Synthesis,crystal structure and optical properties of a novel carbazolyl carboxylic acid ligand
2015-12-05SUNWanZHOUZhihuiXUGuodongZHANGYangLIUWenmoLIShengliWUJieyingTIANYupeng
SUN Wan,ZHOU Zhi-hui ,XU Guo-dong,ZHANG Yang,LIU Wen-mo,LI Sheng-li * ,WU Jie-ying,TIAN Yu-peng,2,3
(1.College of Chemistry and Chemical Engineering,Anhui Province Key Laboratory of Functional Inorganic Materials,Anhui University,Hefei 230039,China;2.State Key Laboratory of Crystal Materials,Shandong University,Jinan 250100,China;3.State Key Laboratory of Coordination Chemistry,Nanjing University,Nanjing 210093,China)
0 Introduction
It has been noticed that the carbazole derivatives have good planarity,super photoelectric effect and electron-transporting capabilities[1]. They show excellent aggregative state luminescence properties and high fluorescent quantum yields owing to the effective radiative decay of their excited intramolecular charge-transfer (ICT)state[2].The particular optical properties for the carbazole carboxylic acid ligand have been investigated with much effort.
Recently,the carbazole derivatives were successfully synthesized for dye-sensitized solar cells[3],highly efficient blue phosphorescent OLEDs[4]and high-efficiency blue-violet electroluminescence[5].These researches have a lot of significance.In consideration of the composite advantages,a new donor-π-acceptor(D-π-A)type ligand (LCOOH)has been designed and prepared(Scheme 1).The carboxyl was used as an electron acceptor and carbazole as an electron donor which has good planarity,electron donating ability,strong fluorescence and optical stability.The D-π-A molecular structure was formed by a conjugatedπbridge and the push-pull electronic effect.In the carbazole component,the modified group(alkyl chain)is introduced in order to improve the lipid solubility of the ligand.The novel photoelectric properties have been studied.
By the experiments and determinations,the title ligand shows excellent luminescence property because the molecules formed the state of aggregation in solution.The intermolecular forces fix the flexible single bond,preventing the way of nonradiative jump and enhancing the molecular luminescence.In addition,the quantum yield is 10.5%and the TPA cross-section(s)is 458.4GM in DMF solution,which has unique advantages such as reduced specimen photo damage,enhanced penetration depth and high spatial resolution.It may be used in 3-D (three-dimensional)fluorescence imaging and photodynamic therapy[6-8],3-D microfabrication[9],photochemical delivery of biological messenger[10],optical data storage[11-12],optical limiting[13]and confocal microscopy[14-16].
1 Experimental
1.1 Materials and instruments
Carbazole,1-bromohexane and phosphorous oxychloride were purchased from Shanghai chemical reagent Co.LTD.Piperidine,malonic acid were purchased from Shanghai Aladdin reagent company.Other solvents were purchased from Sinopharm.All the chemicals and solvents used were analytically pure and used without further purification.The analyses(C,H and N)were made on Perkin Elmer 240Belemental analyzer,1H-NMR and13C-NMR spectroscopic measurements were carried out on a Bruker AM-300NMR spectrometer,the solid infrared spectra(IR)were obtained from a NEXUS-870 vacuum-type FT-IR spectrophotometer by using KBr pellets. Single-crystal X-ray diffraction measurement was carried out on a Bruker Smart 1000CCD diffractometer equipped with a graphite monochromator situated in the incident beam for data collection at room temperature.The determination of unit cell parameters and data collection were performed with MoKαradiation(λ=0.710 73Å).The UV absorption spectra were recorded on a model UV-3600spectrophotometer.Fluorescence measurements were performed on a Model 970CRT spectrofluorimeter.Thermogravimetric analysis (TGA)was carried with a Perkin Elmer pyres-1DMDA-V1thermal analyzer in the range of 20-900℃under nitrogen atmosphere.Z-scan measurement was performed by a femtosecond laser pulse and Ti:95sapphire system(680-1080nm,80MHz,140fs).The laser beam was focused into a 1.0mm thick quartz cell,in which the sample was placed.
1.2 Synthetic route of LCOOH
LCOOH was prepared according to the following method presented in Scheme 1.
Carbazole single aldehyde(1.6g,5mmol)and malonic acid(1.04g,10mmol)were dissolved in pyridine with addition of 0.1mL piperidine,respectively.The mixture was refluxed for 3h,traced by TLC and then column chromatography(silica,petroleum ether:ethyl acetate(V/V)=5∶1),and finally 1.2g white solid were obtained.Yield:37%.0.1g LCOOH was dissolved in 30mL methanol,filtered to 50mL volumetric flask,naturally evaporated for a week,and then the white rectangle crystals formed.Mp.=152℃.Anal.Calcd.for C21H23NO2:C 78.47,H 7.21,N 4.36;Found:C 78.45,H 7.28,N 4.33(%).1H-NMR (400MHz,CD3COCD3):δ=0.828-0.863(t,3H),1.229-1.448(m,6H),1.858-1.930(m,2H),4.447-4.482(t,2H),6.550-6.590(d,1H),7.247-7.284(t,1H),7.485-7.523(t,1H),7.605-7.643(t,2H),7.804-7.825(d,1H),7.877-7.916(d,1H),8.233-8.252(t,1H),8.515(s,1H),10.539(s,1H).13C NMR(CD3COCD3):δ=18.538,27.522,31.758,36.591,47.930,114.660,114.711,120.013,124.656,125.687,126.214,127.918,128.382,130.760,131.698,131.425,146.224,146.941,151.184,172.577.IR(KBr,cm-1):2 923(s),2 683(w),2 581(m),1 674(s),1 611(s),1 590(s),1 480(m),1 307(s),1 277(s),1 201(m),1 134(m),973(m),847(w),800(m),746(m).
2 Results and discussion
2.1 Crystal structure
The X-ray diffraction measurement was performed on a Bruker SMART CCD area detector using graphite monochromated Mo-Kradiation(λ=0.710 73Å)at 298(2)K.Intensity data were collected in the variableω-scan mode.The structure was solved by direct methods and difference fourier syntheses.The non-hydrogen atoms were refined anisotropically and hydrogen atoms were introduced geometrically.Calculations were performed with SHELXTL-97program package.Details of the crystal parameters,data collections and refinements were listed in Tab.1 .Selected bond distances and angles were given in Tab.2 .The molecular structure of LCOOH and the infinite one-dimensional linear chain structure in the crystal were given in Fig.1and Fig.2,respectively.CCDC:992361.
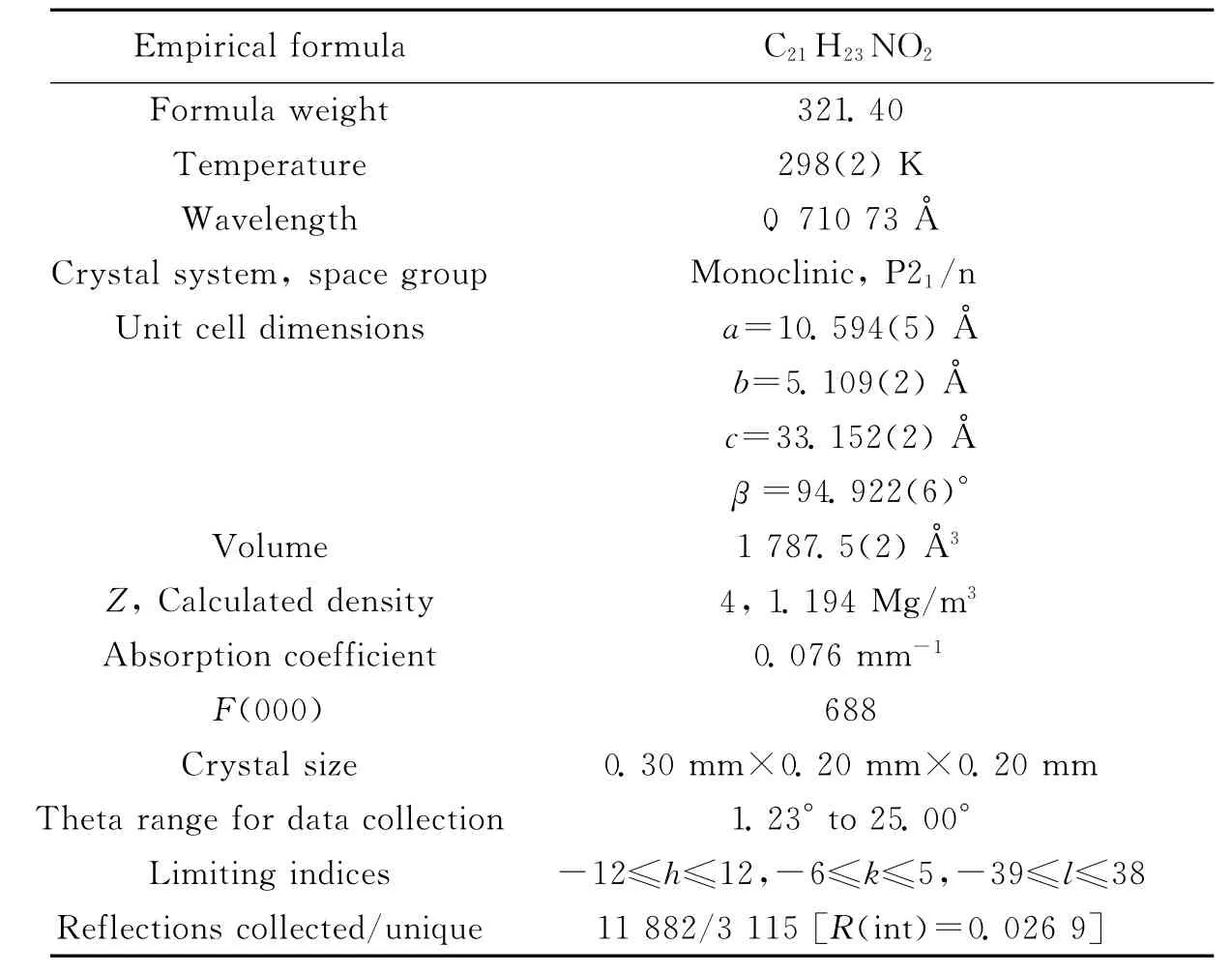
Tab.1 Crystal data and structure refinements for LCOOH
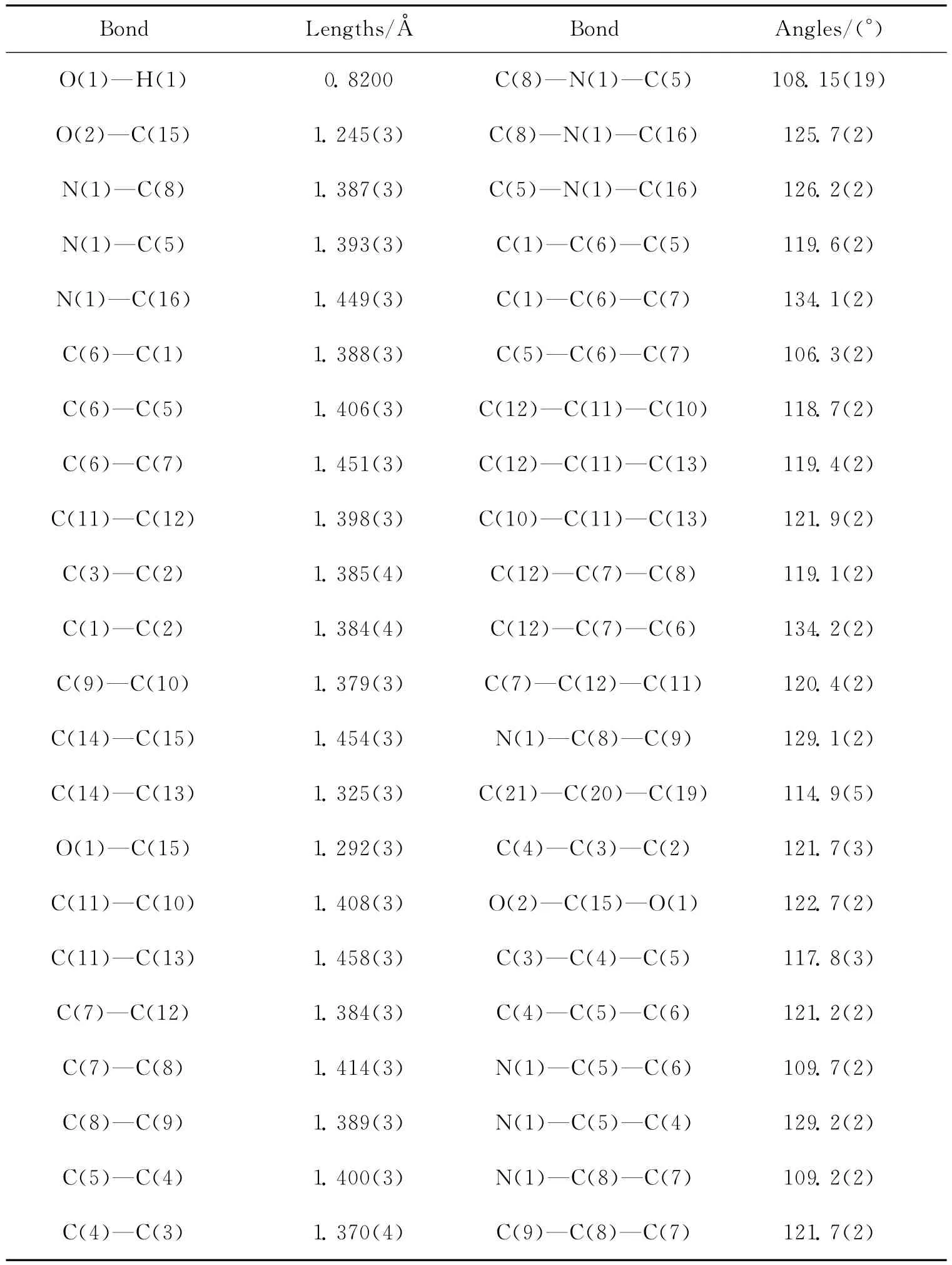
Tab.2 Selected bond lengths and angles of LCOOH
2.2 Linear absorption and time-dependent density functional theory(TD-DFT)
The linear absorption spectra of LCOOH with the concentration of 1.0×10-5mol·L-1in several solvents are shown in Fig.3.In the spectra,the absorptions mainly locate in the range of 270-350nm.An intense absorption at 299nm originates fromπ→π*transition of the carbazole moiety.The low-energy band at 335nm is assigned to the intramolecular charge transfer (ICT)transition.From Tab.3 ,the maximum absorption peaks for the compound showed slight solvatochromism with increasing solvent polarity(benzene,ethyl acetate,DMF).The peaks at about 299nm,which can be attributed to theπ→π*transition from the carbazole moiety,are less affected by solvent polarity.This means the polarity of the solvents has little effect on the ground state and excited state of the carbazole group.
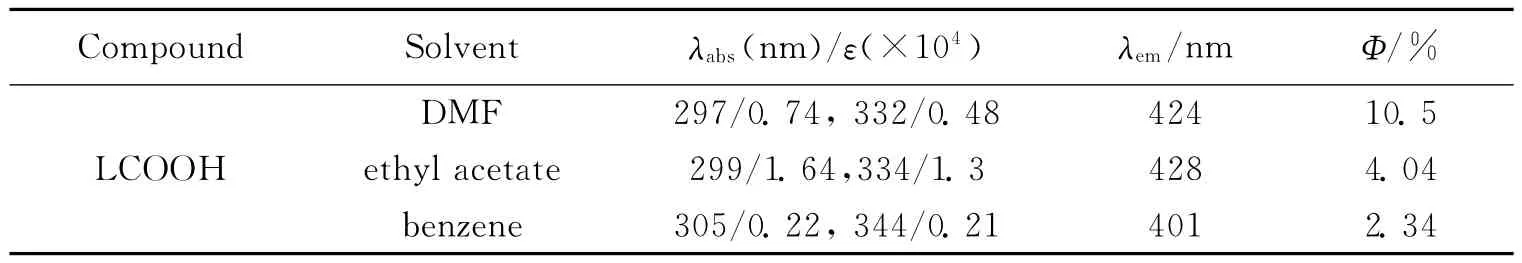
Tab.3 Single-photon-related photophysical properties of LCOOH
Based on TD-DFT,the calculated frontier orbitals of LCOOH was shown in Fig.4and Tab.4 ,respectively.The lowest energy band is calculated at 343.3nm which originates from HOMO→LUMO and can be assigned to ICT transition process.The highest energy band is calculated at 298.5 nm corresponding to HOMO→LUMO+1,which is attributed toπ→π*transition of the ligand.It is observed that the results from the experiments and theoretical calculations are well consistent.
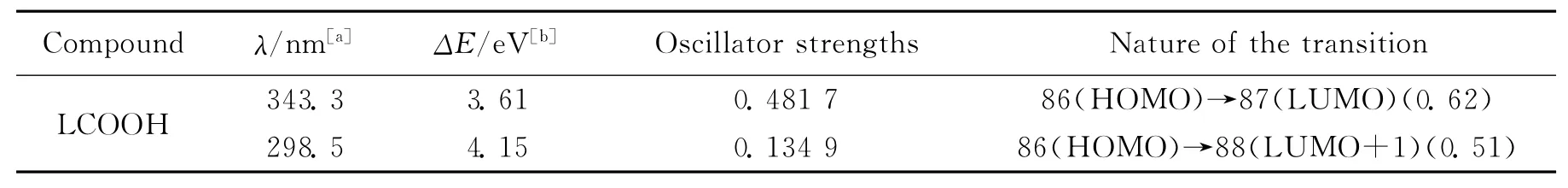
Tab.4 Calculated linear absorption,excitation energy,oscillator strengths and major contribution for LCOOH
2.3 Single-photon excited fluorescence(SPEF)
One-photon excited fluorescence spectra for the compound are summarized in Tab.3 ,including fluorescence quantum yields. With the solvent polarity increasing,the single-photon excited fluorescence(SPEF)spectra show remarkable bathochromic shifts(see Fig.5).Large Stokes shift are observed in three solvents due to the strong solvent-solute dipole-dipole interaction and a manifestation of the large dipole moment.In the ground state,it exhibits a significant dipole moment because of charge separation in carbazole,depending on the electron donating and withdrawing groups.In the excited state,it is likely that the charge separation increases,resulting in a larger dipole moment than that in the ground state,which favorably explains the sensitivity to solvent polarity for the emission spectra of the dipolar compounds[17].In addition,LCOOH has excellent luminescence property of typical AIE (aggregation-induced emission)character due to the formation of aggregates which inhibit the intramolecular rotations to some extent(see Fig.6).
2.4 Nonlinear property
The nonlinear property of the compound was measured by using the open apertureZ-scan technique under a femtosecond laser pulse and Ti:95sapphire system(680-1 080nm,80MHz,140 fs).The experimental spectrum was shown in Fig.7,measured at 720nm for LCOOH in ethyl acetate at a concentration of 1.0.× 10-3mol· L-1.The theoretical data was fitted using the following equations[18-19]whereq0(z)=βI0Leff/(1+x2),x=z/z0,z0=πω02/λ,βis the two-photon absorption (TPA)coefficient of the solution,I0is the intensity of laser beam at focus(z=0),Leff=[1-exp(-α0L)]/α0is the effective length withα0the linear absorption coefficient,Lis the sample length,z0=πω02/λis the diffraction length of the beam withω0the spot size at focus,λthe wavelength of the beam and z is the sample position.Based on Eq.(1),the molecular TPA coefficientβcan be deduced.Furthermore, the TPA cross-sectionσof the sample is determinated by using the following relationship
herehis the Planck constant,νis the frequency of incident laser,NAis the Avogadro number,andd0is the sample concentration.According to Eq.(2),the TPA cross-sectionσcan be got.
Fig.7 shows the normalized transmittance plotted as a function of the sample position.The openaperture transmittance is symmetric with respect to the focus(z=0)where a minimum transmittance locates,indicating the obvious absorption for LCOOH.The single-photon absorption of LCOOH in ethyl acetate is located less than 400nm,and there are no linear absorption at 720nm.Therefore,it can be deduced that the absorption at focus is attributed to the TPA nonlinear effect[20]under the measuring wavelength.
The dataβ(absorption coefficient)andσ(cross section)for LCOOH are calculated and given in Tab.5 .The TPA cross-section is 458.38GM.

Tab.5 Two-photon absorption coefficientβ and cross sectionσ for L
2.5 Thermogravimetric analysis(TGA)and solubility
The thermal stability for LCOOH was evaluated by TGA determination.The compound was heated from 20to 900℃at an increasing rate of 10℃·min-1in a nitrogen atmosphere.It starts to lose weight at ca.210℃,then decompose gradually,and finally leave few residues near to 490℃.The solubility of the ligand was tested to show that,it is soluble in dichloromethane,DMF,THF and ethyl acetate,slightly soluble in acetonitrile and ethyl alcohol,insoluble in petroleum ether.
2.6 Electrochemical property
In order to study the electrochemical property of the ligand,cyclic voltammetry(CV)determination was carried out in the presence of Bu4NClO4(TBAP,0.1M)as a supporting electrolyte with a scan rate of 50mV·s-1and a scanning range of-2.0-+2.0V.Ag wire was used as working electrode,Ag/AgCl and platinum wire acted as a reference and counter electrode,respectively.In Fig.8,the oxidation-reduction potential of LCOOH was showed in the CV response.Furthermore,Tab.6 shows the electrochemical properties of the compound.Comparison of the anodic and cathodic currents(ipaandipc)results inipa/ipc≈1,suggesting that the oxidation-reduction process is well-behaved electrochemically reversible.TheE1/2value(half-wave potential,E1/2=(Epa+Epc)/2)indicates that the compound has a high electron delocalized structure and more likely to lose electrons.

Tab.6 Electrochemical properties of LCOOH
3 Conclusions
In this article,the carbazole carboxylic acid (LCOOH)has been effectively synthesized and characterized by the routine methods.The measurements demonstrate that the ligand has excellent aggregative state luminescence,high quantum yield and photo-stability of single-photon fluorescence.The results based on TD-DFT theoretical calculations are well consistent with that from the linear absorption experiments.Moreover,the nonlinear optical property was determinated byZ-scan technique.The result shows the ligand has a large two-photon absorption cross section,favorably providing a new idea and pathway to design and synthesize novel two-photon optical materials.
[1]Tao Y T,Wang Q,Yang C L,et al.Tuning the optoelectronic properties of carbazole/oxadiazole hybrids through linkage modes:hosts for highly efficient green electrophosphorescence[J].Adv Funct Mater,2010,20(2):304-311.
[2]Nakagawa T,Ku S Y,Wong K T,et al.Electroluminescence based on thermally activated delayed fluorescence generated by a spirobifluorene donor-acceptor structure[J].Chem Commun,2012,48(77):9580-9582.
[3]Tan L L,Huang J F,Shen Y,et al.Highly efficient and stable organic sensitizers with duplex starburst triphenylamine and carbazole donors for liquid and quasi-solid-state dyesensitized solar cells[J].J Mater Chem A,2014,2(24):8988-8994.
[4]Zheng C J,Ye J,Lo M F,et al.New ambipolar hosts based on carbazole and 4,5-diazafluorene units for highly efficient blue phosphorescent OLEDs with low efficiency roll-off[J].Chem Mater,2012,24(4):643-650.
[5]Ye J,Chen Z,Fung M K,et al.Carbazole/sulfone hybrid D-π-A-structured bipolar fluorophores for highefficiency blue-violet electroluminescence[J].Chem Mater,2013,25(13):2630-2637.
[6]Ji S M,Wu W H,Wu W T,et al.Ruthenium(II)polyimine complexes with a long-lived3IL excited state or a3MLCT/3IL equilibrium:efficient triplet sensitizers for low-power upconversion[J].Angew Chem Int Edit,2011,50(7):1626-1629.
[7]Wang X H,Nguyen D M,Yanez C O,et al.High-fidelity hydrophilic probe for two-photon fluorescence lysosomal imaging[J].J Am Chem Soc,2010,132(35):12237-12239.
[8]Horton N G,Wang K,Kobat D,et al.In vivo three-photon microscopy of subcortical structures within an intact mouse brain[J].Photonics,2013,7(3):205-209.
[9]Daicho Y,Murakami T,Hagiwara T,et al.Formation of three-dimensional carbon microstructures via twophoton microfabrication and microtransfer molding[J].Optical Materials Express,2013,3(6):875-883.
[10]Li L,Zhang C W,Chen Grace Y J,et al.A sensitive two-photon probe to selectively detect monoamine oxidase B activity in Parkinson,s disease models[J].Nature Communications,2014,5:3276-3286.
[11]Dvornikov A S,Walker E P,Rentzepis P M.Two-photon three-dimensional optical storage memory[J].J Phys Chem A,2009,113(49):13633-13644.
[12]Iliopoulos K,Krupka O,Gindre D,et al.Reversible two-photon optical data storage in coumarin-based copolymers[J].J Am Chem Soc,2010,132(41):14343-14345.
[13]Chantharasupawong P,Philip R,Narayanan N T,et al.Optical power limiting in fluorinated graphene oxide:an insight into the nonlinear optical properties[J].J Phys Chem C,2012,116(49):25955-25961.
[14]Baek N Y,Heo C H,Lim C S.A highly sensitive two-photon fluorescent probe for mitochondrial zinc ions in living tissue[J].Chem Commun,2012,48(38):4546-4548.
[15]Masanta G,Heo C H,Lim S C,et al.A mitochondria-localized two-photon fluorescent probe for ratiometric imaging of hydrogen peroxide in live tissue[J].Chem Commun,2012,48(29):3518-3520.
[16]Yao S,Belfield K D.Two-photon fluorescent probes for bioimaging[J].Eur J Org Chem,2012(17):3199-3217.
[17]Zhou H P,Zheng Z,Xu G Y,et al.1,3,5-Triazine-cored derivatives dyes containing triphenylamine based twophoton absorption:Synthesis,optical characterization and bioimaging[J].Dyes and Pigments,2012,94(3):570-582.
[18]Li D M,Zhang Q,Wang P,et al.Studies of the isomerization and photophysical properties of a novel 2,2′:6′,2′′-terpyridine-based ligand and its complexes[J].Dalton Trans,2011,40(32):8170-8178.
[19]Geethakrishnan T,Palanisamy P K.Z-scan determination of the third-order optical nonlinearity of a triphenylmethane dye using 633nm He-Ne laser[J].Opt Commun,2007,270:424-428.
[20]Li S L,Wu J Y,Tian Y P,et al.Preparation,characterization,two-photon absorption and optical limiting properties of a novel metal complex containing carbazole[J].Optical Materials,2006,28:897-903.
