Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis
2015-11-30MoniaDeghrigueCarmenFestaLotfiGhribiMariaValeriaAuriaSimonaDeMarinoHichemBenJannetAbderrahmanBouraoui
Monia Deghrigue, Carmen Festa, Lotfi Ghribi, Maria Valeria D’Auria, Simona De Marino,Hichem Ben Jannet, Abderrahman Bouraoui
1Laboratoire de développement chimique, galénique et pharmacologique des médicaments (LR12ES09). Equipe de Pharmacologie marine, Faculté de pharmacie de Monastir, Université de Monastir, 5000 Monastir, Tunisie
2Department of Pharmacy, University of Naples “Federico II”, via D. Montesano 49, I- 80131 Napoli, Italy
3Laboratoire de chimie hétérocyclique, produits naturels et réactivité. Equipe de chimie médicinale et produits naturels (LR11ES39), Faculté des sciences de Monastir, Université de Monastir, 5000 Monastir, Tunisie
1. Introduction
Inflammation is a protective response to tissue injury caused by physical trauma, noxious stimuli by chemical agents, heat, antigenantibody reaction and microbial effect. The signs and symptoms of inflammation, which sometimes may be potentially harmful needs pharmacological treatment[1]. Currently, non-steroidal antiinflammatory drugs are used throughout the world for the treatment of inflammation, pain and fever; however most of these produce several adverse reactions such as ulcers and hemorrhage[2]. The side effects of non-steroidal anti-inflammatory drugs may be associated to the inhibition of cyclooxygenase (COX)-1, enzyme involved in the synthesis of prostaglandins vital to normal cell function besides the inhibition of the cyclooxygenase-2 (COX-2)produced in response to growth factors or inflammatory mediators[3]. In addition to nonsteroidal anti-inflammatory drugs , multiple factors including stress,smoking, nutritional deficiencies, noxious agents such as alcohol,and Helicobacter pylori infection caused gastric ulcer[4]. Actually,drugs used for the treatment of gastric ulcer including histamine H2-antagonists, proton pump inhibitors, and antimuscarinics, as well as acid-independent therapy provided by sucralfate[5], induce adverse generate side effects such as hypersensitivity, arrhythmia,impotence, and hematopoietic changes. Therefore, the development of potent anti-ulcer and anti-inflammatory drugs from natural sources and with fewer side effects is necessary. In recent years,the soft corals and gorgonians of Octocorallia have become a new marine resource for searching of novel bioactive natural products as lead compounds in drug development[6]. The family Gorgoniidae has been demonstrated to contain a wide variety of natural products including steroids, acetogenins, sesquiterpenes and diterpenes[7].Among the substances that currently receive the most attention for drug design include the diterpenes purified from gorgonian,possessing a variety of pharmacologic effects, including anticancer,gastroprotective and anti-inflammatory properties[8]. Despite the biodiversity of the Mediterranean Tunisian coast, minimal efforts on the chemistry and biological activities of marine organisms have been conducted. Therefore, in a program to find new natural sources of anti-inflammatory agents with gastroprotective effect from Tunisian marine invertebrates, we studied the efficiency of organic extract and its fractions from the white gorgonian Eunicella singularis(E. singularis)(Cnidaria: Octocorallia, Esper 1791). The structure elucidation of the isolated compounds from the active fraction was done by 1D and 2D NMR experiments and by comparison with literature data.
2. Materials and methods
2.1. Collection and extraction
E. singularis was collected from the Mediterranean Sea in various areas of the coastal region of Tabarka (Tunisia), in June 2010, at a depth between 20 and 30 m. Identification of specimens was carried out in the National Institute of Marine Sciences and Technologies(Salamboo, Tunisia). After maceration of the powdered material with methanol and dichloromethane (1:1, v/v)for 48 h three times,the organic extract was purified, using C18 cartridges (Sep-pack,Supelco), by gradient elution with different organic solvents in the order of decreased polarity: ethanol, acetone and methanol/CH2Cl2(1:1)to give three semi-purified fractions: ethanol (F-EtOH), acetone(F-Ac)and methanol/CH2Cl2(F-MeOH/CH2Cl2)fractions. Organic solvents were removed from recuperated fractions using rotating evaporator at 40 ℃.
2.2. Purification, isolation and structure elucidation
F-EtOH was fractionated according to the Kupchan partitioning procedure[9]as follow: the ethanolic fraction was dissolved in a mixture of MeOH/H2O containing 10% H2O and partitioned against n-hexane to give 10.3 g of the crude extract. The water content (% v/v)of the MeOH extract was adjusted to 30% and partitioned against CHCl3to give 3.9 g of the crude extract. The aqueous phase was concentrated to remove MeOH and then extracted with n-BuOH(268 mg of crude extract). The n-hexane extract (5 g)was fractioned by silica gel MPLC using a solvent gradient system from CH2Cl2to MeOH. Fraction eluted with CH2Cl2: MeOH 99:1 (307 mg)was purified on a Nucleodur 100-5 C18 (5 μm; 10 mm i.d. × 250 mm)with 99% MeOH: H2O as eluent (flow rate 3 mL/min)to give 2.2 mg of cholesta-5,22-dien-3β-ol (5)(tR=25.5 min).
Fraction eluted with CH2Cl2:MeOH 95:5 (42 mg)was purified on a Nucleodur 100-5 C18 (5 µ, 4.6 mm i.d. × 250 mm)with 87%MeOH:H2O as eluent (flow rate 1 mL/min)to give 0.8 mg of (22E)-cholesta-5,22-diene-3β,7α-diol (3)(tR=26.4 min), 0.6 mg of ergosta-5,24(28)diene-3β,7α-diol (4)(tR=30 min)and 0.6 mg of cholest-5-ene-3β,7α-diol (2)(tR=33.6 min).
The CHCl3extract (3.9 g)was chromatographed by silica gel MPLC using a solvent gradient system from CH2Cl2to CH2Cl2:MeOH 1:1.
Fraction eluted with CH2Cl2: MeOH 95:5 (212 mg)was further purified by HPLC on a Nucleodur 100-5 C18 (5 µ, 4.6 mm i.d. × 250 mm)with 85% MeOH:H2O as eluent (flow rate 1 mL/min)to give 1.5 mg of palmonine F (1)(tR=4.8 min).
NMR spectra were obtained on Varian Inova 400 and Varian Inova 500 NMR spectrometers (1H at 400 and 500 MHz,13C at 100 and 125 MHz, respectively)equipped with a Sun hardware, δ (ppm),J in hertz, and spectra referred to CD3Cl3 (δH=7.27; δC= 70.0)as internal standard. HPLC was performed using a Waters model 510 pump equipped with Waters Rheodine injector and a differential refractometer, model 401.
Medium pressure liquid chromatography was performed on a Buchi apparatus using a silica gel (230-400 mesh column). The purities of compounds were determined to be greater than 95% by HPLC and NMR.
2.3. Pharmacological evaluation
2.3.1. Animals
Wistar rats of either sex, weighing 150-200 g, and swiss albinos mice weighing 18-25 g of both sex were obtained from Pasteur Institute (Tunis, Tunisia). Housing conditions and in vivo experiments were approved according to the guidelines established by the European Union on Animal Care (CCE Council 86/609).
2.3.2. Carrageenan-induced rat paw edema
The anti-inflammatory activity of the organic extract and its semi-purified fractions on carrageenan-induced paw edema was determined according to Hossain et al[1]. The animals were divided into three groups of six rats each. The control group received an intraperitoneal (ip.)dose of saline solution (NaCl 9‰, 2.5 mL/kg),the reference group received acetylsalicylate-lysine (ASL)(300 mg/kg, ip.), and the test groups received the organic extract of E.singularis (50, 100 and 200 mg/kg, ip.)and its semi-purified fractions(25 and 50 mg/kg, ip.). After 30 min, 0.05 mL of a 1% carrageenan suspension was injected into the left hind paw. The paw volume up to the tibiotarsal articulation was measured using a plethysmometer(model 7150, Ugo Basile, Italy). The measures were determined at 0 h (V0)(before carrageenan injection)and 1, 3 and 5 h later (VT)(after carrageenan injection). Paw swelling was determined for each rat and the difference between VTand V0was taken as the edema value. The percentages of inhibition were calculated according to the following formula:
2.3.3. Writhing assay in mice
The analgesic activity was performed according to the method of Ayed et al[10]. Swiss mice were divided into three groups of six mice each. One group served as the control group and was treated with 10 mL/kg of vehicle administered through subcutaneous injection(s/c.). The second group was treated with ASL (200 mg/kg, s/c)as a reference drug, while the third group was treated with the organic extract of E. singularis at different doses (100, 200 and 400 mg/kg, s/c), F-EtOH, F-Ac and F-MeOH/CH2Cl2fractions (25, 50 and 100 mg/kg, s/c). 30 min after the administration of these different substances, all animals received 10 mL/kg (ip.)of 1% acetic acid.The number of abdominal writhing as a pain indicator was counted for 30 min.
随机抽选我院2015年4月~2017年4月收治的40例儿茶酚胺增多症患者资料作为研究对象,其中男性患者32例,女性患者8例,年龄12~43岁,平均年龄(32.6±2.3)岁。
2.3.4. Gastric lesions induced by HCl/ethanol
The gastroprotective activity of the organic fractions of E. singularis was studied in 150 mM HCl/EtOH induced gastric ulcer[4]. Rats were divided into three groups, fasted for 24 h prior receiving an intraperitoneal doses of vehicle (NaCl 9‰, 2.5 mL/kg)for the control group, organic extract and fractions (5, 10, 25, 50 and 100 mg/kg, ip.)for the test group. Reference group, received cimetidine(100 mg/kg, ip.)and omeprazole (30 mg/kg, ip.)as reference drugs.After 30 min, all groups were orally treated with 1 mL/100 g of 150 mM HCl/EtOH solution for gastric ulcer induction. Animals were killed 1 h after the administration of ulcerogenic agent, their stomachs were excised and opened along the great curvature,washed and stretched on cork plates. The surface was examined for the presence of lesions and the extent of the lesions was measured.The summative length of the lesions along the stomach was recorded(mm)as lesion index.
2.3.5. Statistical analysis
Data are presented as the mean ± standard error (SE). Statistical analysis was performed using Student’s t-test. The significance of difference was considered to include values of P<0.05.
3. Results
As shown in Figure 1, 1D and 2D NMR analysis of the ethanolic fraction (F-EtOH)from the gorgonian E. singularis resulted in the identification of five compounds.
Compound 1 was isolated as colorless oil. The molecular formula was determined to be C24H38O6by HRESIMS. The analysis of1H NMR spectrum clearly revealed an eunicellin diterpenoid structure.The13C NMR spectrum revealed 24 carbon signals.1H- and13C NMR assignments were carried out with the aid of the detailed 2D analyses (COSY, HMQC, NOESY, and HMBC)and the resulting NMR evidence revealed 1 to be defined as palmonine F[11].
Compound 2 was isolated as colorless powder. The molecular formula was determined to be C27H46O2. Analysis of1H and,13C NMR data evidenced a △5 dihydroxy-steroid structure with a saturated C8 cholestane side chain. Comparison with literature data allowed to assign the cholest-5-ene-3β,7α-diol structure[12].This compound was also isolated from the sponge Stelodoryx chlorophylla[13], from a soft coral Dendronephthya gigantea[14]and a marine bryozoan Biflustra grandicella[15].
Compound 3 has a molecular formula of C27H44O2as determined by HRESIMS.1H and13C NMR indicated that compound 3 is the △22derivative of compound 2. Therefore compound 3 was identified as (22E)-cholesta-5,22-diene-3β,7α-diol[12](Figure 1).
Compound 4 was isolated as colorless powder. The molecular formula was determined to be C28H46O2and NMR analysis indicated the presence of one exo-methylene function (δH 4.64, br s and 4.78, br s). Its identity was determined by 1D and 2D NMR data as ergosta-5,24(28)diene-3β,7α-diol earlier isolated from the marine sponge Stelodoryx chlorophylla[13]and then reported also from the soft coral Sinularia flexibilis[16].
Compound 5 was isolated as colorless powder. The molecular formula was determined to be C27H44O by HRESIMS data. NMR data disclosed the cholesta-5,22-diene-3β-ol structure[17].
The anti-inflammatory activity of E. singularis organic extract and its semi-purified fractions against acute pedal edema(induced by carrageenan)is shown in Table 1 and the results are comparable to that of the standard drug, ASL, a potent inhibitor of cyclooxygenase-2. Carrageenan induced paw edema remained even 6 h after its injection into the sub plantar region of rat paw. ASL as a reference standard drug inhibited the edema formation due to carrageenan to an extent of 52.78% (at 3 h)at the dose of 300 mg/kg. The extract or fractions from E. singularis significantly inhibited edema formation in rats (P<0.01)in a dose dependent manner. The organic extract at the dose of 200 mg/kg inhibited edema formation to the extent of 64.75% (at 3 h)and the edema was found to be reduced to (19.29±3.40)×10-2mL. The F-EtOH has the highest activity at a dose of 50 mg/kg with a percentage of inhibition of edema of 66.9% at 3 h, while F-Ac and F-MeOH/CH2Cl2at a dose of 50 mg/kg reduced edema with a percentage of 49% and 54%,respectively. The presence of edema is one of the prime signs of inflammation[18]. Peripheral analgesic activity was assessed by acetic- acid induced writhing test, which showed significant(P<0.01)suppression of writhes (Table 2). Injection of acetic acid into the control mice resulted in 86.0 ± 2.5 writhes. Pretreatment with organic extract at doses of 200 and 400 mg/kg reduced the number of writhes to 51.22 (40.44% of inhibition)and 30.65(64.35% of inhibition)respectively. Interestingly, F-MeOH/CH2Cl2(31.42 writhes, 63.46% of inhibition)and F-EtOH (32.56 writhes,62% of inhibition)at a dose of 100 mg/kg registered higher levels of analgesic activity than F-Ac (57.53 writhes, 56.36% of inhibition)and approaches the activity of the standard drug ASL (29.96 writhes,65% of inhibition).
The gastroprotective effect of E. singularis organic extract and fractions against HCl/EtOH induced gastric damage in rats is shown in (Figure 2, 3 and 4)and the results are comparable to that of the standard drugs cimetidine, a histamine H2 receptor antagonist, and omeprazole, a proton pump inhibitor[19]. For the organic extract,the inhibition of gastric lesions varied between 46.7 and 68.8 % at doses of 25 and 100 mg/kg (Figure 2). The F-EtOH had the highest activity, the lesion index was inhibited by 45%, 53%, 70%, 78% and 80% at doses of 5, 10, 25, 50 and 100 mg/kg, respectively (Figure 3),While for the F- MeOH/CH2Cl2, the inhibition of gastric lesions was observed only at doses of 25, 50 and 100 mg/kg (Figure 4)which percentage was 65%, 68% and 73%, respectively. The F-Ac failed to reduce the gastric lesions induced by EtOH/HCl.One diterpenoid: palmonine F (1), and four sterols (2-5): cholest-5-ene-3β,7 α-diol (2); (22E)-cholesta-5,22-diene-3β,7α-diol (3); ergosta-5,24(28)diene-3β,7α-diol (4); and cholesta-5, 22-diene-3β-ol (5).

Table 1Anti-inflammatory effect of E. singularis organic extract and its semi-purified fractions in carrageenan-induced rat paw edema test.
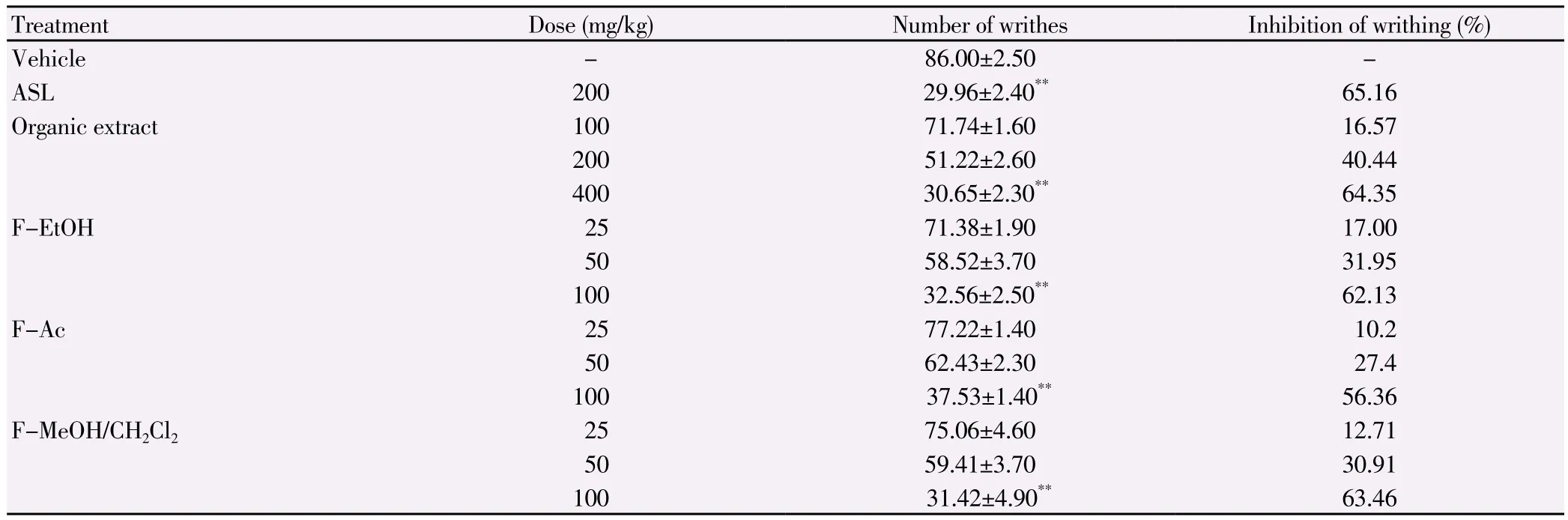
Table 2Analgesic effect of the organic extract of E. singularis and its semi-purified fractions in the acetic acid 1% writhing test in mice.
4. Discussion
The current study was carried out to determine the in vivo antiinflammatory, analgesic and gastroprotective activities of the organic extract of E. singularis and its semi-purified fractions. The chemical composition of the more effective fraction was determined by both 1D and 2D NMR experiments.
Carrageenan induced edema has been commonly used as an experimental animal model for acute inflammation and is believed to be biphasic[20]. The early phase (1-2 h)of the carrageenan model is mainly mediated by histamine, serotonin and increased synthesis of prostaglandins in the damaged tissue surroundings. The late phase (2.5- 6 h)is due to the over production of prostaglandin and nitric oxide with peak at 3h, produced by inducible isoform of cyclooxygenase (COX-2)and nitric oxide synthase[21]. However,treatment with the organic extract or fractions of Eunicella singularis significantly reduced, at dose dependent manner, carrageenan on induced inflammation in both phases (1-6 h)of the experiment.Based on this, it may be that the organic extract/or fractions of E.singularis has a non-selective inhibiting effect on the release or actions of these mediators of inflammation and the suppression of the 1st phase may be due to inhibition of the release of early mediators, such as histamine and serotonin and the action in the second phase may be explained by an inhibition of COX-2.
Peripheral analgesic activity was assessed by acetic- acid induced writhing test. Acetic acid is known to trigger the production of noxious substances within the peritoneum, which induces the writhing response[22]. The effect of organic extract or fractions against the noxious stimulus may be an indication that it depressed the production and release of mediators of pain and there by bringing a reduction in the number of writhes in animals. The writhing induced by chemical substances is due to sensitization of nociceptors by prostaglandins[23]. The results indicate that the analgesic effect of the organic extract of E. singularis and its semi-purified fractions might be mediated by its peripheral effects by inhibiting the synthesis or action of prostaglandins.
Ethanol has been revealed to produce gastric damage and the severity of gastric ulcer could be obviously evaluated by ulcer lesions. In this study, results showed that ethanol/HCl alone intake produced gastric mucosal damage, severe hemorrhage and lesions ulcers. But, pretreatment by organic extract or fractions from E.singularis effectively protected stomach tissues from mucosal damage and gastric lesions ulcers. Moreover, the protective effect of this extract and fractions demonstrated a dose-response relationship. Several mechanisms have been suggested for the effect of gastroprotective compounds, including increase in the gastric hexosamine level and enhancing the strength of the gastric barrier either physically or by blocking the H+, K+/ATPase pump[24]. Among the main classes of chemical bioactive components capable of offering gastroprotection are triterpenes[25]. The chemical analysis of the active fraction (F-EtOH)revealed the presence of an eunicellintype diterpenoid, palmonine F (1)and four sterols: cholest-5-ene-3β,7α-diol (2); (22E)-cholesta-5,22-diene-3β,7α-diol (3);ergosta-5,24(28)diene-3β,7α-diol (4)and cholesta-5,22-diene-3 β-ol (5). Several terpenes or their derivatives have been shown to possess gastroprotective activities in different models of induced gastric lesions in animals[26]. In addition, Ioannou et al[27]reported that steroids of marine origin exhibited an anti-inflammatory activity. There is some study reporting the anti-inflammatory activity of eunicellin-based diterpenoid[28]. This works seems to be related with our results. On the other hand, anti-inflammatory, analgesic and gastroprotective activities may be attributed to various phenolic compounds (alkaloids, glycosides, terpenoids, steroids, and saponins)detected in E. singularis organic extract and fractions[29]. Several studies reported that alkaloids have analgesic, anti-inflammatory and gastroprotective effects. Glycosides, terpenoids and steroids detected in our samples are known to have anti-inflammatory and analgesic properties[30]. The synergic effect of different compounds of E.singularis ethanolic fraction may be responsible for its higher antiinflammatory and analgesic activities with gastroprotective effect.
In conclusion, the ethanolic fraction of E. singularis demonstrated the highest activity in the three tests (anti-inflammatory, analgesic and gastroprotective). The structure elucidation of compounds isolated from this fraction revealed the presence of a eunicellanbased diterpenoid and four sterols which may be responsible for its activity.
Conflict of interest statement
We declare that we have no conflict of interest.
[1]Hossain H, Al-Mansur A, Akter S, Sara U, Ahmed MR, Jahangir AA.Evaluation of anti-inflammatory activity and total tannin content from the leaves of Bacopa monnieri (Linn.). Int J Pharm Sci Res 2014; 5(4): 1246-1252.
[2]Hajhashemi V, Sajjadi SE, Heshmati M. Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydro alcoholic extract in animal models. J Ethnopharmacol 2009; 124: 475-480.
[3]Karoui A, Allouche F, Deghrigue M, Agrebi A, Bouraoui A, Chabchoub,F. Synthesis and pharmacological evaluation of pyrazolopyrimidine derivatives: anti-inflammatory agents with gastroprotective effect in rat.Med Chem Res 2013; 23: 1591-1598.
[4]de Souza Almeida ES, Filho VC, Niero R, Clasen BK, Balogun SO, de Olveira Martins DT. Pharmacological mechanisms underlying the antiulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hill. in animal models. J Ethnopharmacol 2011; 134(3): 630-636.
[5]Bighetti AE, Antonio MA, Kohn LK, Rehder VL, Foglio MA, Possenti A, et al. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz Bip. Phytomed 2005; 12: 72-77.
[6]Wang C, Liu H, Shao C, Wang Y, Li L, Guan, H. Chemical defensive substances of soft corals and gorgonians. Acta Ecologica Sinica 2008;28(5): 2320-2328.
[7]Berrue F, Kerr RG. Diterpenes from gorgonian corals. Nat Prod Rep 2009;26: 681-710.
[8]Ioannou E, Abdel-Razik AF, Alexi X, Vagias C, Alexis MN, Roussis,V. Pregnanes with antiproliferative activity from the gorgonian Eunicella cavolini. Tetrahedron 2008; 64: 11797-11801.
[9]Festa C, De Marino S, Sepe V, Monti MC, Luciano P, D’Auria MV, et al.Perthamides C and D, two new potent anti-inflammatory cyclopeptides from a Solomon Lithistid sponge Theonella swinhoei. Tetrahedron 2009; 65:10424-10429.
[10]Ayed Y, Dellai A, Ben Mansour H, Bacha H, Abid S. Analgesic and antibutyrylcholinestrasic activities of the venom prepared from the Mediterranean jellyfish Pelagia noctiluca (Forsskal, 1971). Ann Clin Microbiol Antimicrob 2012; 11:15.
[11]Ortega MJ, Zubia E, Salva J. Structure and absolute configuration of palmonine F, a new eunicellan-based diterpene from the gorgonian Eunicella verrucosa. J Nat Prod 1994; 57(11): 1584-1586.
[12]Notaro G, Piccialli V, Sica D. New steroidal hydroxyketones and closely related diols from the marine sponge Cliona copiosa. J Nat Prod 1992;55(11): 1588-1594.
[13]de Riccardis F, Minale L, Iorizzi M, Debitus C, Levi C. Marine sterols.Side-chain-oxygenated sterols. Possibly of abiotic origin, from the New Caledonian sponge Stelodoryx chlorophylla. J Nat Prod 1993; 56: 282-287.
[14]Li GQ, Deng ZW, Guan HS, Guo DA, Lin WH. Polyhydroxy sterols from soft coral Dendronephthya gigantea from the South Chin Sea. J Chin Mar Drugs 2004; 1: 1-5.
[15]Yang F, Zhang HJ, Liu XF, Chen WS, Tang HF, Lin HW. Oxygenated steroids from marine bryozoan Biflustra grandicella. Biochem Syst Ecol 2009; 37: 686-689.
[16]Yu S, Deng Z, van Ofwegen L, Proksch P, Lin W. 5,8-Epidioxysterols and related derivatives from a Chinese Soft Coral Sinularia flexibilis. Steroids 2006; 71: 955-959.
[17]Idler DR, Wiseman P. Identification of 22-cis-cholesta-5, 22-dien-3 β-ol and other scallop sterols by gas-liquid chromatography and mass spectrometry. Comparative Biochem Physiol Part A: Physiol 1971; 38(3):581-590.
[18]Yonathan M, Asres K, Assefa A, Bucar F. In vivo anti-inflammatory and anti-nociceptive activities of Cheilanthes farinosa. J Ethnopharmacol 2006; 108: 462-470.
[19]Ishihara M, Ito M. Influence of aging on gastric ulcer healing activities of cimetidine and omeprazole. Eur J Pharmacol 2002; 444: 209-215.
[20]Carey MW, Rao NV, Kumar BR, Mohan GK. Anti-inflammatory and analgesic activities of methanolic extract of Kigelia pinnata DC flower. J Ethnopharmacol 2010; 130: 179-182.
[21]Panthong A, Supraditaporn W, Kanjanapothi D, Taesotikul T, Reutrakul V. Analgesic, anti-inflammatory and venotonic effects of Cissus quadrangularis Linn. J Ethnopharmacol 2007; 110: 264-270.
[22]Bartolini A, Galli A, Ghelardini C, Giotti A, Malcangio M, Malmberg-Aiello MP, et al. Antinociception induced by systematic administration of local anaesthetics depends on a central cholinergic mechanism. Br J Pharmacol 1987; 92: 711-721.
[23]Vasudevan M, Gunnam KK, Parle M. Antinociceptive and antiinflammatory properties of Daucus carota seeds extract. J Health Sci 2006;52: 598-606.
[24]Repetto MG, Llesuy SF. Antioxidant properties of natural compounds used in popular medicine for gastric ulcer. Brazilian J Medd Biol Res 2002; 35:523-534.
[25]Klein JLC, Gandolfi RB, Santin JR, Lemos M, Filho VC, de Andrade SF. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalacea).Naunyn-Schmiedeberg’s Arch Pharmacol 2010; 381: 121-126.
[26]Mequanint W, Makonnen E, Urga, K. In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J Ethnopharmacol 2011; 134: 32-36.
[27]Ioannou E, Abdel-Razik AF, Alexi X, Vagias C, Alexis MN, Roussis,V. 9,11-Secosterols with antiproliferative activity from the gorgonian Eunicella cavolini. Bioorganic & Med Chem 2009; 17: 4537-4541.
[28]Hassan H, Khanfar MA, Elnagar AY, Mohammed R, Shaala LA, Youssef DTA, et al. Pachycladins A-E, prostate cancer invasion and migration inhibitory eunicellan-based diterpenoids from the red sea soft coral Cladiella pachyclados. J Nat Prod 2010; 73: 848-853.
[29]Deghrigue M, Dellai A, Bouraoui A. In vitro antiproliferative and antioxidant activities of the organic extract and its semi-purified fractions from the Mediterranean gorgonian Eunicella singularis. Int J Pharm Pharm Sci 2013; 5 (2): 432-439.
[30]Radjasa OK, Vaske YM, Navarro G, Vervoort HC, Tenney K, Linington RG, et al. Highlights of marine invertebrate-derived biosynthetic products:their biomedical potential and possible production by microbial associants.Bioorganic & Med Chem 2011; 19: 6658-6674.
猜你喜欢
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Effect of Yupingfeng granules on HA and Foxp3+ Treg expression in patients with nasopharyngeal carcinoma
- Rifabutin reduces systemic exposure of an antimalarial drug 97/78 upon co- administration in rats: an in-vivo & in-vitro analysis
- Analysis of good practice of Public Health Emergency Operations Centers
- Effect of Yupingfeng granules on HA and Foxp3+ Treg expression in patients with nasopharyngeal carcinoma
- Antitumor effect of recombinant human endostatin combined with cisplatin on rats with transplanted Lewis lung cancer
- Late cardioprotection of exercise preconditioning against exhaustive exercise-induced myocardial injury by up-regulatation of connexin 43 expression in rat hearts
