基于6-羟基-2-吡啶基膦酸配体的铜配合物的合成、结构及磁性
2015-11-30李海清华敬坤查丽琴马运声唐晓艳谢吉民袁荣鑫
李海清 华敬坤 查丽琴 马运声*, 唐晓艳 谢吉民 袁荣鑫*,
基于6-羟基-2-吡啶基膦酸配体的铜配合物的合成、结构及磁性
李海清1,2华敬坤1查丽琴1马运声*,1唐晓艳1谢吉民2袁荣鑫*,1
(1常熟理工学院化学与材料工程学院,江苏省新型功能材料重点建设实验室,常熟215500) (2江苏大学化学化工学院,镇江212013)
在水热条件下,以6-羟基-2-吡啶基膦酸为主配体,4,4′-联吡啶(bpy)及1,2-二(4-吡啶基)乙烯(bpe)为桥联配体,合成了2个铜膦酸配位聚合物[Cu3(L)2(bpy)2(H2O)2]·2H2O(1),[Cu3(L)2(bpe)2(H2O)3]·2H2O(2)。配合物1中,Cu2+离子由膦酸配体连接成一条链,该链由bpy桥联成二维层,层与层之间通过氢键作用构成三维结构。配合物2与配合物1是同构的,桥联配体是bpe。磁性研究表明,配合物1与2中铜离子之间存在反铁磁性耦合。
铜配合物;有机膦酸;晶体结构;磁性
During the past several decades,copper phosphonates have received considerable attention,not only due to their structural diversity but also for their potential applications in many fields such as ion exchange,sorption,catalysis,sensors,nonlinear optics, as well as magnetism etc[1-7].The study of copper phosphonates started in the 1990s when simple alkyl-[8]and phenyl-phosphonates[9]were prepared with thepurpose to obtain layered host materials for intercalation reactions.To extend intriguing topologies and explore their properties,scientists continually researched the topic of copper phosphonates.Through summarizing the work of predecessors,we find that there are at least three approaches to construct these compounds.The first one is using of different steric hindered phosphonic acids,such as the R-(PO3H2)n(n=1 or 2)ligands(where R=CH3[10],C2H3[11],(CH2)4[12], (CH2)5[12],tert-butyl[13],C6H9[14],trityl[15],etc.).The second is introducing a second auxiliary ligand.For instances,pyrazine(pyz),2,2′-bipyridine(2,2′-bipy), 4,4′-bipyridine(bpy),as well as 1,10-phenanthroline (phen)are common ancillary ligands.[Cu((CH3)2C6H2(PO3H)2)(pyz)(H2O)2]n[16]shows a 3D framework structure.When introducing 2,2′-bipy and bpy,[Cu(2,2′-bipy)(HO3PCH(OH)CO2(H2O)]·H2O[17]and[CuⅠ2CuⅡ(CH3C(OH)(PO3H)2)2(bpy)2]·2H2O[18]were isolated.Both ofthem display 2D layered structures.Incorporating the phen into thereaction,[Cu(phen)(H2O)(O2CC6H4PO3H)][19]was produced,which shows a one-dimensional and composed of helical chains.The third approach is decorating the phosphonate ligands with other coordinating functional groups,such as hydroxy,carboxylate,sulfonate,amino,pyridyl,and pyrimidyl etc.to the phosphonate ligands.Examples for this scheme include Cu4{CH3C(OH)(PO3)2}2(C4H4N2)(H2O)4[20];Cu3[NH2(CH2PO3)2]2[21];[Cu(C6H5CONHPO3H)2]n[22];[Cu (para-HO2CC6H4PO3)]n[23];[Cu2(O3PC2H4SO3)(OH)(H2O)]· 3H2O[24];Cu(2-C5H4NPO3H)2,Cu3(OH)2(2-C5H4NPO3)2· 2H2O and Cu(2-C5H4NPO3)[25];{[(5-C4H4N2PO3H)2Cu2(OH2)5](CF3SO3)2(H2O)}n[26].In this paper,we select 6-hydroxy-2-pyridinephosphonic acid to reactwith copper salt.Two copper phosphonates 1 and 2 were obtained by employing the ancillary bipyridinyl ligands bpy and bpe.The magnetic properties were studied.
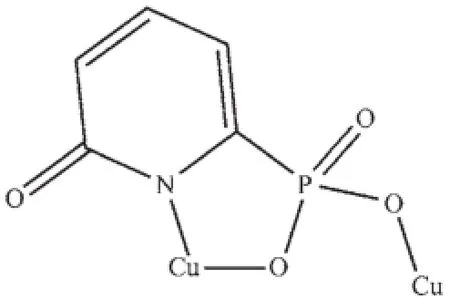
Scheme 1 Coordination mode of the ligand
1 Experimental
1.1 Materials and methods
6-hydroxy-2-pyridinephosphonic acid was prepared according to the literature[27].All other chemicals and solvents were commercially purchased and used as received.Elemental analyses were performed on a PE 240C elemental analyzer.The IR spectra were recorded on a NICOLET 380 spectrometerwith pressed KBr pellets.All the magnetic studies were performed on microcrystalline state.The magnetic susceptibilities were measured on a Quantum Design MPMS SQUID-XL7 magnetometer.Diamagnetic corrections were made for both the sample holder and the compound estimated from Pascal′s constants[28].
1.2 Syntheses
[Cu3(L)2(bpy)2(H2O)2]·2H2O(1):A mixture of Cu2(OH)2CO3(0.012 g,0.05 mmol),H3L(0.017 5 g, 0.10 mmol),and bpy(0.015 6 g,0.10 mmol)was dissolved in 2 mL H2O.The resulting solution was placed in a glass tube,which was sealed and heated at 140℃for 2 days.On cooling the dark green block crystals were obtained(Yield:47%based on Cu). Anal.Calcd.for C30H30Cu3N6O12P2(%):C 39.20,H 3.29,N 9.14;Found(%):C 39.15,H 3.32,N 9.11.IR (KBr,cm-1):3 421.67(vs),1 609.84(s),1 403.87(s), 1 059.11(s),999.04(s),812.28(m),709.75(m),601.40 (w),513.62(w).
[Cu3(L)2(bpe)2(H2O)3]·2H2O(2):The synthesis of 2 is similar to 1 except bpe was chosen as the bridge ligand(Yield:40%based on Cu).Anal.Calcd.for C34H36Cu3N6O13P2(%):C 41.28,H 3.67,N 8.49;Found (%):C 42.03,H 3.55,N 8.63.IR(KBr,cm-1):3 409.42 (vs),3 166.01(vs),1 612.26(s),1 507.64(s),1 061.68(s), 969.37(s),969.37(m),828.83(m),553.99(w),515.14(w).
1.3 X-ray crystallography
Single crystals with dimensions 0.22 mm×0.25 mm×0.30 mm for 1 and 0.22 mm×0.25 mm×0.30 mm for 2 were selected for indexing and intensity data collection with a Rigaku SCX mini CCD diffractometer by using graphite-monochromated Mo Kαradiation (λ=0.710 73 nm)at room temperature.A hemisphere of data was collected in theθrange of 3.2°~27.5°for1 and 3.1°~25.0°for 2 by using a narrow-frame method with scan widths of 0.03°inωand an exposure time of 10 s per frame.The numbers of observed and unique reflections are 8 533 and 3 690 (Rint=0.038)for 1 and 16 187 and 6 637(Rint=0.077) for 2.Cell parameters were refined by using the program CrystalClear[29]on all observed reflections. The collected data were reduced by using the program CrystalClear,and an absorption correction(multiscan) was applied.The reflection data were also corrected for Lorentz and polarization effects.The structures were solved by direct methods and refined on F2by full-matrix least squares using SHELXTL[30].All the non-hydrogen atoms were located from the Fourier maps and were refined anisotropically.All H atoms were refined isotropically with the isotropic vibration parameters related to the non-H atom to which they are bonded.Crystallographic and refinement details of 1~2 are listed in Table 1.Selected bond lengths and angles are given in Table 2 for 1~2,respectively.
CCDC:1044790,1;1044791,2.
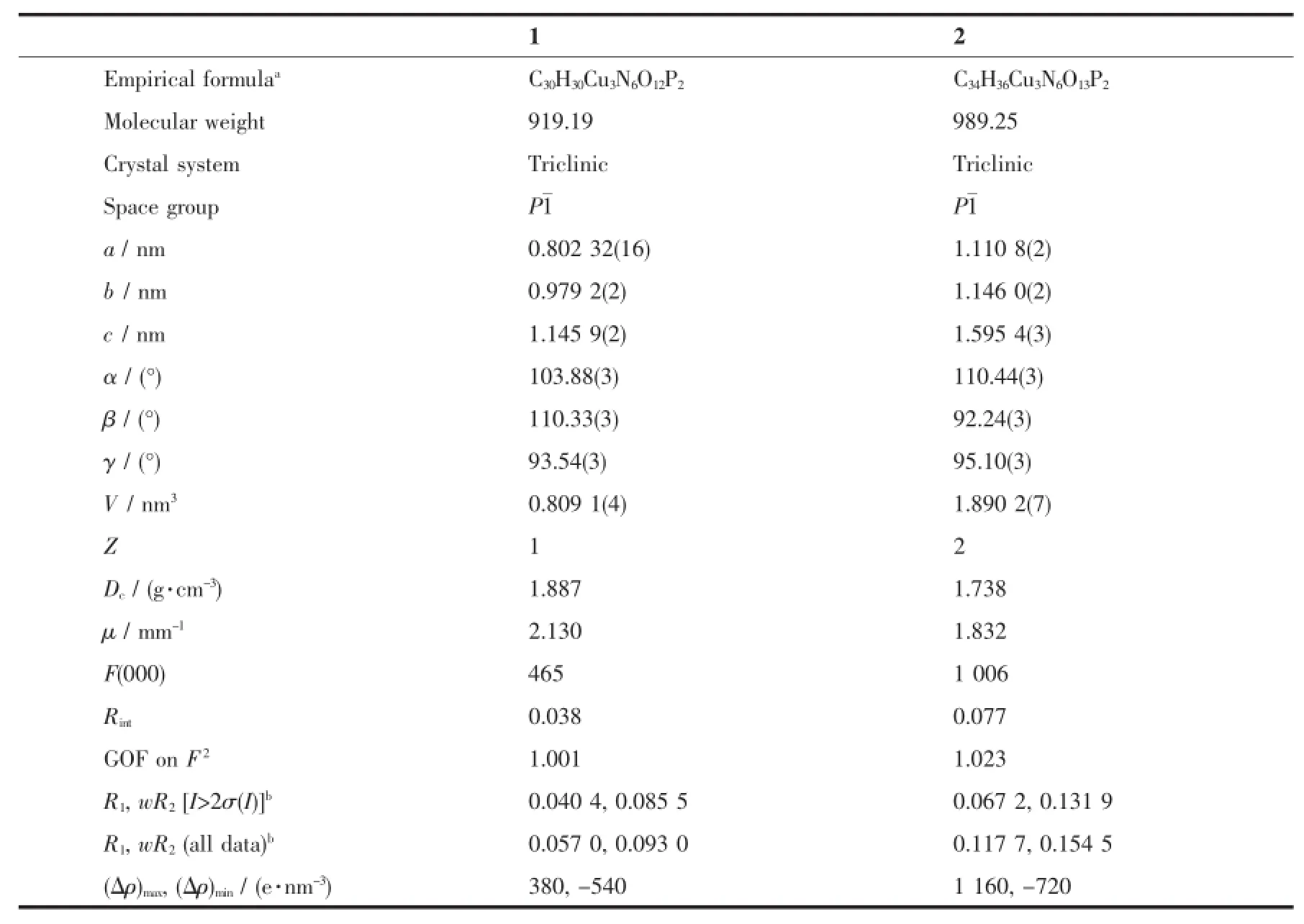
Table 1 Crystal data and structure refinements for 1 to 2
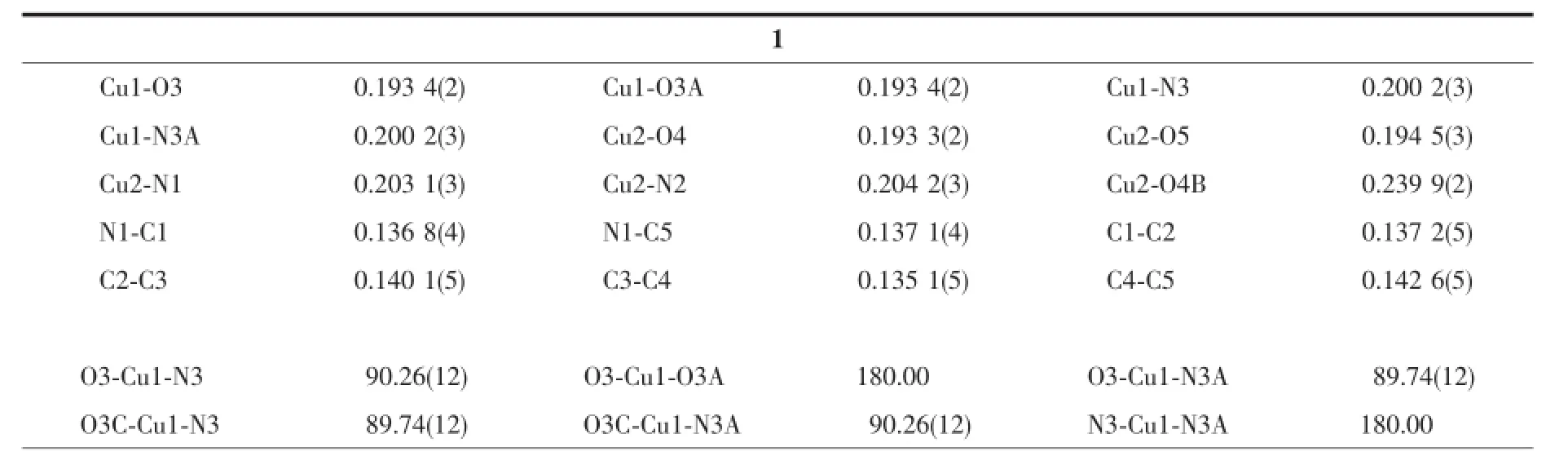
Table 2 Selected bond lengths(nm)and angles(°)for 1 and 2
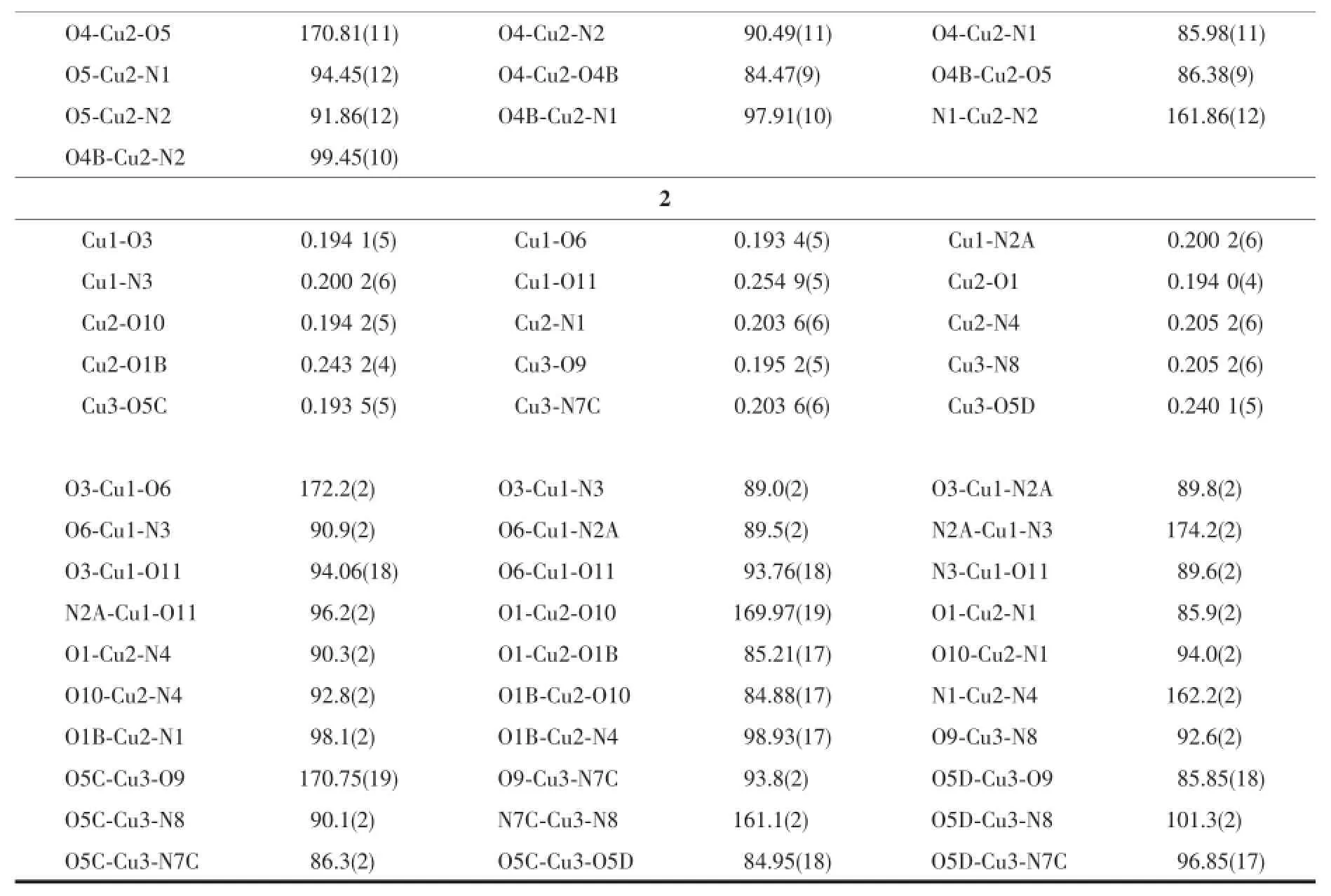
Continued Table 2
2 Results and discussion
2.1 Structure description
X-ray single crystal diffraction reveals that compound 1 crystallizes in the triclinic space group P1(Table 1).As shown in Fig.1,the asymmetric unit contains one and a half copper atoms,one L3-,one bpy,one coordinated water,and one lattice water.A curious feature of the structure is the presence of two distinct Cu2+geometries.Cu1 locates on an inversion center with 0.5 occupations and has square-planar coordination geometry.The atoms around Cu1 atom are(O3 and O3A)from two different L3-ligands and (N3 and N3A)from two separate bpy.The Cu1-O and Cu1-N bond lengths are 0.193 4(2)nm and 0.200 2(3) nm(Table 2),respectively.Cu2 is square-pyramidally coordinated,surrounded by(O4,O4B,O5,N1 and N2)from two phosphonate ligands,bpy and water.The O4B fills on the axial position with the Cu2-O4B distance of0.239 9(2)nm.The Cu2-O/N lengths on the planar position range from 0.193 3(2)to 0.204 2(3) nm,which are in consistent with that observed in othercopperphosphonatessuch as Cu(2-C5H4NPO3H)2[25]. The Cu2 and Cu2B atoms are bridged togetherthrough phosphonate oxygen atoms to form a dimer {Cu2O2}with Cu2…Cu2B distance of 0.322 3 nm.The Cu2-O4-Cu2B bond angle is 95.53°.Furthermore,the dimers and Cu1 atoms are linked together through the O-P-O groups to form one infinite chain along the caxis(Fig.2a).The chains are joined together by bpy to form a layer,which are stabilized by hydrogen bonding interactions(Table 3)into a 3D network(Fig. 2b).Charge balance calculation shows that the ligand is minus trivalent(Scheme 1).The anionic ligand is coordinated to the copper in the lactam form,as evidenced by the C5-O1 bond length,0.127 6(4)nm, which is shorter than thatin the free ligand(0.130 6 nm)[27].Thus we assume that the C5-O1 is characteristic ofa double bond.The C-C distances in the ligand are different and correspond to single and double bonds[31].
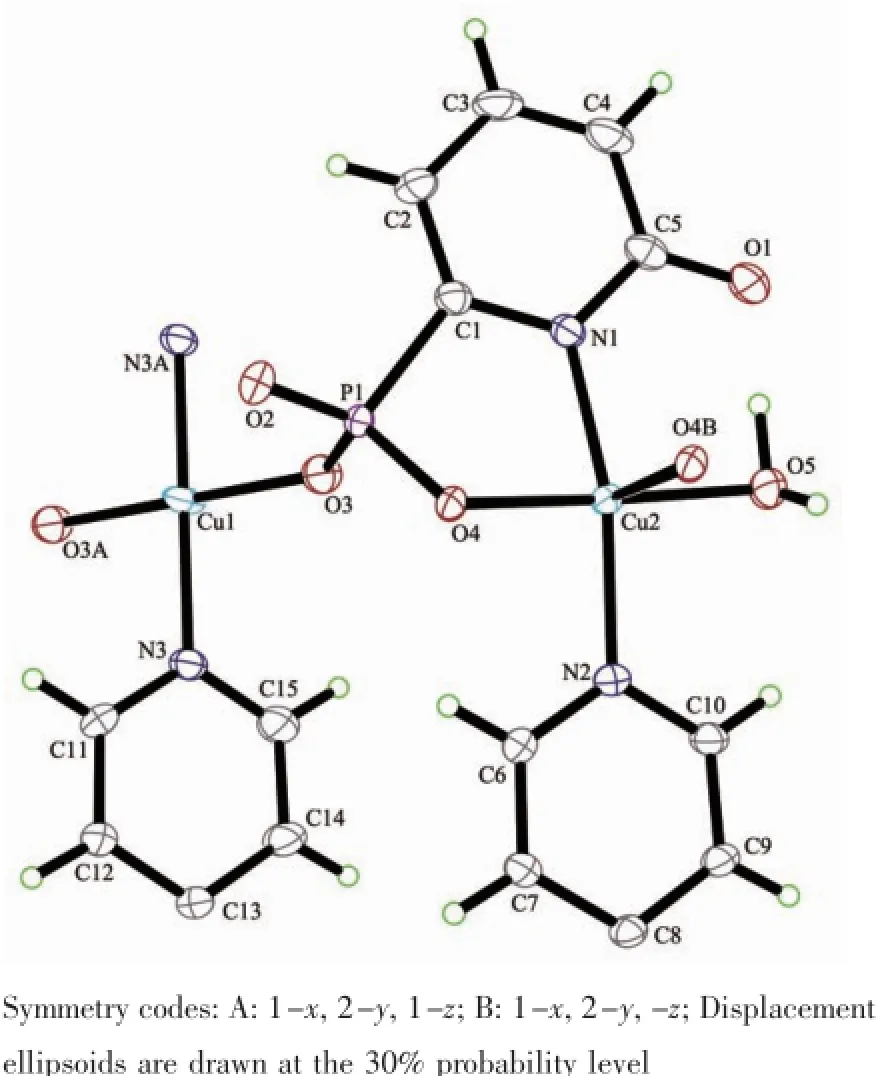
Fig.1 View of the coordination environments of Cu2+ions in compound 1
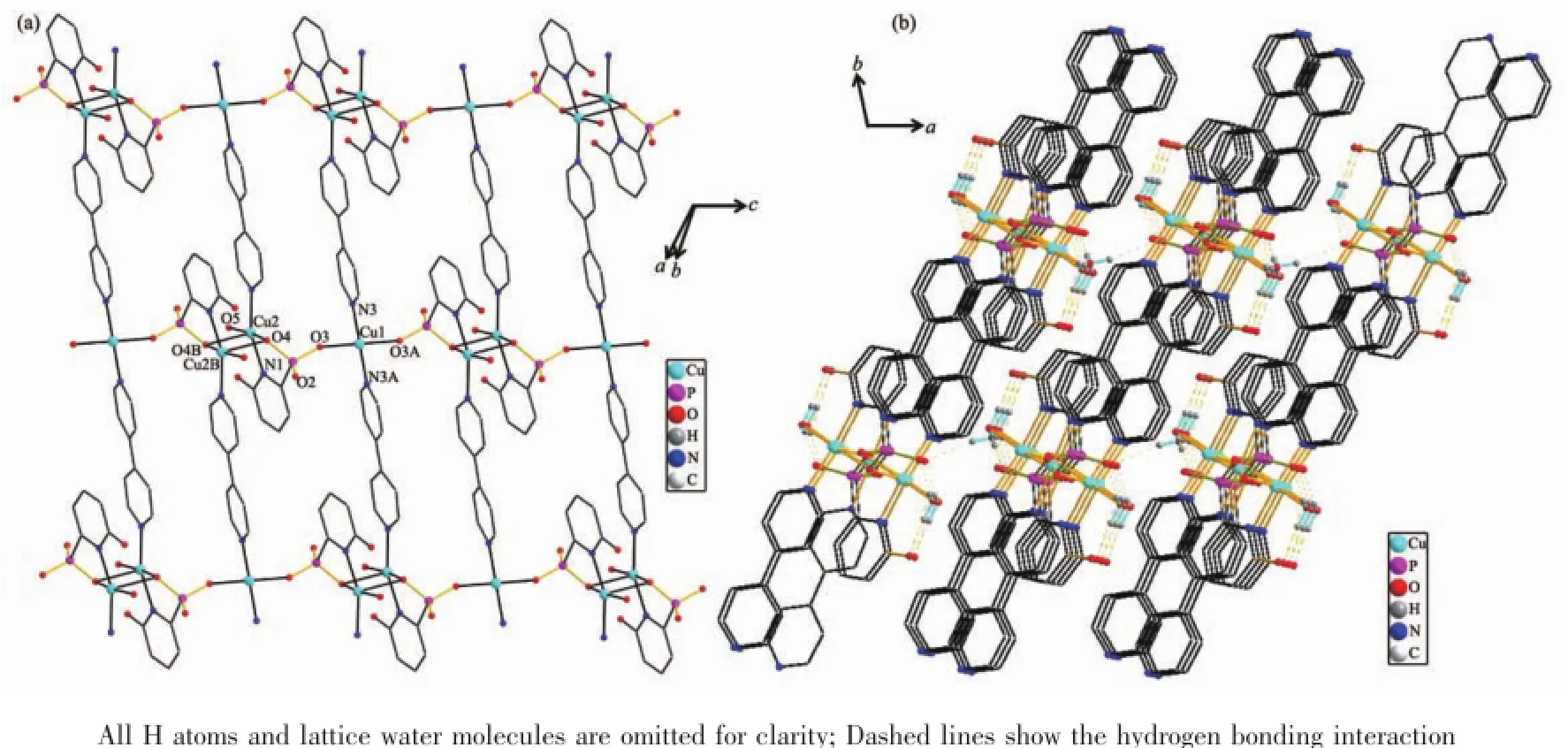
Fig.2(a)View of the 2D layered structure of compound 1;(b)View of the 3D framework for compound 1
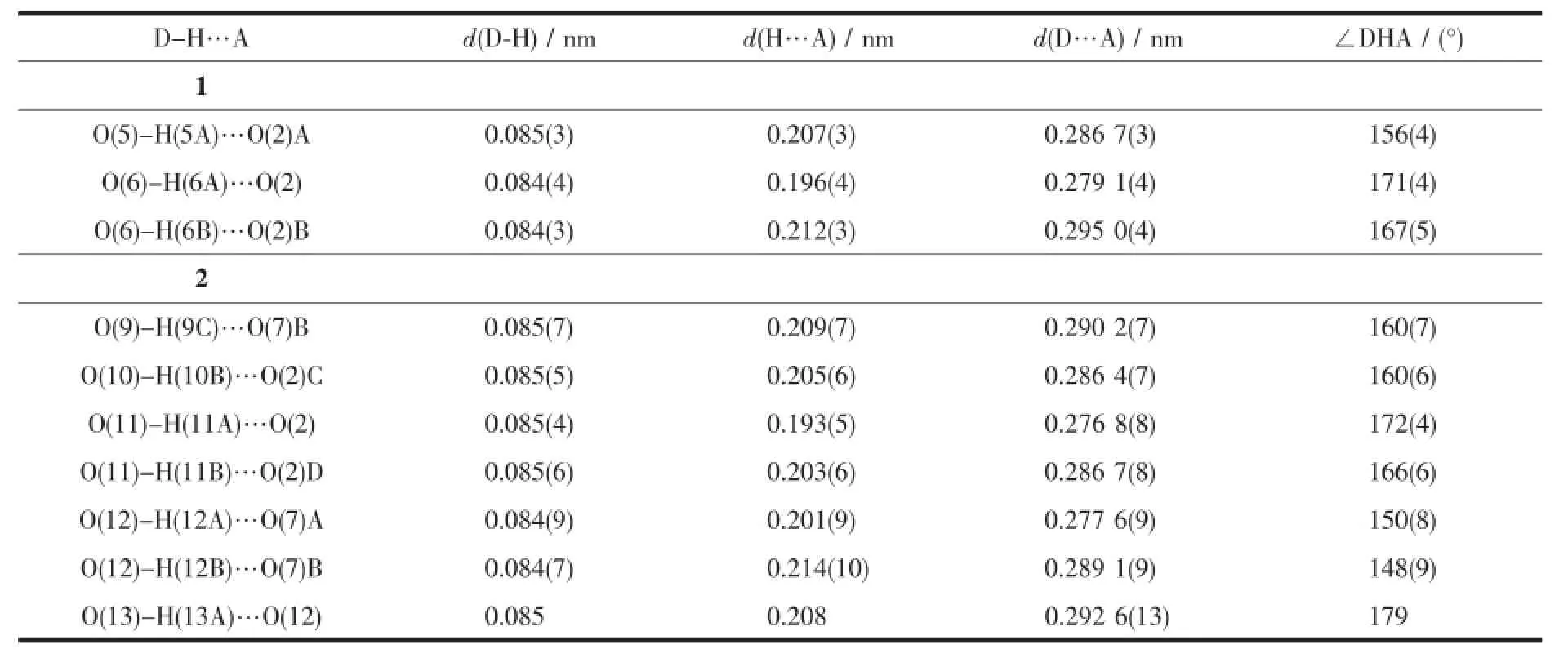
Table 3 Hydrogen bonds in 1 and 2
Compound 2 crystallizes in the triclinic space group P1.There are three Cu atoms,two phosphonates,two bpe ligands,three coordinated water,and two lattice water(Fig.3).Cu1 is square-pyramidal coordinated by(O3,O6,O11,N3,N2A)from two phosphonates,two bpe ligands and one coordinated water.The O11 locates on the axialposition.The Cu1-O3,Cu1-O6 and Cu1-O11 are 0.194 1(5),0.193 4(5) and 0.254 9(5)nm,respectively.The Cu-N bond lengths are 0.200 2(6)nm.The Cu2 and Cu3 atoms also have square-pyramidal environment.The five coordinationsites around the Cu2 atom are occupied by pyridyl nitrogen(N1),bpe nitrogen(N4),phosphonate oxygen (O1,O1B)atoms from two equivalent phosphonates and(O10)from water.Those around Cu3 atoms are provided by pyridyl and bpe nitrogen(N7C,N8)and phoshonate oxygen(O5C,O5D)atoms from two different phosphonate groups and oxygen atom(O9) from water.The Cu2-O1B(0.243 2(4)nm)and Cu3-O5D(0.240 1(5)nm)bond lengths on the axialposition are longer than thatin the planar positions(0.193 5(5) to 0.205 2(6)nm)(Table 2).The overall structure of 2 is isostructural to compound 1(Fig.4a).In the onedimensional chain,the Cu2…Cu2B and Cu3…Cu3E distances are 0.323 6 and 0.321 4 nm,similar to that in compound 1.The Cu2-O1-Cu2E and Cu3-O5CCu3E bond angles are 94.79°and 95.05°.The adjacent layers are connected by the classic hydrogen bonds (Table 3)to form a 3D supramolecular framework (Fig.4b).
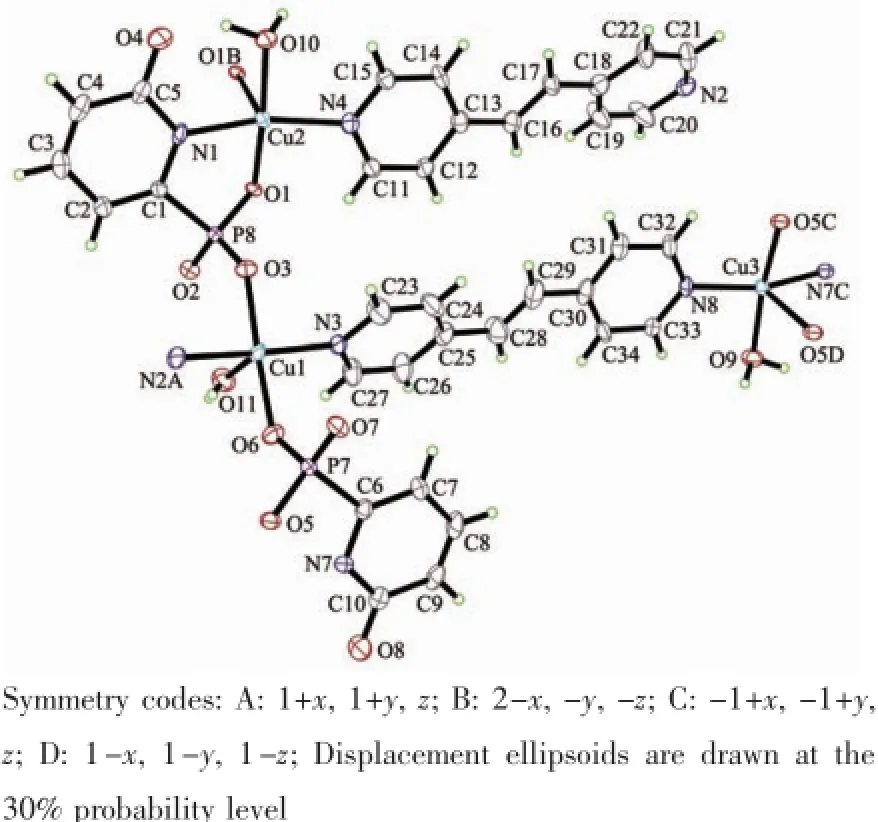
Fig.3 View of the coordination environments of Cu2+ions in compound 2
The 2D layered structures of 1 and 2 are remarkably different from[Cu3(O2CC5H3NPO3)2(H2O)2] and[Cu(HO2CC5H3NPO3H)2]with 2-phosphonic isonicotinic acid as ligand.Compound[Cu3(O2CC5H3NPO3)2(H2O)2]has a pillar-layered structure,while,[Cu(HO2CC5H3NPO3H)2]has a chain-like structure[32].The structure of 1 can be compared with Cu[(HO3PCH2)2C6H4(4,4′-bipy)]·2H2O,in which the phosphonate-copper chains are bridged by bpy into a 2D layer[33].This research demonstrates that the 6-hydroxy-2-pyridinephosphonic acid ligand can be used to construct structurally diverse coordination polymers.
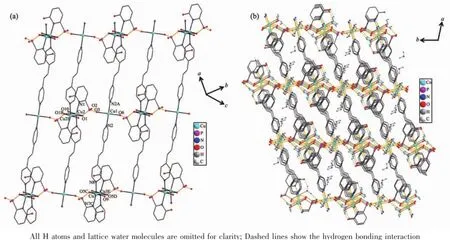
Fig.4(a)View of the 2D layered structure of compound 2;(b)View of the 3D framework for compound 2
2.2 Magnetic properties
The variable-temperature dc magnetic susceptibilities measurementfor 1 and 2 are shown in Fig.5 and Fig.6.The room temperatureχMT values are 1.24 and 1.21 cm3·K·mol-1for 1 and 2,respectively.These values are consistent with that of the expected forthree isolated copper ions 1.125 cm3·K·mol-1(g=2)[28]. On lowering the temperature,χMT slowly decreases until 50 K and then falls drastically reaching a value of0.40 and 0.40 cm3·K·mol-1for 1 and 2,respectively, at 1.8 K.The decrease ofχMT as the temperature is lowered indicating antiferromagnetic coupling between the Cu2+centers.According to the structure of both compounds,the magnetic exchange between the Cu ions may be propagated through theμ-O(P)bridges within the{Cu2O2}dimer and the O-P-O and bpy/bpe bridges.It is known that comparing theμ-O bridge, O-P-O and bpy/bpe bridges play a negligible role in propagating the magnetic exchange.Therefore the susceptibility data were analyzed by Bleaney-Bowers expression based on a Heisenberg Hamiltonian H= -2JS1S2and an isolated Cu2+ion[28]:
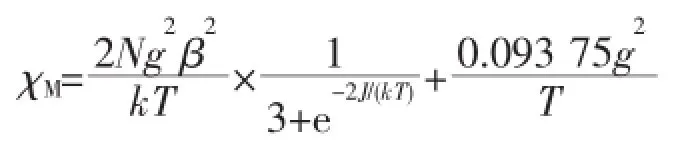
Where 2J is the singlet-tripletenergy gap and N,g,β, k have their usual meaning.Considering the intermolecular interaction(zJ′),good fit results were obtained in the solid line in Fig.5 and Fig.6,with the parameters g=2.1,J=-2.0 cm-1,zJ′=-0.6 cm-1for 1, and g=2.1,J=-1.7 cm-1,zJ′=-0.7 cm-1for 2.The minus J values observed here indicate that antiferromagnetic interactions are propagated among the Cu centers.
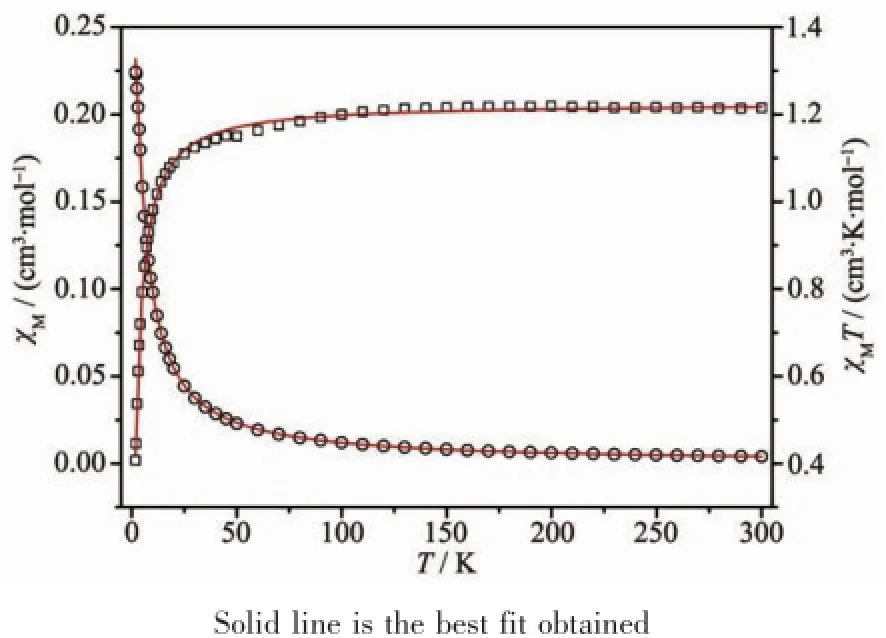
Fig.5χMandχMT vs T for 1
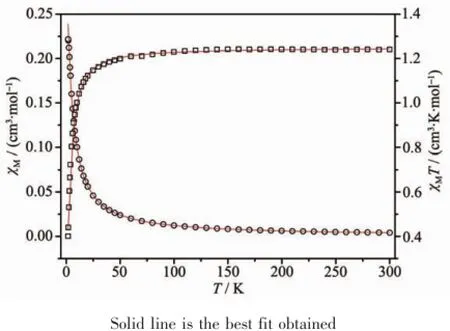
Fig.6χMandχMT vs T for 2
3 Conclusions
Through hydrothermal reactions,two copper phosphonates[Cu3(L)2(bpy)2(H2O)2]·2H2O and[Cu3(L)2(bpe)2(H2O)3]·2H2O were constructed by using 6-hydroxy-2-pyridinephosphonic acid as ligand.Both compounds show 2D layered structures with bpy/bpe as bridges.Although the hydroxyl group does not coordinate with mental centers,it experiences a deprotonation reaction under the hydrothermal reaction condition.Both compounds show antiferromagnetic interactions among the Cu2+centers.Further work is in progress by employing the longer bipyridinyl bridge ligand in the reaction system.
[1]Gagnon K J,Perry H P,Clearfield A.Chem.Rev.,2012,112: 1034-1054
[2]Zhang B L,Clearfield A.J.Am.Chem.Soc.,1997,119:2751 -2752
[3]Gallagher L A,Serron S A,Wen X G,et al.Inorg.Chem., 2005,44:2089-2097
[4]Liu J W,Chen L F,Cui H,et al.Chem.Soc.Rev.,2014,43: 6011-6061
[5]Kreno L E,Leong K,Farha O K,etal.Chem.Rev.,2012,112: 1105-1125
[6]Wang C,Zhang T,Lin W B.Chem.Rev.,2012,112:1084-1104
[7]Zhang W,Xiong R G.Chem.Rev.,2012,112:1163-1195
[8]Bideau J L,Bujoli B,Jouanneaux A,et al.Inorg.Chem., 1993,32:4617-4620
[9]Zhang Y P,Clearfield A.Inorg.Chem.,1992,31:2821-2826
[10]Bideau J L,Payen C,Palvadeau P,et al.Inorg.Chem., 1994,33:4885-4890
[11]Menaa B,Kariuki B,Shannon I J.New J.Chem.,2002,26: 906-909
[12]Arnold D I,Ouyang X,Clearfield A.Chem.Mater.,2002,14:2020-2027
[13]Chandrasekhar V,Nagarajan L,Gopal K,et al.Dalton Trans.,2005:3143-3145
[14]Yao H C,Li Y Z,Gao S,et al.J.Solid State Chem.,2004, 177:4557-4563
[15]Baskar V,Shanmugam M,Sańudo E C,etal.Chem.Commun., 2007:37-39
[16]Tang S F,Li L J,Wang C,et al.CrystEngComm,2014,16: 9104-9115
[17]Demadis K D,Panera A,Anagnostou Z,et al.Cryst.Growth Des.,2013,13:4480-4489
[18]Zheng L M,Yin P,Xin X Q.Inorg.Chem.,2002,41:4084-4086
[19]Zima V,Svoboda J,Yang Y C,et al.CrystEngComm,2012, 14:3469-3477
[20]Yin P,Zheng L M,Gao S,et al.Chem.Commun.,2001: 2346-2347
[21]Kong D Y,Li Y,Clearfield A,et al.Chem.Commun.,2003: 1720-1721
[22]Murugavel R,Singh M P.Inorg.Chem.,2006,45:9154-9156
[23]Pütz A M,Carrella L M,Rentschler E.Dalton Trans.,2013, 42:16194-16199
[24]Feyand M,Hübner A,Rothkirch A,etal.Inorg.Chem.,2012, 51:12540-12547
[25]Ma Y S,Song Y,Du W X,et al.Dalton Trans.,2006:3228-3235
[26]Samanamu C R,Zamora E N,Richards A F,et al. CrystEngComm,2008,10:1372-1378
[27]Ma Y S,Zha L Q,Cai W S,et al.Inorg.Chim.Acta,2013, 406:217-222
[28]Kahn O.Molecular Magnetism.New York:VCH Publishers Inc.,1993.
[29]CrystalClear,Rigaku Corporation,Tokyo,Japan,2005.
[30]Sheldrick G M.Acta Crystallogr.,Sect.A,2008,64:112
[31]Sousa-Pedrares A,Durán M L,García-Vázquez J A,et al. Inorg.Chem.,2009,48:4031-4043
[32]Yang Y F,Ma Y S,Guo L R,et al.Cryst.Growth Des., 2008,8:1213-1217
[33]Taddei M,Costantino F,Vivani R,et al.Cryst.Growth Des., 2012,12:2327-2335
Syntheses,Structures and Magnetic Properties of Two Copper Phosphonates Based on 6-Hydroxy-2-pyridinephosphonic Acid
LIHai-Qing1,2HUA Jing-Kun1ZHA Li-Qin1MA Yun-Sheng*,1TANG Xiao-Yan1XIE Ji-Min2YUAN Rong-Xin*,1
(1School of Chemistry and Materials Engineering,Changshu Institute of Technology,Changshu,Jiangsu 215500,China)
(2School of Chemistry and Chemical Engineering,Jiangsu University,Zhenjiang,Jiangsu 212013,China)
Two copper phosphonates[Cu3(L)2(bpy)2(H2O)2]·2H2O(1)and[Cu3(L)2(bpe)2(H2O)3]·2H2O(2)(H3L=6-hydroxy-2-pyridinephosphonic acid,bpy=4,4′-bipyridine,bpe=trans-1,2-bis(4-pyridyl)ethylene)were synthesized under hydrothermal conditions.In compound 1 the Cu2+ions are bridged by the phosphonate ligand into one chain,which are further bridged by bpy into a 2D layer.The layers are interlinked by hydrogen bonds into 3D supramolecular network.Compound 2 is isostructuralto compound 1,however,the bridge ligand is bpe.Magnetic measurements indicate antiferromagnetic interactions are propagated among the Cu2+centers in compound 1 and 2.CCDC:1044790,1;1044791,2.
copper compound;phosphonic acid;crystal structure;magnetic properties
O614.121
A
1001-4861(2015)07-1417-08
10.11862/CJIC.2015.184
2015-02-03。收修改稿日期:2015-05-07。
江苏省高校自然科学重大项目(No.12KJA150001,14KJA150001),江苏省科技基础研究计划(No.BK2012643),江苏省六大人才高峰计划资助项目。
*通讯联系人。E-mail:myschem@cslg.edu.cn,yuanrx@cslg.edu.cn
