通过1,3-偶极环加成反应合成3-吡咯螺环氧化吲哚的研究进展
2015-12-02张文会彭礼军黄俊飞刘雄利余章彪
周 英,张文会,张 敏,彭礼军,黄俊飞,杨 超,刘雄利,余章彪
(贵州大学药学院贵州省中药民族药创制工程中心,贵州 贵阳 550025)
1 引言
由于吡咯螺环氧化吲哚类化合物具有明确或潜在的生物和药物活性,近年来已被大家广泛关注[1]。例如,spirotryprostatin A(1)[2],pteropodine(2)[3],alstonisine(3)[4],elacomine(4)[5],horsfiline(5)[6],formosanine(6)[7],rychnophylline(7)[8]等都是经典的含具有吡咯螺环氧化吲哚骨架的天然生物碱(见图1)。同时,对这些化合物进行药理研究发现具有显着的生物和医药活性。并且,各种含天然吡咯螺环氧化吲哚骨架类化合物及其衍生物也已经被人工通过新颖有效的方法大量合成。这里,我们主要综述近年来以α-氨基酸和羰基化合物为原料原位产生亚胺叶立德,再和缺电子烯烃发生[3+2]1,3-偶极环加成反应合成各种3-吡咯螺环氧化吲哚的合成方法。

图1 具有吡咯螺环氧化吲哚骨架的代表性天然生物碱Fig.1 Typical alkaloids with pyrrolo spirooxindole skeleton
2 研究方法
通常,亚胺叶立德和吸电子烯烃的1,3-偶极环加成反应是合成吡咯环最常用的方法。而α-氨基酸和靛红通过热力学原位去羰基化产生亚胺叶立德,和缺电子烯烃发生的1,3-偶极环加成反应则是合成吡咯螺环氧化吲哚骨架的一种方法(路线 A,图表2)[9-16]。实际上,通过路线 A 合成吡咯螺环氧化吲哚化合物是目前报道最多的方法。靛红衍生的Knoevenagel缩合烯烃,通过和α-氨基酸、醛原位去羰基化产生的亚胺叶立德发生1,3-偶极环加成是合成吡咯螺环氧化吲哚化合物的另一种有效方法(路径B,图表2)。

图2 吡咯螺环氧化吲哚化合物的合成路线A和路线B图Fig.2 Synthetic pathway A and B of pyrrolo spirooxindoles
无环α-氨基酸如肌氨酸[17]或环状α-氨基酸如脯氨酸[18]、噻唑 -4- 羧酸[19]、八氢 -1H- 吲哚 -2- 羧酸[20]、1,2,3,4- 四氢异喹啉 -3- 羧酸[21]等可以用作生成亚胺叶立德的原料。此外,分别以无环缺电子烯烃和环外缺电子烯烃作为亲偶极体,也可以得到螺环和双螺环结构。通常,该环加成反应有高度的区域选择性和立体选择性,这种选择性的原因可以用B3LYP/6-31G密度泛函理论和半经验AM计算规则阐明[22]。
2007年,Scheidt课题组[23]以一价金属铜盐为催化剂,催化亚胺、重氮化合物和靛红衍生的亲偶极体的三组份1,3-偶极环加成反应(图3)。该反应能提供四个手性中心相连高官能团化的单一异构体产物吡咯螺环氧化吲哚(8)。该产物的2,5位是反式结构,源于反应过中的E-环外过渡态产生。

图3 构建四个手性中心相连高官能团化的单一异构体吡咯螺环氧化吲哚Fig.3 Construction of single isomer of pyrrolo spirooxindole connected by 4 chiral centers
2008年,Li课题组[24]按照合成路线A报道了第一个四组份参与的1,3-偶极环加成反应合成了双螺环吡咯氧化吲哚(9)。该反应采用1,3-茚二酮和芳基醛原位产生Knoevenagel缩合烯烃,同时和肌氨酸与靛红原位产生的亚胺叶立德进行反应,具有高效,操作简单等优越性。类似地,3-腈乙酰基-吲哚也能和芳基醛原位产生Knoevenagel缩合烯烃,得到相应的螺环吡咯氧化吲哚(10)(图4)[25]。
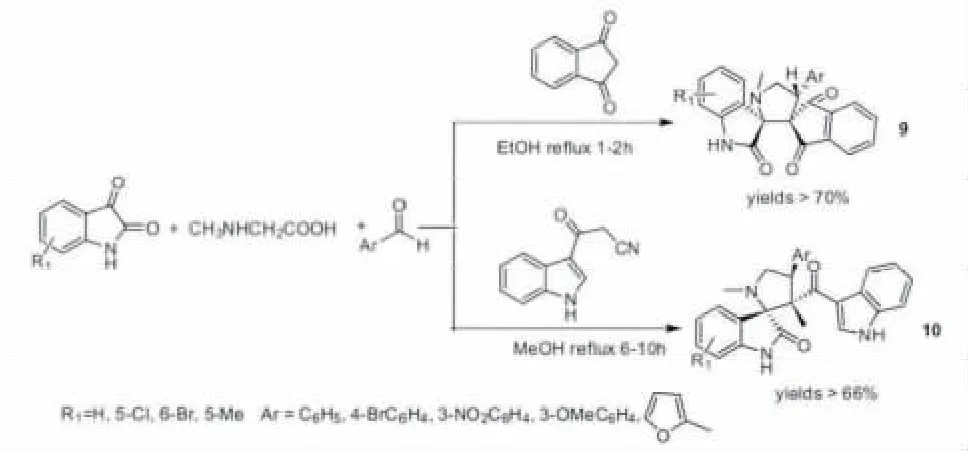
图4 四组份参与的1,3-偶极环加成反应构建螺环吡咯氧化吲哚Fig.4 Pyrrolo spirooxindoles synthesis via 1,3-dipolar cycloaddition
2009 年,Taheri等[26]报道了以芳基醛和二级α-氨基酸如肌氨酸生成亚胺叶立德,和靛红衍生的Knoevenagel缩合烯烃发生环加成反应,生成了低区域选择性的1∶1异构体(11,12)的吡咯螺环氧化吲哚(图5)。如果用脯氨酸做底物,也得到类似的结果。
2010年,Perumal课题组报道了合成一系列包含三环或四环吡咯稠合苯并2,3-二氢噻吩-1,1-二氧化物的螺环氧化吲哚(13,14)。该反应以靛红、苯并噻吩-1,1-二氧化物、肌氨酸或脯氨酸为原料,在甲苯回流条件下发生区域选择性的多组份1,3-偶极环加成,通过使用环内烯烃作为亲偶极体,以一步反应有效得到包含螺环和稠环复杂拼接产物(图 6)[27]。

图5 构建低区域选择性异构体的吡咯螺环氧化吲哚Fig.5 Construction of isomers of pyrrolo spirooxindoles with low regioselectivity
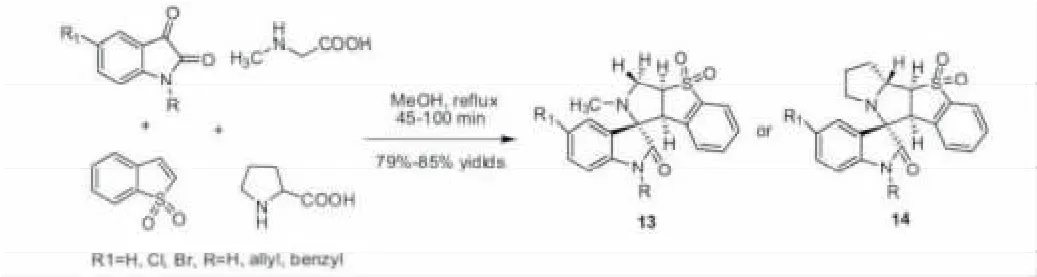
图6 构建包含三环或四环吡咯稠合苯并2,3-二氢噻吩-1,1-二氧化物的螺环氧化吲哚Fig.6 Constraction of spirooxindoles
2006-2010年,Sriram等课题组以苯基甘氨酸、肌氨酸或脯氨酸和靛红作为起始原料原位产生亚胺叶立德[28],同时与3,5-二烯哌啶 -4-酮发生1,3-偶极环加成反应高产率合成了具有抗微生物活性的螺环吡咯氧化吲哚哌啶-4-酮(15-17)。该反应底物亲偶极体虽然有两个α,β-不饱和烯键,由于双加成产物会产生很强的立体位阻作用,所以仅仅一个单螺环加成产物产生[29-31]。有意思的是,单加成产物中的α,β-不饱和烯键能进一步与腈氧化产物发生第二次环加成生产三螺环产物(18)(图 7)[32]。

图7 构建具有抗微生物活性的螺环吡咯氧化吲哚哌啶-4-酮Fig.7 Construction of pyrrolo spirooxindole piperidin-4-one with antibacterial activity
2012年,Farag课题组[33]报道了以肌氨酸和靛红作为起始原料原位产生亚胺叶立德,同时与马内酰胺发生1,3-偶极环加成反应高产率合成了螺环吡咯氧化吲哚哌啶-4-酮(19)。经检测,所有的化合物都对人肿瘤细胞株HCT116,MCF-7和HepG2表现了中等至很好的抑制活性。其中化合物(R1=4-MeC6H4,R2=OMe)对人肝癌细胞株HepG2 IC50为12.16 μM,活性接近于阳性对照药阿霉素(IC50=7.36 μM)(图 8)。

图8 构建具有抗肿瘤活性的螺环吡咯氧化吲哚Fig.8 Construction of pyrrolo spirooxindoles with antitumor activity
2013 年,Perumal课题组[34]报道了以肌氨酸和靛红作为起始原料原位产生亚胺叶立德,同时与亲偶极体含双吲哚结构的Knoevenagel缩合烯烃发生环加成反应,生成含三吲哚三季碳相连的双吡咯螺环氧化吲哚(20)。这系列化合物都表现了重要的抗微生物活性。其中4个化合物(R1=H,R2=Me;R1=H,R2=C6H3;R1=H,R2=CH2CCH;R1=Me,R2=5-NO2)还对A549人肺腺癌细胞株表现了非常好的抑制活性(图9)。

图9 构建具有生物活性的含三吲哚三季碳相连的双吡咯螺环氧化吲哚Fig.9 Construction of dipyrrolo spirooxindoles with biological activity
2014年,我们课题组[35]报道了以多聚甲醛和肌氨酸生成亚胺叶立德,和靛红衍生的Knoevenagel缩合烯烃发生1,3-偶极环加成反应,得到了相应的吡咯螺环氧化吲哚(21)。该反应通过在氮原子上采用吸电子基团如叔丁氧羰基保护,解决了底物3-烯键亲偶极体活性不足的问题,最高可达85%的产率和大于99∶1的非对映选择性(图10)。

图10 构建具有抗微生物活性的螺环吡咯氧化吲哚哌啶-4-酮Fig.10 Construction of pyrrolo spirooxindole piperidin-4-one with antibacterial activity
此外,1,3-偶极环加成反应亲偶极体可以扩展到其他吸电子基烯烃,如Baylis-Hillman加合物[36-40],二茂铁基 α,β -不饱和酮[41-43],16- 亚芳基雌酮衍生物[44],穿心莲内酯[45]等。并且1,3-偶极[3+2]环加成反应也可以通过超声辐照提高产量和缩短反应时间[46-48]。
3 结论与展望
本文对通过原位产生亚胺叶立德参与1,3-偶极环加成反应合成3-吡咯螺环氧化吲哚的研究进行了较全面的综述。在氧化吲哚3位成功构建了季碳吡咯螺环中心,以较高产率和非对映选择性获得了多官能团的氧化吲哚衍生物,这为进一步设计合成生理活性物质奠定了基础。我们相信,这类反应会在有机合成界,特别是天然产物的合成中起到重要的促进作用;这类反应产生的具有3-吡咯螺环氧化吲哚骨架的化合物会在今后的药物设计和研发中占据重要地位。
[1]Da Silva J F,M Garden,S J Pinto.The Chemistry of Isatins:a Review from 1975 to 1999 [J].J.Braz Chem Soc,2001,12:273.
[2]Sannigrahi M.Stereocontrolled synthesis of spirocyclics[J].Tetrahedron,1999,55:9 007.
[3]Kang T H,Matsumoto K,Tohda M,Murakami,Y Takayama,H Kitajima,M Aimi,Watanabe H.Pteropodine and isopteropodine positively modulate the function of rat muscarinic M1 and 5-HT2 receptors expressed in Xenopusoocyte [J].Eur J Pharmacol,2002,444:39.
[4]Garnick R L,LeQuesne P.W.Biomimetic transformations among monomeric macroline-related indole alkaloids[J].J Am Chem Soc,1978,100:4 213.
[5]James M N G,Williams G J B.The Molecular and Crystal Structure of an Oxindole Alkaloid(6-Hydroxy-2'-(2-methylpropyl)-3,3'-spirotetrahydropyrrolidino-oxindole)[J].Can J Chem,1972,50:2 407.
[6]Jossang A,Jossang P,Hadi H A,Sevenet T.Horsfiline,an oxindole alkaloid from Horsfieldia superba[J].J Org Chem,1991,56:6 527.
[7]Marti C,Carreira E M.Construction of Spiro[pyrrolidine-3,3'-oxindoles]Recent Applications to the Synthesis of Oxindole Alkaloids[J].Eur J Org Chem,2003:2 209.
[8]Angenot L.Plant Med Phytother[M]London:1978.
[9]Kumar R R,Perumal S,Senthilkumar P,Yogeeswari P,Sriram D.A facile synthesis and antimycobacterial evaluation of novel spiro-pyrido-pyrrolizines and pyrrolidines[J].Eur J Med Chem,2009,44:3 821.
[10]Li X F,Zheng A T,Liu B,Yu X Y.Synthesis of Novel Spiro Pyrrolidine and Pyrrolizine Derivatives by 1,3-Dipolar Cycloaddition[J].Chin J Chem,2010,28:1 207.
[11]Liu,H,Dou D L,Shi D Q.Regioselective synthesis of novel spiropyrrolidines and spirothiapyrrolizidines through multicomponent 1,3-dipolar cycloaddition reaction of azomethine ylides[J].J Comb Chem,2010,12:633.
[12]Karthikeyan K,Sivakumar P M,Doble M,Perumal P T.Synthesis,antibacterial activity evaluation and QSAR studies of novel dispiropyrrolidines[J].Eur J Med Chem,2010,45:3 446.
[13]Ghandi M,Taheri A,Abbasi A.A facile synthesis of chromeno[3,4-c]spiropyrrolidine-oxindoles via 1,3-dipolar cycloadditions[J].Tetrahedron,2010,66:6 744.
[14]Lakshmi N V,Thirumurugan P,Perumal P T.An expedient approach for the synthesis of dispiropyrrolidine bisoxindoles,spiropyrrolidine oxindoles and spiroindane-1,3-diones through 1,3-dipolar cycloaddition reactions[J].Tetrahedron Lett,2010,51:1 064.
[15]Li X F,Yu X Y,Feng Y Q.Regio-and Stereo-selective Synthesis of Novel Spirooxindole Compounds[J].Chin J Org Chem,2009,29:1 129.
[16]Thangamani A.Regiospecific synthesis and biological evaluation of spirooxindolo-pyrrolizidines via[3+2]cycloaddition of azomethine ylide[J].Eur J Med Chem,2010,45:6 120.
[17]Poornachandran M,Muruganantham R,Raghunathan R.Synthetic Communications:An International Journal for Rapid Communication of Synthetic Organic Chemistry[J].Synth Commun,2006,36:141.
[18]Poornachandran M,Raghunathan R.Synthetic Communications:An International Journal for Rapid Communication of Synthetic Organic Chemistry[J].Synth Commun,2007,37:2 507.
[19]Maheswari S U,Balamuragan K,Perumal S,Yogeeswari P.A facile 1,3-dipolar cycloaddition of azomethine ylides to 2-arylidene-1,3-indanediones:Synthesis of dispiro-oxindolylpyrrolothiazoles and their antimycobacterial evaluation [J].Bioorg Med Chem Lett,2010,20:7 278.
[20]Murugan R,Raghunathan R,Narayanan S S.Synthetic Communications:An International Journal for Rapid Communication of Synthetic Organic Chemistry[J].Synth Commun,2010,40:3 135.
[21]Surash Babu A R,Raghunathan R.TiO2– silica mediated one pot three component 1,3-dipolar cycloaddition reaction:a facile and rapid synthesis of dispiro acenaphthenone/oxindole[indanedione/oxindole]pyrroloisoquinoline ring systems[J].Tetrahedron,2007,63:8 010.
[22]Suresh Babu A R,Raghunathan R,Gayatri G,Sastry N G.A highly regioselective synthesis of 1-n-methyl-spiro-[2,3]-oxindole-spiro-[3,2]indane-1,3-dione-4-arylpyrrolidines through 1,3-dipolar cycloaddition protocol[J].J Heterocycl Chem,2006,43:1 467.
[23]Galliford C V,Martenson J S,Stern C,Scheidt K A.A highly diastereoselective,catalytic three-component assembly reaction for the synthesis of spiropyrrolidinyloxindoles[J].Chem Commun,2007,35:631.
[24]Li M Yang,W L,Wen L R,Li F Q.A First Resource-Efficient and Highly Flexible Procedure for a Four-Component Synthesis of Dispiropyrrolidines[J].Eur J Org Chem,2008,24:2 751.
[25]Zhao K,Zhu S L,Shi D Q,Xu X P.Synthesis[M].Newyork:2010.
[26]Ghandi M,Yari A,Rezaei S J T,Taheri A.Synthesis of novel spiropyrrolidine/pyrrolizine-oxindole scaffolds through 1,3-dipolar cycloadditions[J].Tetrahedron Lett,2009,50:4 724.
[27]Lakshmi N V,Thirumurugan P,Jayakumar C,Perumal P T.An Easy Access to Novel Spiro-Fused Pyrrolo Benzo[b]thiophene 1,1-Dioxide Derivatives via 1,3-Dipolar Cycloaddition Using Benzo[b]thiophene 1,1-Dioxide [J].Synlett,2010,15:955.
[28]Kumar R R,Perumal S,Senthilkumar P.Discovery of Antimycobacterial Spiro-piperidin-4-ones:An Atom Economic,Stereoselective Synthesis,and Biological Intervention[J].J Med Chem,2008,51:5 731.
[29]Girgis A S.Regioselective synthesis and stereochemical structure of anti-tumor active dispiro[3H-indole-3,2'-pyrrolidine-3',3″-piperidine]-2(1H),4″-diones[J].Eur J Med Chem,2009,44:1 257.
[30]Li X F,Yu X Y,Yi P G.Synthesis of Novel Trispiroheterocycles through 1,3-Dipolar Cycloaddition of Azomethine Ylides and Nitrile Oxide[J].Chin J Chem,2010,28:434.
[31]Sridhar G,Raghunathan R.Synthetic Communications:An International Journal for Rapid Communication of Synthetic Organic Chemistry[J].ynth Commun,2006,36:21.
[32]Li X F,Zheng A T,Liu B,Yu X Y,Yi P G.Synthesis of trispiro[oxindole-yrrolidine]-cyclopentanone-isoxazolines by 1,3-dipolarcycloaddition[J].Heterocycl Chem,2010,47:1 157.
[33]Girgis A S,Stawinski J,Ismail N S M,Farag H.Synthesis and QSAR study of novel cytotoxic spiro[3H-indole-3,2'(1'H)-pyrrolo[3,4-c]pyrrole]-2,3',5'(1H,2'aH,4'H)-triones[J].Eur.J.Med.Chem,2012,47:312.
[34]Arun Y,Bhaskar G,Balachandran C.Facile one-pot synthesis of novel dispirooxindole-pyrrolidine derivatives and their antimicrobial and anticancer activity against A549 human lung adenocarcinoma cancer cell line [J].Bioorg.Med.Chem.Lett,2013,23:1 839.
[35]Yu Z B,Liu X L,Pan B W,Chen B.Synthetic Communications:An International Journal for Rapid Communication of Synthetic Organic Chemistry[J].Synthetic Communications,2014,44:530-539.
[36]Ramesh E,Kathiresan M,Raghunathan R.Solvent-free microwave-assisted conversion of Baylis– Hillman adducts of ninhydrin into functionalized spiropyrrolidines/pyrrolizidines through 1,3-dipolar cycloaddition [J].Tetrahedron Lett 2007,48:1 835.
[37]Karthikeyan K,Saranya N,Kalaivani A,Perumal P T.Synthesis of Spiropyrrolidines and Spiropyrrolizidines by Azomethine Ylide Cycloaddition of Baylis-Hillman Adducts Derived from N-Methyl Maleimide[J].Synlett,2010,6:2 751.
[38]Shanmugam P,Viswambharan B,Selvakumar K,Madhaven S.A facile and efficient synthesis of highly functionalised 3,3'-dispiropyrrolidine-and 3,3'-dispiropyrrolizidine bisoxindoles via[3+2]cycloaddition[J].Tetrahedron Lett,2008,49:2611.
[39]Shanmugam P,Viswambharan B,Madhaven S.Synthesis of Novel Functionalized 3-Spiropyrrolizidine and 3-Spiropyrrolidine Oxindoles from Ba ylis Hillman Adducts of Isatin and Heteroaldehydes with Azomethine Ylides via[3+2]-Cycloaddition [J].Org Lett,2007,9:4 095.
[40]Kathiravan S,Raghunathan R.A facile one-pot three-component synthesis of ferrocene-grafted dispiro pyrrolidine/pyrrolizidine scaffolds through intermolecular[3+2]cycloaddition reaction of ferrocenyl Baylis– Hillman adduct[J].Tetrahedron Lett,2009,50:6 116.
[41]Suresh Babu A.R,Raghunathan R,Baskaran S.An expedient synthesis of ferrocene grafted spirooxindolopyrrolizidines via[3+2]-cycloaddition of azomethine ylides[J].Tetrahedron,2009,65:2 239.
[42]Suresh Babu A R,Raghunathan R.Synthesis of ferrocenyl monospirooxindolopyrrolidines-a facile[3+2]-cycloaddition of azomethine ylides[J].Tetrahedron Lett,2008,49:4 487.
[43]Jayashankaran J,Durga R,Manian R S,Raghunathan R.Synthetic Communications:An International Journal for Rapid Communication of Synthetic Organic Chemistry[J].Synth Commun,2006,36:979.
[44]Suresh Babu A R,Raghunathan R.An easy access to novel steroidal dispiropyrrolidines through 1,3-dipolar cycloaddition of azomethine ylides[J].Tetrahedron Lett,2008,49:4 618.
[45]Hazra A,Paira P,Sahu K B,Naskar S,et al.Chemistry of andrographolide:formation of novel di-spiropyrrolidino and di-spiropyrrolizidino-oxindole adducts via one-pot three-component[3+2]azomethine ylide cycloaddition[J].Tetrahedron Lett,2010,51:1 585.
[46]Rezaei S J T,Nabid M R,Yari A,Ng S W.Ultrasound-promoted synthesis of novel spirooxindolo/spiroacenaphthen dicyano pyrrolidines and pyrrolizidines through regioselective azomethine ylide cycloaddition reaction[J].Ultrason Sonochem,2011,18:49.
[47]Ge S Q,Hua Y Y,Xai M.Ultrasound-promoted synthesis of novel dispirocyclic frameworks from aza-Claisen rearrangements of Baylis– Hillman amines[J].Ultrason Sonochem,2009,16:232.
[48]Azizian J,Saffar-Teluri A,Asadi A.A Facile One-Pot Synthesis of New Spiro Pyrrolidine-Oxindoles Under Ultrasonic Irradiation in DMSO-H2O[J].Lett Org Chem,2006,3:887.
