand of which the crystal structure is determined and the optimized structure and preferred conformation are inferred by using DFT method[33].Then,a coordination compound, namely[Co(3,3′-Hbpt)2(H2O)4]·(ad)·6H2O(ad= adipate)(2),has been obtained under hydrothermal condition and structurally characterized in detail,in which the structure and conformation of the ligand are comparable with the calculated results.The thermo stability of 2 is tested and discussed as well.
1 Experimental
1.1Materials
Commercially available reagents were used as received without further purification.1H NMR spectra were taken with a Varian 400 spectrometer using tetramethylsilane(TMS)as an internal standard. Elemental analyses(C,H and N)were performed on a Vario EL III analyzer.Infrared spectra were obtained from KBr pellets1on a BEQ VZNDX 550 FTIR instrument within the 400~4 000 cm-1region.Thermal analysiswasperformedonNetzschSTA449C microanalyzerunderflowingN2atmosphereata heatingrateof10℃ ·min-1.X-raypowder diffractometer were received on a Bruker Advanced-D8 instrument at room temperature.The calculations have been carried out using Gaussian03 program[34],at the B3LYP[35-36]level of theory with 6-31G(d,p)basis set.
1.2Synthesis of 3,3′-Hbpt·2H2O(1)
3,3′-Hbpt·2H2O was synthesized through advan-ced methods reported[37].A mixture of magnesium(0.6 g)and iodine(0.2 g)in methanol(110 mL)was refluxed for 0.5 h to prepare dry methanol(50 mL). Later a mixture of sodium(0.1 g)and 3-cyanopyridine (2.1 g)was added with stirring for 1 h,then 3-pyridyl carbonyl hydrazide(2.1 g)was added followed by glacial acetic acid(0.1 g).The resulting yellow precipitate was filtered off and washed thoroughly with boiling ethanol.Drying in vacuo gave 1.8 g(75%)of analytically pure 3,3′-Hbpt as a white powder.The product(10 mmol)was dissolved in the mixed solution of H2O(2 mL)and MeOH(20 mL),and the resulting solution was placed in a silicagel desiccator at room temperature for several weeks to give colorless crystals as compound 1.Anal.Calcd.for C12H13N5O2(%):C, 55.54;H,5.01;N,27.01.Found(%):C,55.69;H, 5.10;N,27.08.m.p.:509~510 K;IR(cm-1,KBr):3 327(s),3 156(s),3 048(m),2 843(m),1 620(s),1 594 (s),1 482(w),1 406(s),1 308(s),1 196(w),1 051(m), 821(m),709(m),625(w).1H NMR(400 MHz,DMSO-d6):7.534~7.552(2H,m),8.408~8.427(2H,d,J=7.6 Hz),8.723~8.743(2H,dd,J=8.0 Hz),9.272(2H,s), 14.926(1H,s,Triazole-H).
1.3Synthesisof[Co(3,3′-Hbpt)2(H2O)4](ad)6H2O(2) A mixture containing Co(OAc)2·4H2O(7.5 mg, 0.03 mmol),3,3′-Hbpt(6.6 mg,0.03 mmol),adipic acid(8.8 mg,0.06 mmol)and water(6 mL)was sealed in a 15 mL Teflon-lined stainless steel vessel and heated at 150℃for 3 days,and then cooled to room temperature at a rate of 5℃·h-1.Pink plate-like crystals of 2 were collected in a yield of 30%(based on Co).Anal.Calcd.for(CoC30N10O14H42)(%):C,43.64; H,5.13;N,16.96.Found(%):C,43.72;H,5.15;N, 16.99.IR(cm-1,KBr):3 208(s),3 057(m),2 964(w), 2 941(w),2 643(w),1 692(m),1 550(s),1 483(m),1 450 (m),1 402(s),1 292(m),1 186(m),1 146(m),1 127 (m),1 048(w),986(m),952(w),821(w),748(m),703(s), 695(s),475(w).
1.4Crystallographic data collection and structure determination
All diffraction data of compounds were collected on a Bruker Smart ApexⅡ CCD diffractometer with graphite monochromated Mo Kα radiation(λ=0.071 073 nm)at 293(2)K.Absorption corrections were applied using SADABS[38].All structures were solved by the direct methods using the SHELXS program of the SHELXTL-97 package and refined with SHELXL -97[39-40].All non-hydrogen atoms were refined anisortropically.Hydrogen atoms were placed in geometrically calculated positions.Crystallographic details are summarized in Table1.Selected bond(nm)and bond angles(°)of compound 2 are shown in Table S1. Hydrogen bond geometries in compounds 1 and 2 are listed in Table S2 and Table S3,respectively.
CCDC:1028283,1;1002548,2.

Table1 Crystal data and structure refinement details for compounds 1 and 2

Continued Table1
2 Results and discussion
2.1Quantum chemical calculation for 3,3′-Hbpt
DFT method was carried out to get an insight of the electronic structures and bonding properties of 3,3′-Hbpt.The following calculation and discussion are resulted from the optimized structure.The full geometry optimization is performed without constraints on symmetry.The optimized geometries of different conformations have been obtained at the B3LYP/6-31G(d,p)level and shown in Fig.1.Selected bond lengths and angles of cis-Ⅰand cis-Ⅱconformations are summarized in Table2.For cis-Ⅰ conformation, the bond lengths of N1-C5,N2-C6,N3-N4 and N5-C12 are calculated to be 0.133 527,0.136 689, 0.134 913 and 0.135 133 nm,respectively,while the corresponding bond lengths of cis-Ⅱ are 0.133 896, 10.326 55,0.135 322 and 0.133 389 nm.

Fig.1 Optimized structures of four conformations for 3,3′-Hbpt
The molecular total energies,zero-point energies and frontier orbital energies and the energy gaps of four different conformations had been calculated and listed in Table3.The total energies of four different conformations are calculated to be-736.464 0,-736.462 5, -736.463 9,-736.462 8 a.u.,respectively.Obviously, the cis-Ⅰconformation provides relatively lower energy, representing more stable geometry.The surfaces of HOMO-1,HOMO,LUMO and LUMO+1 for four conformationsareshowninFig.2.Thefrontier molecular orbitals of four kinds of conformations are mainly composed of p atomic orbitals,and the electronic transitions are mainly assigned to n→π*and π→π* electronic transitions.The same electron distribution of the frontier orbitals indicates similar electronic transition model.As listed in Table3,the energy gap of cis-Ⅱ conformation(ΔE(LUMO-HOMO)=4.71 eV)is lower than that of other three conformations,suggesting the greater ability of electron transition under external stimuli.To estimate the possible coordinated condition of the cis-Ⅱ conformation,the natural charges and electronconfigurationsoftheatomshavebeen calculatedbyusingnaturalbondorbital(NBO) analysis.As shown in Table4,mulliken charges andnatural charges of N1,N2,N3,N4 and N5 atoms are calculated as negative values.The charge of N2 atom shows more negative than that of N3 and N4 atoms in the triazole ring.The natural charge of N1 and N5 atoms in pyridine rings are indentified to be-0.440 60 and-0.449 06.The results above demonstrate N1,N2and N5 atoms are prone to coordinate with metal ions.

Fig.2 Isodensity surfaces of HOMO-1,HOMO,LUMO and LUMO+1 for 3,3′-Hbpt

Table2 Experimental and theoretical bond lengths(nm)and angles(°)for 3,3′-Hbpt in two compounds

Table3 Calculated total energies,zero-point energies(ZPE)and frontier orbit energies of 3,3′-Hbpt

Table4 Natural configurations and natural charges for the selected atoms of cis-Ⅱ
2.2Structure of 3,3′-Hbpt·2H2O(1)
Single crystal X-ray analysis reveals that the elementaryunitofcompound1consistsofone discrete 3,3′-Hbpt molecule and two lattice water(Fig. 3a).The 3,3′-Hbpt ligand performs cis-Ⅰ conformation in the crystal structure,which is in agreement with calculated molecular total energies of four kinds of conformations.The dihedral angles between the two pyridine planes and triazole are 2.012°and 8.640°, respectively.Thesupramoleculararchitectureis formed by hydrogen-bonding interactions including O1-H21…O2(0.276 1(3)nm),O1-H22…N5i(2.804 nm),O2-H24…N1ii(0.291 0(3)nm),O2-H23…N2iii(0.309 6(3)nm)and N4-H1…O1iv(0.270 7(3)nm)(Fig. 3b).The comparisons of observed and calculated geometric parameters for compound 1 are listed in Table3.

Fig.3 (a)Structure of 1 with 50%thermal ellipsoids;(b)Supramolecular structure assembled by hydrogen bonds throughthe free water molecules
2.3Structure of[Co(3,3′-Hbpt)2(H2O)4]·(ad)· 6H2O(2)
Compound 2 is a 0D motif with one Cocation, two 3,3′-Hbpt molecules,four coordinated water molecules,one free adipate anion and six lattice water molecules.As illustrated in Fig.4a,the Cocenter presents a slightly distorted octahedron by two axial nitrogen atoms from two different 3,3′-Hbpt groups (Co-N 0.211 8(2)nm)and four oxygen atoms of four water molecules(Co-O 0.2055 2~0.2108 5(19)nm). Adjacent mononuclear molecules are integrated by diverse hydrogen-bonding interactions derived from the free water molecules and 3,3′-Hbpt ligands(O1-H1A…O5iv0.270 7(3)nm;O5-H5B…N2v0.294 9(3)nm; O5-H5A…O6 0.273 2(4)nm;O6-H6B…N3 0.284 1(4) nm;O7-H7B…N5vi0.292 9(7)nm;N4-H4N…O7vii0.279 2(3)nm).Subsequently,the 3D supramolecular network is constructed by the non-covalent interactions between the oxygen atoms in coordinated water and adipate anions(O2-H2A…O3iii0.266 8(3)nm;O2-H2B…O3ii0.265 1(3)nm;O1-H1B…O4ii0.267 4(3) nm)(Fig.4c),in which the rectanglar channels are filled by free adipate anions.
In addition,all of the 3,3′-Hbpt ligand keeps uniform cis-Ⅱ conformation in compound 2,corresponding to the lower energy gap value from frontier orbitals obtained by theoretical calculation.In the structure of 2,the ligands display identical coordination mode where the pyridine N1 atom connects to Coions,which is in good agreement with the calculated results of atomic charge density.As shown in Table3,the experimental bond lengths of N1-C1,N2-C7,N3-N4 and N5-C12 and the bond angles of C6-N2-C7,C6-N3-N4,C7-N4-N3 and C11-N5-C12 are well in accordance with the calculated values.In this case,the satisfying result is that the calculated geometry slightly differs from the observed parameters only by an average of 0.000 5 nm,emphasizing that the calculation method and basis set are habituated to the present system.

Fig.4 (a)Coordination environment of cobalt ion with 50%thermal ellipsoids;(b)Hydrogen-bonding between two mono-molecules; (c)Supramolecular network with rectangle channels encapsulated by adipate anions
2.4Thermaldecompositionprocessofcompound2 Before to study the thermal stability,the phase purity of the bulk materials of compound 2 was confirmed by comparison of powder X-ray diffraction (PXRD)with that calculated from the single-crystal study(Fig.S1).Then,the thermal behavior of 2 was determined using TG measurement with the linear heatingrateof10℃ ·min-1undernitrogen atmosphere.As shown in Fig.5,the first stage occurs with weight loss of 22.09%between 71 and 137℃, coinciding with the loss of coordinated water and lattice water molecules(Calcd.21.80%).The second process of weight loss appears from 290℃to 424℃, and the weight loss of 16.78%is attributed to the release of adipate anions(Calcd.17.44%).After that, theconsecutivethirdstepisconsideredasthe decompositionofthe3,3′-Hbptligands.The remainder might be CoO since the weight(10.22%)is in agreement with the calculated value of 9.08%.

Fig.5 TG curve for compound 2
3 Conclusions
In summary,3,3′-Hbpt·2H2O(1)and its coordination compound,[Co(3,3′-Hbpt)2(H2O)4]·(ad)·6H2O (2),have been successfully synthesized and structurally investigated.Single-crystal structure analysis reveals thatcompound2possessesa0Dmononuclear structure which can be extended to the 3D supramolecular network with rectanglar channels packed by free adipate anions.The optimized structure,preferred conformation,chargedistributionandfrontier molecular orbitals of 3,3′-Hbpt ligand are obtained by theoretical calculation,which is in good agreement with the experimental results.The crystalline 3,3′-Hbpt maintains the cis-Ⅰconformation which displays the lowest molecular total energy,while the cis-Ⅱconformation with minimum energy gaps of frontier molecularorbitalappearsinthecoordination compound when the ligand combines with the metal ion.The present work shows a practical case for the combination of theory and experiment,leading to the sophisticated concepts for the design and synthesis of desired coordination polymers.
[1]Batten S R,Robson R.Angew.Chem.Int.Ed.Engl.,1998, 37:1460-1494
[2]Yaghi O M,O′Keeffe M,Ockwig N W,et al.Nature,2003, 423:705-714
[3]Liu X Y,Cen P P,Li H,et al.Inorg.Chem.,2014,53:8088-8097
[4]Mahata P,Prabu M,Natarajan S.Cryst.Growth Des.,2009, 9:3683-3691
[5]Seo J S,Wang D,Lee H,et al.Nature,2000,404(6781):982-986
[6]You Z L,Qiu X Y,Xian D M,et al.Inorg.Chem.Commun., 2012,26:11-16
[7]Liu X Y,Cen P P,Zhou H L,et al.Inorg.Chem.Commun., 2015,53:11-14
[8]Xu Q F,Ge G F,Zhou Q X,et al.Dalton Trans.,2011,40: 2805-2814
[9]Wang H S,Zhao B,Zhai B,et al.Cryst.Growth Des.,2007, 7(9):1851-1857
[10]Kitchen J A,White N J,Gandolfi C,et al.Chem.Commun., 2010,46:6464-6466
[11]Li,B Y,Peng,Y,Li,G H,et al.Cryst.Growth Des.,2010, 10:2192-2201
[12]Herchel R,Travnícek Z,et al.Inorg.Chem.,2011,50:12390 -12392
[13]Fan L M,Li D C,Wei H,et al.J.Coord.Chem.,2011,64: 3031-3040
[14]Ouellette W,Hudson B S,Zubieta J.Inorg.Chem.,2007,46 (12):4887-4904
[15]Li B,Wei Q,Yang Q,J,et al.Chem.Eng.Data,2011,56: 3043-3046
[16]Huang F P,Tian J L,Gu W,et al.Cryst.Growth Des.,2010, 10:1145-1154
[17]Huang F P,Tian J L,Chen G J,et al.CrystEngComm, 2010,12:1269-1279
[18]Huang F P,Zhang Q,Yu Q,et al.Cryst.Growth Des., 2012,12:1890-1898
[19]Huang F P,Tian J L,Li D D,et al.CrystEngComm,2010, 12:395-400
[20]Zhang J P,Lin Y Y,Huang X C,et al.Cryst.Growth Des., 2006,6:519-523
[21]Xie X F,Chen S P,Gao S L,et al.Polyhedron,2009,28: 679-688
[22]Wen G L,Wang Y Y,Wang H.J.Mol.Struct.,2009,928: 125-131
[23]Lin J B,Zhang J P,Zhang W X,et al.Inorg.Chem.,2009, 48:6652-6660
[24]Huang F P,Bian H D,Yu Q,et al.CrystEngComm,2011, 13:6538-6548
[25]Huang F P,Tian J L,Yu Q,et al.Polyhedron,2012,34: 129-135
[26]Liu X Y,Su,Z Y,Ji W X,et al.J.Phys.Chem.C,2014, 118:23487-23498
[27]Li B,Chen S P,Yang Q,et al.Polyhedron,2011,30:1213-1218
[28]Sheu C F,Shih,C H,Sugimoto K,et al.Chem.Commun., 2012,48:5715-5717
[29]WANG Jun-Mei(王俊梅),LI Bing(李冰),SUN Lin(孙琳),et al.Chinese J.Inorg.Chem.(无机化学学报),2014,30(3): 683-688
[30]Kobrsi I,Zheng W J,Knox J E,et al.Inorg.Chem.,2006, 45:8700-8710
[31]Zhai Q G,Hu M C,Wang Y,et al.Inorg.Chem.Commun., 2009,12:286-289
[32]Su Z Y,Liu X Y,Yang Q,et al.CrystEngComm,2014,16: 4245-4253
[33]Dreizler R M,Gross E U K.Density Functional Theory. Heidelberg,Germany:Spinger-Verlag,1990.
[34]Frisch M J,Trucks G W,Schlegel H B,et al.Gaussian 03, Revision B.05,Gaussian,Inc.,Pittsburgh,PA,2003.
[35]Becke A D.J.Chem.Phys.,1993,98:5648-5652
[36]Lee C,Yang W,Parr R G,et al.Phys.Rev.B,1988,37: 785-789
[37]Browne E.Aust.J.Chem.,1975,28(11):2543-2546
[38]Sheldrick G M.SADABS,An Empirical Absorption Correction Program,Bruker Analytical X-ray Systems,Madison,WI, 1996.
[39]Sheldrick G M.SHELXL97,Program for Crystal Structure Determination,University of Göttingen,Göttingen,Germany, 1997.
[40]Sheldrick G M.SHELXL97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Germany, 1997.
SUN LinLIU Huai-XianZHOU Hui-LiangLIU Xiang-Yu*SONG Wei-MingLI Bing*HU Qi-Lin
(School of Chemistry and Chemical Engineering,Ningxia University,Yinchuan 750021,China)
Based on the versatile ligand 1H-3-(3-pyridyl)-5-(3′-pyridyl)-1,2,4-triazole(3,3′-Hbpt,(1),a coordination polymer,[Co(3,3′-Hbpt)2(H2O)4]·(ad)·6H2O(2),has been hydrothermally isolated(ad=adipate).The crystal structure of ligand has been investigated.Density functional theory(DFT)is employed to explicate the optimized geometry,preferred conformation and electronic properties of 3,3′-Hbpt ligand.Structural analysis reveals that compound 2 is a zero-dimensional mononuclear molecule,and the 3D supramolecular network is constructed through hydrogen-bonding interactions,in which the rectangular channels are filled by free adipate anions.Notably,the geometry and conformation of the ligand in compound 2 are corresponding with the calculated results.In addition,thermostability of compound 2 is investigated by TG.CCDC:1028283,1; 1002548,2.
1H-3-(3-pyridyl)-5-(3′-pyridyl)-1,2,4-triazole;theoreticalcalculation;coordinationcompound;crystalstructure; thermostability
O614.81+2
A
1001-4861(2015)06-1207-08
10.11862/CJIC.2015.172
2015-01-24。收修改稿日期:2015-04-13。
国家自然科学基金(No.21463020、21263019)和宁夏自然科学基金(No.NZ13029)资助项目。
*通讯联系人。E-mail:xiangyuliu432@126.com;nxdaxue@126.com