颜色可区分的氰桥五边形带经亲铜作用聚集的异金属多形体
2015-12-01秦英恋李士利姜宁张献明
秦英恋 李士利 姜宁 张献明*,
(1山西师范大学化学与材料科学学院,分子磁体与磁信息材料重点实验室,临汾041004)
(2运城学院应用化学系,运城044000)
颜色可区分的氰桥五边形带经亲铜作用聚集的异金属多形体
秦英恋1,2李士利1姜宁1张献明*,1
(1山西师范大学化学与材料科学学院,分子磁体与磁信息材料重点实验室,临汾041004)
(2运城学院应用化学系,运城044000)
水热条件下,环境友好的K2[Ni(CN)4]能缓慢水解形成cis-[Ni(CN)2(H2O)4]单元和氰基配体,并进一步与金属铜(Ⅱ)原子组装形成异金属的五边形带。这些五边形带通过亲铜聚集作用形成二维有色的超分子多形体[(CuCN)2Ni(CN)2(H2O)4](1和2)。研究发现,低温条件下形成了密集态的深蓝色的化合物1,然而高温反应条件形成疏松态的紫色化合物2,这一现象与高温高压的反应条件形成密集态的物质这一规律相违背。结构的进一步分析发现五边形环尺寸的微小改变和二维超分子层间距离的差异是引起这一反常的原因。除此之外,这两个新的化合物也是少见的由亲铜性聚集作用诱导的着色异常的多形体的例子,显示了从深蓝色到紫色的颜色改变。磁性研究证实了平面正方形的[Ni(CN)4]2-中的金属镍(Ⅱ)转换成了有着基态自旋S=1的八面体配位几何中心。
亲铜作用;多形体;异金属;五边形;聚集
0 Introduction
The attractive interactions between closed-shell d10metal ions have received considerable attention because they play an important role in the structural,optical,and electronic properties that are associated with the presence of metallophilicity effect[1].This behavior has beenfrequentlyobservedinAu,withtheterm“aurophilicity”being used to describe AuⅠ-AuⅠinteractionsthataretheoreticallyattributedto correlation and relativistic effects[2-5].The occurrence of analogous metallophilic effects has been found even in lighter Cu atoms,while most structurally characterized complexes featuring CuⅠ-CuⅠinteractions,namely,“cuprophilicity”,are associated with the other structurecontrolling factors,such as ligand bridging,H bonding, electrostatic interaction,π-π stacking,etc[6-8].It is well recognizedthatonlyafewreliablecasesof cuprophilicity have been reported and the phenomenon of cuprophilicity is usually encountered in a classic familyofoligomers,dimers,trimersandchains complexes[9-11].Theoreticalstudies reveal thatthe strength of cuprophilicity is up to 17 kJ·mol-1,indicating cuprophilicity may be used as synthons for control over supramolecular polymorphs[12].Recently,we and others have endeavored to modulate multidimensional CuCN-basedsupramolecularframeworksviacuprophilic interactions[13-14].In some rare cases,CuCN-based supramolecular isomers with cuprophilic interactions havebeenprepared.
On the other hand,chromism is a process that induces a change in the colors of compounds.In most cases,chromism is based on a change in the electron states of molecules,so this phenomenon induced by various external stimuli e.g.,solvents,applied stress, pH value,temperature,etc[15-16].Differing from the above external stimulation,supramolecular cuprophilicity can induce aggregation of various of coordination geometry of copper(Ⅱ)to give broad range of colors crystal forms in the solid state and show interesting absorption and luminescence effect related to electron modulation by cuprophilic interactions[9-11].The sensitivity ofthe electronic absorption and luminescence properties to the degree of cuprophilic interactions may offer an attractive option for the exploitation of color change of different crystal forms in the solid state[17-18].To date, althoughthecuprophilicity-inducedCuCN-based monometallic polymorphic phenomena are well known, most systems are confined to studies of their structures and crystal packings in the solid state,with corresponding studies on crystal color-varied phenomena arising from cuprophilic aggregation less explored[13-14]. Asanextensionofourworkoncuprophilicity controlled complexes,we report two heterometallic cyano-bridged color-varied isomorphisms[(CuCN)2Ni (CN)2(H2O)4](1 and 2),both of which have two dimensionalsupramoleculararraysconstructed pentagonal heterometallic ribbons via different extent of CuⅠ…CuⅠinteractions.Quite interestingly,dark blue isomorphism 1 synthesized a lower temperature and pressure has weaker CuⅠ…CuⅠinteraction but larger density while purple isomorphism 2 synthesized a higher temperature and pressure has stronger CuⅠ…CuⅠinteractions but smaller density.This kind of abnormality between pressure and density can be rationalized by analyses of the size of pentagonal rings and distances between adjacent 2D layers.
1 Experimental
1.1Materials and methods
Allchemicalswereanalyticallypurefrom commercialsourcesandusedwithoutfurther purification.Elemental analyses were performed on a Vario EL-Ⅱanalyzer.FTIR spectra were recorded from KBr pellets in the range 4 000~400 cm-1on a Perkin-Elmer Spectrum BX FTIR spectrometer.Powder X-ray diffraction(PXRD)data were collected in a Bruker D8 advance diffractometer.The magnetic measurements were made with Quantum Design SQUID MPMS XL-5 instruments.Thediamagneticcorrectionforeach sample was applied using Pascals constants.Scanning electronmicroscopeEnergy-disperseX-ray spectroscopy analysis(SEM-EDS)were measured on a JSM-7500FequippedwithanEDAXCDUleap detector.The thermogravimetric analysis(TG/DTA) were carried out in air atmosphere using SETARAMLABSYS equipment at a heating rate of 10℃·min-1. The UV-Vis diffuse reflection curves were performed on the powder samples in the room temperature through TU-1901 double beam spectrophotometer.
1.2Syntheses of compounds 1 and 2
1.2.1Synthesis of compound 1
A mixture of CuCl2·2H2O(0.068 g,0.4 mmol),K2[Ni(CN)4](0.084 g,0.2 mmol),aqueous ammonia(25%, 2 mL)in a molar ratio of 2.0:1.0:14.8 and CH3CN(3 mL),and water(3 mL)was sealed in a 15 mL Teflonlined stainless container,which was heated to 140℃and held for 5 days.After cooling to room temperature, dark-blue block-like crystals of 1 were obtained with a yield of 45%.Elemental analysis Calcd.for 1(%):C, 13.27;H,2.22;N,15.48;Found(%):C,13.34;H,2.20, N,15.58.IR(KBr,cm-1):2 104(s),1 608(s),1 247(s), 1 180(m),678(m),3393(w),3278(w).
1.2.2Synthesis of compound 2
The preparation condition of 2 was same to 1 except that the temperature was increased to 145℃. After cooling to room temperature,purple block crystals of isomorphism 2 were recovered in 50%yield. Elemental analysis Calcd.for 2(%):C,13.27;H,2.22; N,15.48;Found(%):C,13.24;H,2.18,N,15.45.IR (KBr,cm-1):2 107(s),1 612(s),1 245(s),1 180(m), 681(m),3 390(w),3 284(w).
1.3X-raydatacollectionandstructure determination
Data were collected at 298 K on a Bruker Apex diffractometer(Mo Kα,λ=0.071 073 nm).Lorentzpolarization and absorption corrections were applied. The structures were solved with direct methods and refinedwithfull-matrixleast-squarestechnique (SHELX-97)[19].Analytical expressions of neutral-atom scatteringfactorswereemployed,andanomalous dispersion corrections were incorporated.All nonhydrogen atoms were refined anisotropically.Hydrogen atoms of organic ligands were geometrically placed and refinedwithisotropictemperaturefactors.The crystallographic data and selected bond lengths and bond angles are listed in Table 1 and Table 2.
CCDC:1046750,1;1046751,2.
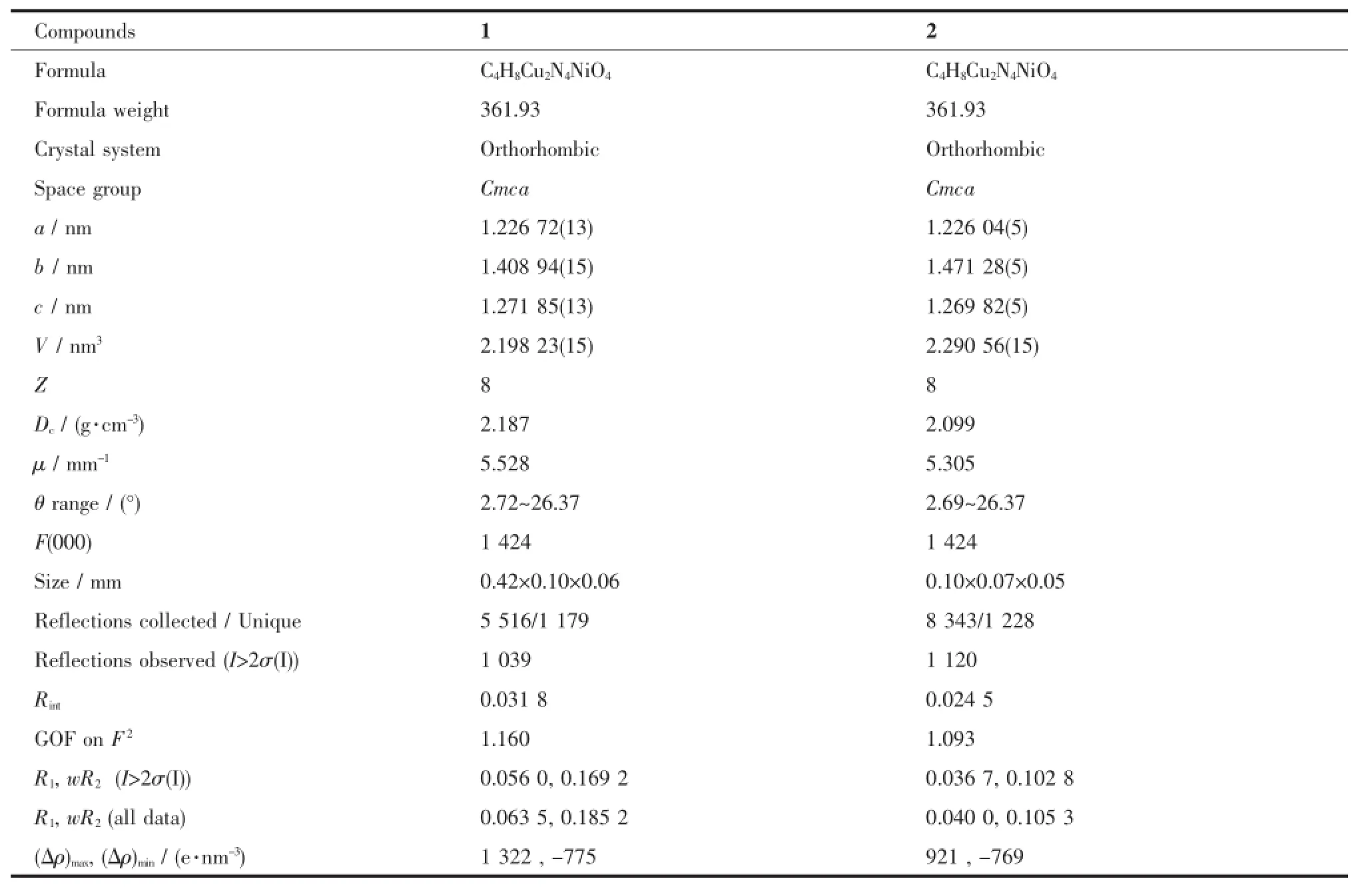
Table 1Crystallographic data for compounds 1 and 2

Table 2Selected bond lengths(nm)and angles(°)for compounds 1 and 2
2 Results and discussion
2.1Description of crystal structures
2.1.1Crystal structure of compound 1
Single-crystal X-ray analysis shows that darkblue 1 and purple 2 are isostructural,and thus only the structure of 1 is described herein in detail.Darkblue and denser 1 crystallizes in the orthorhombic space group Cmca,which has a larger calculated density of 2.187 g·cm-3.The asymmetric unit consists of one crystallographically independent Cu(Ⅱ)centers, half Ni(Ⅱ)atom,two cyanides and two water molecules as shown in Fig.1a.All atoms except for Cu(1),C(1), N(1)and O(2W)localize in general positions.The indistinguishable C(2),N(2),C(3)and N(3)atoms occupy same sites and have a site occupancy of 0.5.The Ni(1),O(1W)andO(3W)atomsarewithin crystallographic mirror and thus have site occupancy of 0.5.The C(1)-N(1)distance of 0.1134(10)nm is the typical C≡N bond length of cyanide.The Cu(1) adopts a trigonal geometry,coordinated by N(1),C(2)/ N(2)and C(3)/N(3)from three different cyanides.The Cu(1)-N(N=cyanide)bond lengths are in the range of 0.192 9(7)~0.198 3(6)nm.The N-Cu(1)-N(N= cyanide)bond angles are in the range of 110.4(3)°~124.1(3)°,which is close to 120°that can be viewed as close to trigonal-planar structure.The Ni(1)atom shows an octahedral coordination environment,being occupied by two cis-carbon atoms from cyanides and four terminal water molecules.The Ni(1)-C(1) bond length is equal 0.203 8(6)nm while the Ni(1)-O(W)bond distances are in the range of 0.201 1(11)~0.215 0(10)nm.The cis-and trans-L-Ni(1)-L(L=C,O) angles are in the range of 80.9(9)°~97.4(4)°and 171.1(5)°~173.7(5)°,suggesting a distorted octahedral geometry.The octahedral cis-[Ni(CN)2(H2O)4]units can beconsideredas2-connectedbridgingwithan approximate angle of 97.4(4)°.

Fig.1 View of the coordination environments of metal atoms(a),1-D pentagonal ribbon of 15-membered[Cu4NiⅡ(CN)5]rings(b), the 2-D supramolecular array showing unsupported CuⅠ…CuⅠinteractions(green dashed lines)(c)and the ABAB stacking of adjacent layers(d)along the b axis in compound 1
Each Cu center is connected to two adjacent Cu centers and one Ni center via linearly coordinated cyanidestogeneratepentagonalneutralribbon [(CuCN)2Ni(CN)2(H2O)4](Fig.1b).The basic units in the ribbon are 15-membered[Cu4Ni(CN)5]ring pointing in opposite directions,inwhichthefivesidesare 0.500 13,0.060 8,0.506 08,0.508 80 and 0.508 80 nm as well as the internal angles are 97.4(4)°,110.4(3)°, 110.4(3)°,120.6(3)°and 120.6(3)°,showing some deviation from ideal 108°internal angles of regular pentagon.The pentagon in 1 is also different from pentagonal Cairo tiling which has internal angles of 90° and 120°.It should be noted that there exist weak unsupported CuⅠ…CuⅠinteractions between adjacent ribbons with distances of 0.283 5(2)nm,by which the pentagonalribbonsareextendedinto2-D supramolecular array(Fig.1c).The adjacent layers are stacked in an ABAB fashion,and the average interlayer distance is 0.704 47 nm along the b axis in 1(Fig.1d). The terminal water molecules of Ni(1)atoms protrude out on two sides of the pentagonal with shortest interribbon O…O distance of 0.332 1 nm,indicating negligible O-H…O hydrogen bonds and genuine unsupported cuprophilicity.The structure and formula of 1 are reminiscent of cyanide-based mixed-valence copper complex{CuⅡ(NH3)3CuⅠ(CN)[CuⅠ(CN)3]}n[20],in which cyanides bridge between CuⅡand CuⅠions to form a centipede-like belt containing 15-memebered[CuⅠ4CuⅡ(CN)5]pentagonal rings.In contrast,the compound 1 exhibitscyanide-basedheterometallicpentagonal ribbons composed of heterometallic 15-membered basic units[CuⅠ4NiⅡ(CN)5],in which octahedral coordinated cis-[NiⅡ(CN)2(H2O)4]units formed by square planar[Ni (CN)4]2-slowly hydrolyze upon hydrothermal treatment.
2.1.2Crystal structure of compound 2
Keeping the same molar ratio and increasing the temperature to 145℃,purple 2 was obtained,which also crystallizes in the orthorhombic space group Cmca.Careful structure analysis has revealed that the two compounds are identical in chemical composition and coordination modes,as well as extended 2-D supramolecular arrays and packing modes of adjacent layers,but they possess different bond lengths and angles and strength of unsupported CuⅠ…CuⅠinteractions(0.283 5(2)nm in 1 and 0.273 81(11)nm in 2)(Table 2).Notably,the significant changes in bond lengthsandanglesmayresultinalittle expanded[Cu4Ni(CN)5]pentagonal rings in 2,which can be seen from five side distances(0.501 84, 0.515 91,0.515 91,0.507 75 and 0.507 75 nm)(Fig. 2a).Furthermore,slightly expanded pentagonal rings also respond to the average interlayer distance of 0.735 64 nm in 2 longer than 0.704 47 nm of 1 along the b axis(Fig.2b).Considering the above points,we conclude that the larger size of pentagonal rings and relatively longer distance of interlayer responsible for a looser structure and a smaller density of 2.099 g· cm-3in 2 than 2.187 g·cm-3of 1,which is in accordance with the increased cell volume by 4.2% (Table 1).Similarly,the shortest inter-ribbon O…O distance is 0.362 5 nm for 2,fully eliminating O-H…Ohydrogeninteractionandexhibitinggenuine cuprophilicity.
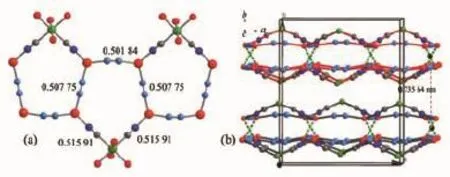
Fig.2 1-D pentagonal ribbon formed by larger size of 15-membered[Cu4NiⅡ(CN)5]pentagonal rings compared with 1(a),the ABAB stacking of adjacent layers along the b axis showing longer distances of interlayer in 2(b)
Although the theoretical and experimental studies of metallophilicity for the closed-shell d10metal ions have been widely documented,examples of ligand unsupported CuⅠ…CuⅠinteractions are relatively few, as compared to those of ligand unsupported AuⅠ-AuⅠand AgⅠ-AgⅠinteractions[9-11].The examples of complexes with ligand-unsupported cuprophilicity are very important for the study of this kind of optics and electricity related weak interactions.The cuprous cyanide system could give an opportunity to investigate metallophilic interactions because the ligand steric hindrance and inter-chain copper-ligand interactions are decreased in the system.It should be noted that the CuⅠ…CuⅠdistances of 0.283 5 nm in 1 and 0.273 8 nm in 2,closer and slightly shorter than the sum of van der Waals radii for dicoordinated Cu(Ⅱ)atoms(0.280 nm), arenotassociatedwithligand-bridged,hydrogen bonded,electrostatic attracted and π-π stacked effects, and thus they indicate reliable unsupported CuⅠ…CuⅠinteractions.To date,there are only a few ligandunsupported cuprophilic interactions;these are usually constructed by linear two-coordinate Cu(Ⅱ)centers, such as[Cu(NH3)Cl](0.297 9(1)nm)[9a],[Cu(NH3)2]Br (0.293 1(1)nm)[9a],([CuCl2]-)2(0.292 2(2)nm)[9b]and [tBuCu(CN)Li·(OEt2)2]8(0.271 3(1)nm)[9c].To our knowledge,only few examples featuring trigonal Cu(Ⅱ) centers have been reported in[Cu3{2-[3(5)-pz]py}3]2(0.290 5(3)nm)[9d]and[Cu2(obpy)2]2(0.298 6and 0.299 3 nm)[6a].Differently,cuprophilic interactions in 1 and2areformedbetweeninter-ribbonthreecoordinated copper centers and have shorter CuⅠ…CuⅠdistances.Thepreparationandstructural characterization of 1 and 2 show that cuprophilicity as one kind of weak supramolecular force,can be used as synthons for control over supramolecular polymorphous compounds.
2.2Relationship between synthesis and structure chemistry
Hydrothermal reactions of CuCl2,K2[Ni(CN)4]and aqueous ammonia in the same molar ratio synthesized denser dark-blue 1(2.187 g·cm-3)at a lower temperature of 140℃and looser purple polymorph 2 (2.099 g·cm-3)at a higher temperature of 145℃(Scheme 1).As can be seen,5℃difference in reaction temperature is enough to affect strength of unsupported CuⅠ…CuⅠinteractions and overall supramolecular arrays.The CuⅠ…CuⅠdistances in 1 and 2 are ca.0.283 5 and 0.273 8 nm,respectively, which means a stronger cuprophilicity in 2.As confirmed by crystallographic study,the coordination geometries for metal ions,pentagonal ribbons and packing fashion of ribbons are very similar in 1 and 2. Thus one may expect that 2 has a larger density due to shorter and stronger CuⅠ…CuⅠcontact.However, this is not the case.Compound 2 is lighter than 1 by 4.2%.Whatistheabnormalreason?Careful examinations reveal that the larger size of[Cu4Ni(CN)5] pentagonalringsandrelativelylongerdistances between 2D layers in 2 contribute to the abnormal phenomenon,whichisinaccordancewiththe deviation distance of b axis from 1.408 to 1.471 nm. Generally,polymorphs with close formation entropy are obtained in mixture.Interestingly,1 and 2 can be easily synthesized and separated due to quite different colors.Besides,the bulk purity of polymorphs 1 and 2 was verified by contrast of recorded and simulated XRPDpatterns(Fig.3).TheirsimulatedXRPD patterns are very similar and the small differences are caused by the small different unit-cell parameters. The evidence of bridging cyanide groups is provided bythepresenceofcyanidegroupstretching absorptions at 2 107 cm-1for 1 and at 2 104 cm-1for 2 in the infrared spectra(Fig.4).The presence of heterometallic Ni(Ⅱ)atoms in 1 and 2 was confirmed by EDS analyses(Fig.5 and Table 3)and magnetic analysis.
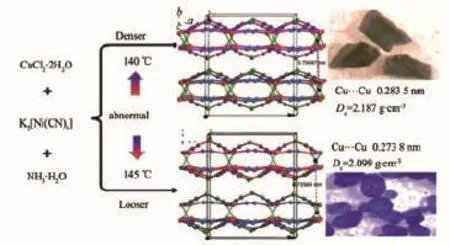
Scheme 1View of syntheses and supramolecular packing,and photographs in polymorphs

Fig.3 Simulated and measured XRPD patterns for 1 and 2
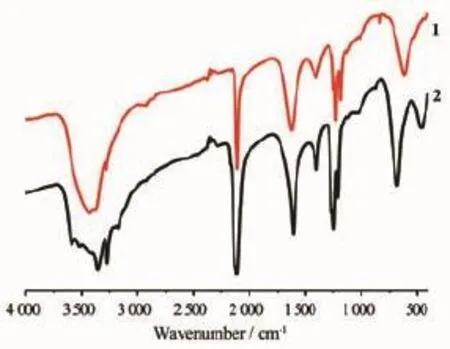
Fig.4 IR spectra of 1 and 2

Fig.5 SEM images and EDS plots for 1 and 2

Table 3Elemental analysis C,H and N and EDS data for ratio of Cu and Ni in heterometallic 1 and 2
2.3Possible mechanism
Compared with traditional cyanide sources of KCN and CuCN,environmentally friendly cyanidometallate K2[Ni(CN)4]can slowly release cyanide and act as heterometallic source upon heat treatment, which is a key for the formation of heterometallic 1 and 2.This course can be expressed by the following equation:
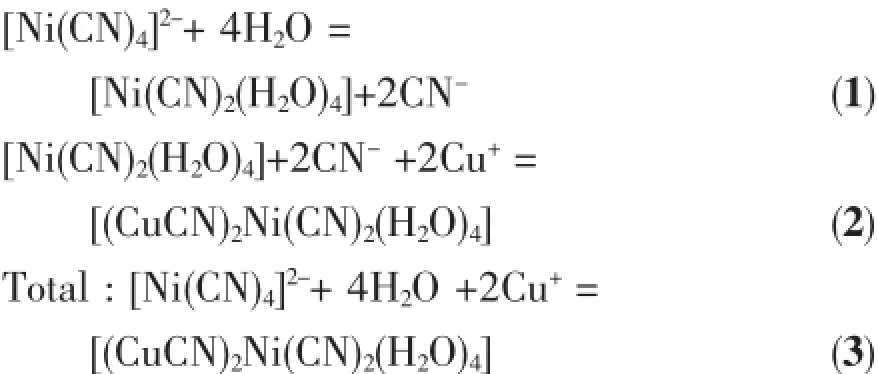
From a thermodynamic point of view,the stability constant of square planar[Ni(CN)4]2-is very large(K= 2.0×1031),indicating high chemical stability[21].In spite of high stability,there exists a hydrolysis equilibrium between[Ni(CN)4]2-and cis-[Ni(CN)2(H2O)4]as shown in Equation 1.This equilibrium seems to be critical in the syntheses of 1 and 2,and it should be noted that this course is accompanied by transition of strong square planar field to weak octahedral field for Ni(Ⅱ) atoms.The in situ formed minor cis-[Ni(CN)2(H2O)4] and CN-ions in the presence of Cu(Ⅱ)atoms can generatepolymericpentagonalribbonsthatare aggregated via unsupported cuprophilic interaction intosolidsof1and2.Crystallizationunderhydrothermalconditioncanbeanonequilibrium course,which is helpful to hydrolysis of[Ni(CN)4]2-into cis-[Ni(CN)2(H2O)4].It is noteworthy that both chloride and cyanide can act as reducing agent to reduce Cu(Ⅱ)into Cu(Ⅱ).TD-DFT calculations were performed using the B3LYP method[22]and the 6-31G (d,p)basis set[23],which have also been usedto theoretically predict the Equation 1 reaction and functionasanassistantmethodtoqualitatively analyse the formation of the 1 and 2.The modified neutral model building blocks of K2[Ni(CN)4],H2O,[Ni (CN)2(H2O)4],and HCN adapted from the X-ray data have been used as structure component unit for calculationsbyusingtheGaussian03program package[24].Relative bonding energies analysisfor block unit K2[Ni(CN)4],H2O,[Ni(CN)2(H2O)4]and HCN are-1792.62,-329.99,-2210.16 and-459.58 kJ· mol-1.It is interesting to find that the energy difference between the product and reactants is about -16.74 kJ·mol-1,which is consistent with our deductions that the reaction product should possess more stable energies than reactants(Table 4).
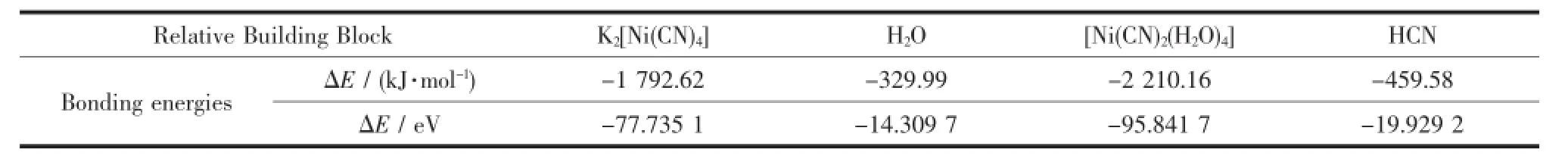
Table 4Relative bonding energies(ΔE)of the optimized building block K2[Ni(CN)4],H2O,[Ni(CN)2(H2O)4]and HCN and all of the calculations were performed at the DFT level by using the Gaussian 03 program package
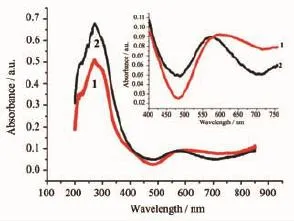
Fig.6 Solid-state UV-Vis absorption spectra of 1 and 2 at room temperature
2.4Colored polymorphs
Althoughisostructural1and2havesame building units and similar pentagonal ribbons and 2D supramoleculararrays,surprisingly,theyshow significantly different color:dark-blue 1 and purple 2. The solid state diffuse-reflectance UV-Vis spectra using BaSO4powder as a 100%reflectance reference wererecordedtorevealthisphenomenon.The absorption data was calculated from the reflectance. As shown in Fig.6,the absorption spectra of 1 and 2 have a very similar high energy UV absorption peaks at about 272 nm and 274 nm,which are assigned to cyano centered π-π*charge transfer(LC)processes. Thebandsinvisibleregionareimportantand responsible for the significant color difference.There is an absorption,which is started from 480 nm and extended to IR region with maxima at 590 nm for 1. For 2,there is also a visible absorption started from 480 nm and extended to IR region but the maximum absorption is ca.570 nm,which is blue-shifted by 20 nm and has larger absorption intensity compared with 1.This may explain the color difference of 1 and 2. The visible absorption band at 590 nm and 570 nm that imparted the dramatic color difference has been tentatively assigned as a metal→metal 3d→(4p,4s) transition modified by cuprophilic interactions[17].The UV-Vis spectra are in agreement with the colordifference observed by naked eyes in our experiment. The band gaps of 3.00 eV and 2.92 eV for 1 and 2 have been measured by diffuse reflectivity for the powder sample,which indicate that they are potential semiconductor materials(Fig.7).
2.5Thermogravimetry analysis
TG/DTAanalysesfor1and2inanair atmosphere and under 101 kPa pressure at the heating rate of 10℃·min-1were performed on polycrystalline samples,which showed similar thermal stability of 1 and2.Thismaybeattributedtotheirsimilar supramolecular structures.As shown in Fig.8,TGA traces of 1 and 2 exhibit two main steps of weight loss.The initial weight loss of 17.71%for 1 and 18.1%for 2 occur in the temperature range of 129~280℃and 122~297℃,which correspond to the loss of four water molecules(Calcd.19.91%).The second weight loss of 29.5%for 1 and 27.4%for 2 occur in the temperature range of 349~1 080℃and 361~1 085℃,which correspond to the removal of cyanides,and an intermediate of copper and nickel are formed by deduction from empirical composition (Calcd.31.4%).DTA traces of 1 and 2 exhibit similar exothermic peak at ca.425℃.

Fig.8 TGA-DTA curves of 1 and 2 in air atmosphere and at the heating rate of 10℃·min-1

Fig.9 Magnetic susceptibility data per Ni1 unit presented as plots of χmT vs T and χm-1vs T under an applied 1 000 Oe field for compounds 1(a)and 2(b)
2.6Magnetism
The temperature dependence of the magnetic susceptibilities for 1 and 2 in the temperature range of 2~300 K under 1 000 Oe applied field were measured with a Quantum Design SQUID MPMS XL-5.The χmT value at 300 K is 1.25 cm3·K·mol-1for 1,which is slightly larger than that expected 1.00 cm3·K·mol-1for an uncoupled Ni(Ⅱ)ion with g=2.0.The χmT value decreases slowly with decreasing temperature until 50 K.After that,the χmT value rapidly decreases with lowering temperature down to a value of 0.34 cm3·K· mol-1for 1 at 1.29 K.The tting of Curie-Weiss law in the temperature range of 20~300 K gives C=1.272 cm3·K·mol-1,θ=-6.11 K(Fig.9a).The negative θ values indicate antiferromagnetic coupling dominating these systems.The χmT value at 300 K is 1.10 cm3·K· mol-1for 2,which is also slightly larger than that expected 1.00 cm3·K·mol-1for an uncoupled Ni(Ⅱ)ion with g=2.0.The χmT value monotonically decreases to a value of 0.29 cm3·K·mol-1at 2 K upon cooling.Thetting of Curie-Weiss law in the temperature range of 25~300 K gives C=1.122 cm3·K·mol-1,θ=-7.92 K (Fig.9b).The field-dependent magnetization at 2 K increases slowly and linearly with the applied field, and no saturation is observed.The magnetization value at the highest field of 50 kOe are 1.52Nβ for 1 and 1.46Nβ for 2,close to the saturation value of 2Nβ expected for one spin-only Ni(Ⅱ)species(Fig.10). Quite a few examples of bimetallic systems containing diamagnetic cyanometalate bridgingunitsbetween paramagnetic ions have been reported so far[25-26],in which weak antiferromagnetic exchange interactions are more likely due to a combination of intra-and inter-chain interactions.

Fig.1 0Field dependence of the magnetization per Ni1 unit at 2 K for 1(a)and 2(b)
3 Conclusions
In summary,we have demonstrated the preparation andcharacterizationoftwonovelheterometallic polymorphs by using environmentally friendly K2[Ni (CN)4]as cyanide source.Both consist of pentagonal heterometallic[(CuCN)2Ni(CN)2(H2O)4]ribbons that are linked into 2D supramolecular arrays via unsupported cuprophilic interactions.Quite interestingly,dark blue 1 synthesized at a lower temperature and pressure is denserthanpurple2synthesizedatahigher temperature and pressure.This observation is abnormal to general rule that a higher reaction temperature and pressure tends to form a dense phase,which can be rationalized by analyses of size of pentagonal rings and relative distances between adjacent layers.The size of [Cu4Ni(CN)5]pentagons in 2 is a little larger than that in 1 and the distances between adjacent layers in 2 is also largerthanthatin1.Interestingcolor-varied phenomenon is observed in the two polymorphs,which is confirmed by remarkable color change from darkbluetopurple.UV-Visabsorptionspectraare characteristic of two bands,in which the visible low energy one is responsible for different color and arising from CuI…CuIinteractions.In situ slow hydrolysis of[Ni(CN)4]2-to form cis-[Ni(CN)2(H2O)4] units and cyanides are key to formation of the two compounds,during which diamagnetic square planar Ni(Ⅱ)atom is transformed into octahedral coordinated Ni(Ⅱ)with ground state spin S=1.This work not only provideanenvironmentallyfriendlyrouteto heterometallic cyanide polymorphs but also exhibit genuine cuprophilicity modulated optical behavior.
References:
[1](a)Khlobystov A N,Blake A J,Champness N R,et al.Coord. Chem.Rev.,2001,222:155-192
(b)Yam V W W,Lo K K W.Chem.Soc.Rev.,1999,28:323-334
[2](a)Zheng S L,Nygren C L,Messerschmidt M,et al.Chem. Commun.,2006,3711-3713
(b)Tong M L,Chen X M,Ye B H,et al.Angew.Chem.Int. Ed.,1999,38:2237-2239
(c)Ray L,Shaikh M M,Ghosh P.Inorg.Chem.,2008,47: 230-240
[3](a)Burini A,Fackler J P,Galassi R,et al.J.Am.Chem. Soc.,2000,122:11264-11265
(b)Streb C,Ritchie C,Long D L,et al.Angew.Chem.Int. Ed.,2007,46:7579-7582
[4](a)Liu X,Guo G C,Fu M L,et al.Inorg.Chem.,2006,45: 3679-3685
(b)Sun D F,Cao R,Weng J B,et al.J.Chem.Soc.Dalton Trans.,2002:291-292
[5](a)Tong M L,Chen X M,Ye B H,et al.Inorg.Chem., 1998,37:5278-5281
(b)Chen X M,Thomas C W M.J.Chem.Soc.Dalton Trans., 1991:3253-3258
(c)Codina A,Fernández E J,Jones P G,et al.J.Am.Chem. Soc.,2002,124:6781-6786
[6](a)Zhang J P,Wang Y B,Chen X M,et al.Chem.Eur.J., 2005,11:552-561
(b)Chui S S Y,Ng M F Y,Che C M,Chem.Eur.J.,2005,11: 1739-1749
(c)Sundararaman A,Zakharov L N,Jäkle F,et al.Chem. Commun.,2005:1708-1710
[7](a)Peng R,Li D,Wu T,et al.Inorg.Chem.,2006,45:4035-4046
(b)Kang Y,Yao Y G,Qin Y Y,et al.Chem.Commun., 2004:1046-1047
[8](a)Cotton F A,Dikarev E V,Petrukhina M A,Inorg. Chem.,2000,39:6072-6079
(b)Liu X,Guo G C,Liu B,et al.Cryst.Growth Des.2005, 5:841-843
(c)Huang X C,Zhang J P,Chen X M,J.Am.Chem.Soc., 2004,126:13218-13219
[9](a)Margraf G,Bats J W,Bolte M,et al.Chem.Commun., 2003:956-957
(b)Köhn R D,Seifert G,Pan Z,et al.Angew.Chem.Int. Ed.,2003,42:793-796
(c)Boche G,Bosold F,Marsch M,et al.Angew.Chem.Int. Ed.,1998,37:1684-1686
(d)Singh K,Long J R,Stavropoulos P.J.Am.Chem.Soc., 1997,119:2942-2943
[10](a)Liu X,Guo G C,Wu A Q,et al.Inorg.Chem.,2005,44: 4282-4286
(b)Zhang X M,Tong M L,Gong M L,et al.Chem.Eur.J., 2002,8:3187-3194
(c)Huang X C,Zhang J P,Chen X M.Cryst.Growth Des., 2006,6:1194-1198
(d)Zheng S L,Messerschmidt M,Coppens P.Angew.Chem. Int.Ed.,2005,44:4614-4617
(e)Zhang J X,He J,Yin Y G,et al.Inorg.Chem.,2008,47: 3471-3473
[11](a)Gao G F,Li M,Zhan S Z,et al.Chem.Eur.J.,2011,17: 4113-4117
(b)Jin K,Huang X,Pang L,et al.Chem.Commun.,2002: 2872-2873
[12]Hermann H L,Boche G,Schwerdtfeger P,Chem.Eur.J., 2001,7:5333-5342
[13](a)Tronic T A,deKrafft K E,Pike R D,et al.Inorg.Chem., 2007,46:8897-8912 (b)Lim M J,Murray C A,deButts J C,et al.Inorg.Chem., 2008,47:6931-6947
(c)Hibble S J,Eversfield S G,Cowley A.R,et al.Angew. Chem.Int.Ed.,2004,43:628-630
(d)Park K M,Lee S,Kang Y,et al.Dalton Trans.,2008, 6521-6523
[14](a)Zhang X M,Hao Z M,Wu H S.Inorg.Chem.,2005,44: 7301-7303
(b)Qin Y L,Liu J,Zhang X M,et al.Cryst.Growth Des., 2012,12:6068-6073
(c)Zhang X M,Qin Y L,Wu H S.Inorg.Chem.,2008,47: 2255-2257
(d)Qin Y L,Hou J J,Zhang X M,et al.Cryst.Growth Des., 2011,11:3101-3108
[15](a)Reichardt C.Chem.Rev.,1994,94:2319-2358
(b)Kahr B,Gurney R W.Chem.Rev.,2001,101:893-952
(c)Sun J K,Cai L X,Zhang J,et al.Chem.Commun.,2011, 47:6870-6872
(d)Nakai H,Isobe K.Coord.Chem.Rev.,2010,254:2652 -2662
[16](a)Xu G,Guo G C,Huang J S,et al.Angew.Chem.Int. Ed.,2007,46:3249-3251
(b)Létard J F,Guionneau P,Nguyen O,et al.Chem.Eur. J.,2005,11:4582-4589
(c)Dias H V R,Diyabalanage H V K,Omary M A,et al.J. Am.Chem.Soc.,2003,125:12072-12073
[17](a)Fu W F,Gan X,Che C M,et al.Chem.Eur.J.,2004,10: 2228-2236
(b)Che C M,Mao Z,Miskowski V M,et al.Angew.Chem., Int.Ed.,2000,39:4084-4088
(c)Mao Z,Chao H Y,Che C M,et al.Chem.Eur.J.,2003,9: 2885-2894
[18](a)CheCM,LaiSW.Coord.Chem.Rev.,2005,249:1296-1309
(b)He J,Yin Y G,Li D,et al.Chem.Commun.,2006:2845 -2847
(c)Yam V W W,Wong K M C,Zhu N.J.Am.Chem.Soc., 2002,124:6506-6507
(d)Fu W F,Chan K C,Che C M,et al.Chem.Eur.J. 2001,7:4656-4664
(e)Wong K M C,Yam V W W.Acc.Chem.Res.,2011,44: 424-434
[19]Sheldrick G M.SHELX-97,Program for X-ray Crystal Structure Solution and Refinement,Göttingen University, Germany,1997.
[20]Song Y,Xu Y,Wang T W,et al.J.Mol.Struct.,2006,788: 206-210
[21]ZHANG Xiang-Lin(张祥麟),KANG Heng(康衡). Coordination Chemistry(配位化学).Changsha:ZhongnanIndustrial University Press,1986.
[22](a)Becke A D.J.Chem.Phys.,1993,98:5648
(b)Miehlich B,Savin A,Preuss H,et al.Chem.Phys.Lett., 1989,157:200
(c)Stephens P J,Devlin F J,Chabalowski C F.J.Phys. Chem.,1994,98:11623
[23]Petersson G A,Bennett A,Shirley W A,et al.J.Chem. Phys.,1988,89:2193
[24]Frisch M J,Trucks G W,Cheeseman J R.Gaussian 03, Revision D.01,Gaussian,Inc.,Wallingford,CT,2004.
[25]Ohba M,Usuki N,Okawa H,et al.Inorg.Chem.,1998,37: 3349-3354
[26]Rogez G,Marvilliers A,Mallah T,et al.Angew.Chem.Int. Ed.,2000,39:2885-2887
Distinguishable Polymorphs in Color:Cyano-Bridged Heterometallic Pentagonal Ribbons via Cuprophilic Aggregation
QIN Ying-Lian1,2LI Shi-Li1JIANG Ning1ZHANG Xian-Ming*,1
(1School of Chemistry&Material Science,Key Laboratory of Magnetic Molecules and Magnetic Information Material,Ministry of Education,Shanxi Normal University,Linfen,Shanxi 041004,China)
(2Department of Applied Chemistry,Yuncheng University,Yuncheng,Shanxi 044000,China)
By using environmentally friendly K2[Ni(CN)4]that slowly hydrolyse upon hydrothermal treatment,cis-[Ni(CN)2(H2O)4]units and cyanides were generated and further assembled with Cu(Ⅱ)atoms into pentagonal heterometallic ribbons.Subsequently,these pentagonal ribbons are induced aggregation via cuprophilic interactions into 2D supramolecular coloured polymorphs[(CuCN)2Ni(CN)2(H2O)4](1 and 2).Dark blue 1 synthesized at a lower temperature is a denser phase while purple 2 synthesized at a higher temperature is a looser phase,which is abnormal to general rule that a higher reaction time and pressure tends to form a denser phase.Careful examination on structures reveals that slight size difference in pentagons and different interlayer distances of 2D supramolecular arrangement contribute to the abnormality.Besides,the titled compounds could be rare cuprophilicity drived examples of coloured polymorphs,which exhibit remarkable colour difference from dark-blue to purple.Magnetic measurements confirmed that diamagnetic Ni(Ⅱ)atom in square planar[Ni(CN)4]2-is transformed into octahedral coordinated Ni(Ⅱ)with ground state spin S=1.CCDC:1046750,1;1046751,2.
cuprophilicity;polymorph;heterometa;pentagon;aggregate
O614.121
A
1001-4861(2015)09-1785-13
10.11862/CJIC.2015.249
2015-04-20。收修改稿日期:2015-06-05。
国家重点基础研究发展计划(973项目2012CB821701),中国教育部项目(No.IRT1156),国家杰出青年科学基金(No.20925101),运城学院青年教师科研启动项目(No.YQ-2013013)资助。
*通讯联系人。E-mail:zhangxm@dns.sxnu.edu.cn
