Effects of Three Different Diluents on Quality of Boar Semen Stored at 17℃
2015-11-25HuShanZhangXiaogangHanCongWeiShuaiyiXieDongqiDuRenrangandHuJianhong
Hu Shan, Zhang Xiao-gang, Han Cong, Wei Shuai-yi, Xie Dong-qi, Du Ren-rang, and Hu Jian-hong
College of Animal Science and Technology, Northwest A&F University, Yangling 712100, Shaanxi, China
Effects of Three Different Diluents on Quality of Boar Semen Stored at 17℃
HuShan,ZhangXiao-gang,HanCong,WeiShuai-yi,XieDong-qi,DuRen-rang,andHuJian-hong*
CollegeofAnimalScienceandTechnology,NorthwestA&FUniversity,Yangling712100,Shaanxi,China
Toinvestigatetheeffectsofdifferentdiluentsonthequalityoftheboarsemenstoredat17℃,andassesstherelationship betweenspermmotilityandtherelativelevelsofenzymes,threecommercialdiluents(DiluentⅠ,DiluentⅡ andDiluentⅢ)andthree boarbreedsemens(Yorkshire,LandraceandDuroc)wereutilized.Thespermmotility,effectivesurvivaltime,survivalindex,catalase (CAT),thetotalanti-oxidativecapacity(T-AOC)andmalondialdehyde(MDA)levelswereevaluated.Theresultsshowedthatthere weresignificantinteractioneffectsbetweendiluentsandbreedsontheboarspermmotility(P<0.001),survivaltime(P<0.001),CAT levels(P<0.001)andT-AOClevels(P<0.001),butneithereffectsnorinteractioneffectsbetweendiluentsandbreedsonsurvival index(P>0.05).Alloftheparametersvariedsignificantlywiththeincreaseofthestoragetime(P<0.001).Thesurvivaltimeincreased 12.9%inYorkshireboarsemendilutedwithDiluentⅢthanwithDiluentⅡ,whilethesurvivaltimeincreased6.6%inLandraceboar semendilutedwithDiluentⅡthanwithDiluentⅢ.BothCATandT-AOClevelsweresignificantlypositivecorrelatedwithsperm motilityinallthethreeboarbreeds(P<0.001),whileMDAlevelsweresignificantlynegativecorrelatedwithspermmotility(P<0.001). TheseresultsindicatedthatDiluentⅢandDiluentⅡ weretheoptimalcommercialdiluentsforYorkshireandLandraceboarsemen storedat17℃,respectively.
boarsemen,storage,differentdiluents,survivalindex,enzymeactivity
Introduction
Artificialinsemination(AI)isamomentoustoolin pigindustrytoimprovethegeneticgenewithfrozen semen(Castellanoet al.,2010).Comparedwithother animalspecies,boarspermisfragiletocoldshockfor differentphospholipids,suchasunsaturatedfattyacids (UFAs),compositioninspermmembranes(Fraczek et al.,2001;Zhanget al.,2012;Silvaet al.,2015). Frozenboarsemenhasbeenperformedsince1975,but thecommercialdiluenthasnostablebreakthroughfor thesusceptibilityofboarspermatozoa(Zhanget al., 2012).Asthebarrieroflowfecundityratescausedby AIusingfreeze-thawsemen,extensiveworkhasbeen doneonpreservationofboarsemenwithdiluentat normaltemperature(15-20℃)(Althouseet al.,1998; ZouandYang,2000;Vytet al.,2007;Perez-Llano et al.,2009).Ithasbeenconsideredthattheliquid boarsemenwouldmaintainhigherspermfertility comparedwithfrozensemen(ClarkeandJohnson, 1987).Therefore,storageofboarsemeninnormaltemperaturewasincreasinglyexploitedinAI.
However,therearestillmanydeficienciesinnormal temperaturestorage.Duringthestorageprocessof boarsemen,boarspermissubjectedtodecrease motilityandviability.Inthemeantime,thesperm membranepermeabilitywouldbealteredpartly byoxidativedamagethatcausedbyinappropriate formationofreactiveoxygenspecies(ROS)(Wang et al.,1997;GuthrieandWelch,2010).Thesystems ofprotectiveanti-oxidantinspermatozoa,stem fromthecytoplasm,areconsistedofglutathione peroxidase(GSH-Px),catalase(CAT)andsuperoxide dismutase(SOD)(Bilodeauet al.,2001).Ithasbeen authenticatedthatthissystemswhichareessentialto maintainspermmotilityandviabilitydefendagainst thelipidperoxidation(Huet al.,2009).
Keepingpacewiththedevelopmentofthe preservationboarsemenfrom15℃to17℃,many researchersareinterestedinthediluentbreakthrough. ThediluentwillfacilitatetobalancepH,maintain spermmembranesintegrity,inhibitspermcapacitation anddefendtheoxidativedamage(Watson,1990; Weitze,1991;Huet al.,2009).Theeffectsofdiluent onthespermqualityparameters,duringthestorage processwerewelldocumented(Estienneet al., 2007;KusterandAlthouse,1999;Vytet al.,2004; Waterhouseet al.,2004;Martín-Hidalgoet al., 2013).Asthediluentneedtobeadaptedtoindividual differencesamonganimals(Gadea,2003;Levis, 2000),Martín-Hidalgoet al.(2013)studiedtheeffects ofdifferentdiluentsonspermmotilityandviability, reactiveoxygenspecieswerethoughttobethe importantreasonthatproducedanddamagedsperm cellmotilityandgenomicintegrity(Clarkson,1988; Storey,1997;Aitkenet al.,1998;Bilodeauet al., 2000;Footeet al.,2002),andtheresultsrequired abetterunderstandingthatdifferentdiluentshad differenteffectsonsemenfromdifferentboarbreeds.
Toinvestigatetheeffectsofdifferentdiluentson thespermmotilityandthelevelsofenzymesfrom differentboarbreedsstoredat17℃,andassessedthe relationshipbetweenspermmotilityandtherelative levelsofenzymes,threedifferentdiluentsandthree boarbreedsemens(Yorkshire,LandraceandDuroc) wereutilizedinthepresentstudy.Thespermmotility, ROSrelatedenzymelevelsofcatalase(CAT),thetotal anti-oxidativecapacity(T-AOC)andmalondialdehyde (MDA)duringthestorageweremeasured,the correlationbetweenspermmotilityandeachenzyme levelwasalsoanalyzed.
Materials and Methods
Thecareandproceduresofboarthatusedtocollect semenwereapprovedbytheAnimalCareand UseCommitteeofFujianAgricultureandForestry University.
Semen collection and diluent prepare
Thesexuallymatureboars(1.5-2years)ofYorkshire (n=5),Landrace(n=5)andDuroc(n=5)fromShanjia Co.,Ltd.wereemployedinthisstudyfromSeptember of2012toSeptemberof2013.Total150-200mL semenwascollectedfromeachboarineachtimeby glovedhandtechniqueandfilteredthroughfourlayers ofsterilecottongauze.Subsequently,thesemenwas transferredintoasteriletube.Thevolume,sperm concentrationandpercentmotilespermatozoawere assessedinsperm-richfractions.Whensemenhad morethan80%ofspermmotilityandnormalsperm morphologywouldbeused.Toeliminatevariability betweentheevaluated,theejaculateswerepooledfor eachbreedboarinthepresentstudy,respectively.All theexperimentswererepeatedthreetimes.
Diluentswerepurchasedfromthreecommercial companiesandthecompositionswerenotclearforthe protectionofintellectualproperty.Theyweredesigned asthegroupsofDiluentⅠ,DiluentⅡandDiluentⅢanddissolvedinthedouble-distilledwaterwiththe samevolume.
Semen processing
Afterthesemenqualitytomeettherequirements, thecollectedboarsemenwasequallydividedintothreeparts.Subsequently,theratioofsemendiluted byDiluentⅠ,DiluentⅡandDiluentⅢwas2:1.The temperatureofdiluentsweresamewithboarsemen. Thedilutedsemenwasgentlymixed,andallthetubes werewrappedby10layersofsterilegauze.They wereslowlycooledto17℃.Finally,theywerestored inthe17℃thermostat.Weshooksemenonceevery 12handdetectedtheparametersevery24huntil spermmotilitydecreasedto50%.
Assessment of sperm motility, effectively survival time and survival index
Spermmotilityreferstotheabilityorintensityof spermactivity.Phasecontrastmicroscopywasrecommended for motility evaluation. Five μL semen sampleswereplacedonglassandvisuallyexamined thepercentageoflinearmotilespermbylight microscopeat400×.Thepercentageofspermmotility washigherthan50%,whichwasconsideredasthe effectivelysurvivaltime.Thesurvivalindexofsperm wascalculatedasthefollowingequation:I=(MA+MB)× 2/D(Iindicatedthesurvivalindex,MAandMBdemonstratedthespermmotilityinAdayandBday,respectively,andDwasconsideredastheintervaltime betweenAdayandBday.
Biochemical assay
Semenwaspouredinto0.5mLcentrifugetubes andcentrifugedat1000r•min-1for10min.The supernatantwasaspirated,thenadded1mLof1% Triton,mixedwellandplacedafter20min,then centrifugedat4000r•min-1for30min.Thenthe supernatantwasextractedfordetectingenzyme activity.TheenzymaticactivitiesofCAT,T-AOCand MDAweredetectedbythekit(NanjingJianchengCo., Ltd.,China)followingthedescription,respectively.
CAT
CATactivityassaywasperformedaccordingtothe methodofGoth(1991).Total0.3mLofpretreated semenwasincubatedin1.7mLofsubstrate(pH7.0, 65 μmol •L-1hydrogenperoxidein50mmol•L-1phosphatebuffer)at37℃for60s.CATdecomposition ofhydrogenperoxidereactioncanberapidlysuspendedbytheadditionof1.0mLof32.4mmol•L-1ammoniummolybdate.Theremaininghydrogenperoxide couldreactwithammoniummolybdateandproduce akindoflightyellowcomplexcompound.Then, measuredtheproductionamountat405nmagainst blankcontainingallthecomponentsexcepttheenzyme onapectrophotometer.subsequently,calculatedCAT concentration.ThevalueofCATwasexpressedin U•mL-1.
T-AOC
T-AOCactivityassaywasperformedaccordingto themethodofdoubleantibodysandwich.Wesetthe standardhole,thesampleholeandblankholeinthe microtiterplatecoatedbyT-AOCantibody.Added 50 μL standard solution in standard hole, added 10 μL sample and 40 μL sample diluent in sample hole,withoutinblankhole.Inadditiontotheblank hole, each hole added 100 μL detection antibody thatmarkedbyHRP,sealedtheholesandincubatedat37℃for60min.Thenwashedtoremove unboundcomponents.Eachwellweresequentially added the substrates of A and B, each 50 μL. Gently shook30sandincubatedat37℃for15min. Subsequently,ODvalueofeachholethatwasadded 50 μL terminated liquid was measured at 520 nm. Finally,calculatedT-AOCconcentrationbythe standardcurve.
MDA
MDAactivityassaywasperformedaccordingtothe methodofdoubleantibodysandwich.Afteradded thesampletowellofmicrotiterplatecoatedbyMDA antibody,reactionformacomplex.Thenwashed completely.Subsequently,addedTBAsubstrate solution,andmeasuredODvalueat532nm.Finally, calculatedMDAconcentrationbythestandardcurve.
Statistical analyses
Allresultswereexpressedasmeanvalues±SD.andP<0.05wasusedasthelevelofstatisticalsignificance. SPSSStatistics17.0wasusedforthedataanalyses. Theeffectsofthediluentonsurvivaltimeandsurvival indexinsemenfromdifferentboarbreedswere analyzedwithatwo-wayanalysisofthevariance (ANOVA).Theeffectsofthediluentandstoragetime onspermmotility,CAT,T-AOCandMDAlevelsin semenfromdifferentboarbreedswereanalyzedwith athree-wayANOVA.ANOVAwasfollowedbya Duncanmultiple-comparisontestifitwasnecessary toevaluatethedifferencesbetweenthevaluesof differentexperimentalgroups.APearsoncorrelation wasusedtodeterminetherelationshipbetweensperm motilityandeachenzymelevel.
Results
Effect of different diluents on sperm motility and autioxidat enzyme level
Theeffectsofthepreservationtime,diluenton motilityandrelativeenzymaticlevelsofsemenare showninTable1.

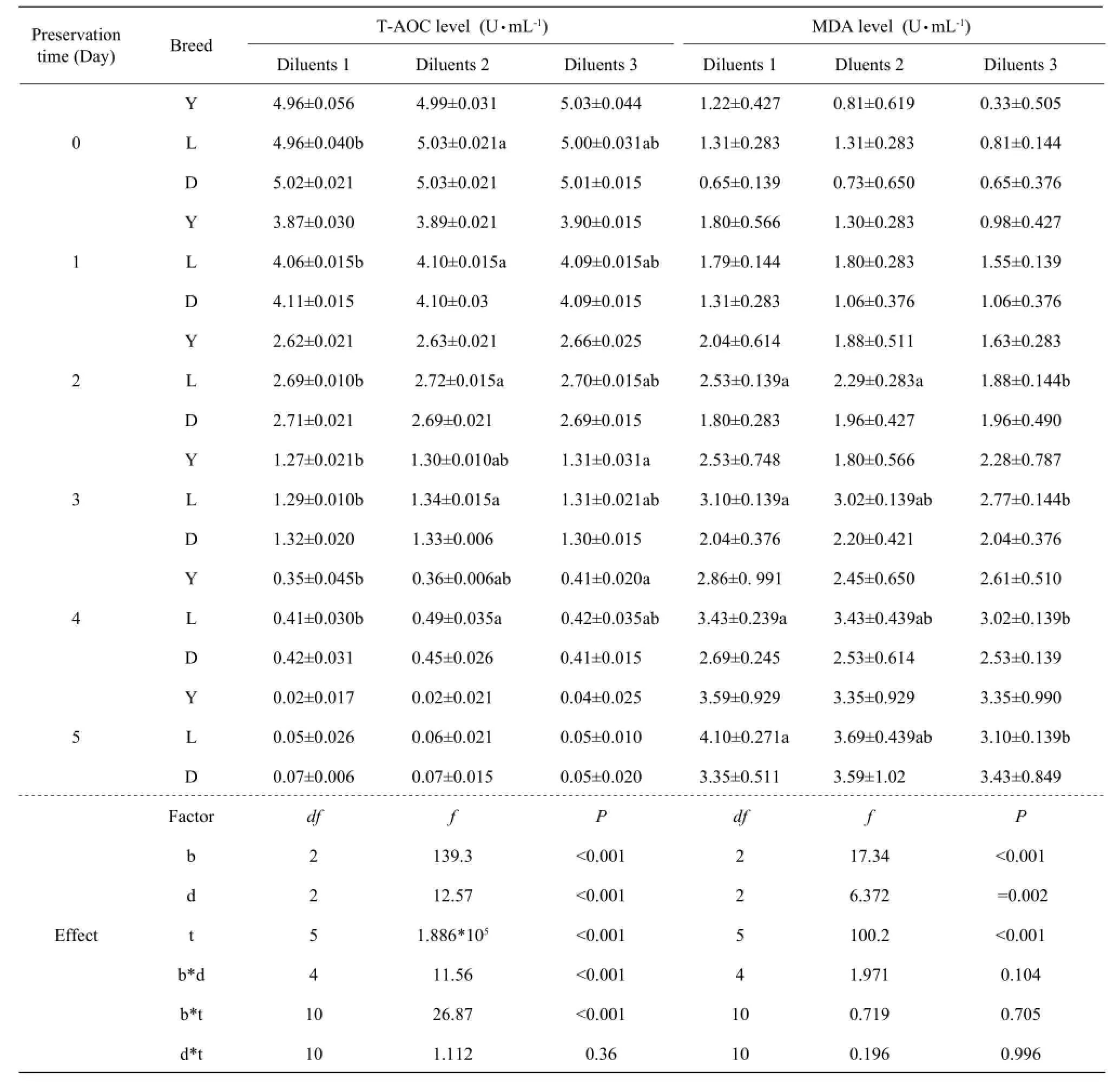
Table 1 Effects of preservation time and diluents on motility and relative enzymatic levle of semen from different boar breeds at 17℃
Continued
ItwasobviouslyobservedfromTable1thatthere weresignificantinteractioneffectsbetweenbreeddiluentandsemenbreed-timeonspermmotility (P<0.001,P=0.039),CAT(P<0.001,P<0.001)and T-AOC(P<0.001,P<0.001)levels.Intheprocessof preservation,thespermmotility,CATandT-AOC contentsdecreasedsignificantly,butMDAcontent increased.However,therewereneithersignificant effectsnorsignificantinteractioneffectsbetween breed-diluentandsemenbreed-timeonMDAlevels. Furtherone-wayANOVAindicatedthatthestorage timesignificantlyaffected(P<0.001)thespermmotility,CAT,T-AOCandMDAlevelsineachbreed.
InYorkshireboarsemen,thespermmotilityand CATlevelsofdilutedwithDiluentⅢweresignificant higherthanthosewithDiluentⅡafter3daysand during0-3days,respectively(P<0.05),andT-AOC levelsofboarsemendilutedwithDiluentⅢwere significanthigherthanthosewithDiluentⅠduring3-4 days.However,therewerenosignificantdifferences ofMDAlevelsamongthediluents.
InLandraceboarsemen,thespermmotilityand CATlevelsdilutedwithDiluentⅡweresignificant higherthanthosewithDiluentⅢ(P<0.05)after 3daysandduring2-3days,respectively.CATand T-AOClevelsofLandraceboarsemendilutedwith DiluentⅡweresignificanthigherthanthosewith DiluentⅠ during0-3days(P<0.05),butMDAlevels ofLandraceboarsemendilutedwithDiluentⅠwere significanthigherthanthosewithDiluentⅢafter 2days(P<0.05).InDurocboarsemen,therewereno significanteffectsofdiluentonspermmotility,CAT, T-AOCandMDAlevels.
Effect of different diluents on sperm survival index and survival time
Thesurvivaltimeandsurvivalindexofspermare showninTable2.
ItwasclearlyseenfromTable2thatthediluents andbreedshadnoeffectsandnointeractioneffects onsurvivalindex.Thereweresignificantinteraction effectsbetweendiluentsandbreedsonsurvivaltime. Furtherone-wayANOVAindicatedtheeffective survivaltimeofYorkshireboarsemendilutedwith DiluentⅢwassignificantgreaterthanthatwith DiluentⅡ(P=0.038,Table2),andLandraceboar semendilutedwithDiluentⅡwassignificantgreater thanDiluentⅢ(P=0.040,Table2),butnosignificant effectswereobservedinothergroups.
Relationship of sperm motility and autioxidant enzyme level
BothCATandT-AOClevelsweresignificantly positivecorrelatedwithspermmotilityinallthethree boarbreeds(Fig.1),PearsoncorrelationofCAT andspermmotilityofYorkshire,Landrace,Duroc were0.988,0.962,and0.988,respectively,Pearson correlationofT-AOCandspermmotilityofYorkshire, Landrace,Durocwere0.987,0.993and0.998, respectively.However,MDAlevelweresignificant negativecorrelatedwithspermmotility(Fig.1), PearsoncorrelationofMDAandspermmotilityof Yorkshire,LandraceandDurocwere–0.932,–0.941 and–0.958,respectively.
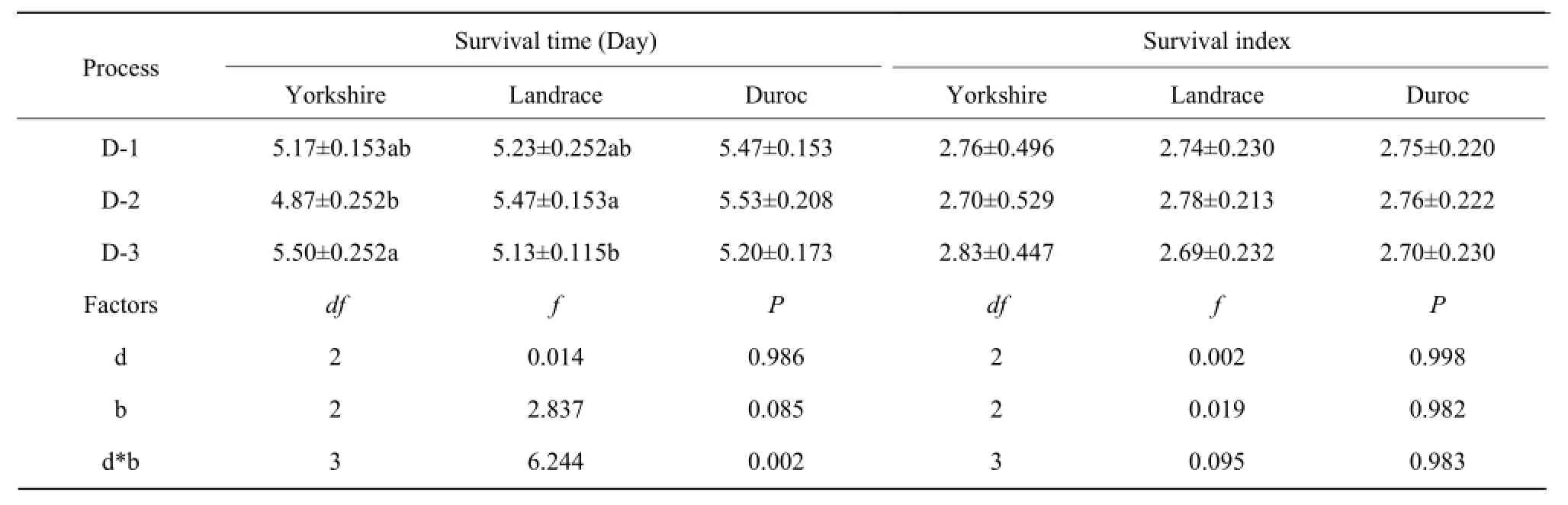
Table 2 Survival time and index of sperm from three boar breeds in diluents at 17℃

Fig. 1 Relationship of sperm motility and enzyme level
Discussion
Thecommercialdiluentswereproducedtomaintain andprotectthefertilityofspermatozoaduringstorage process(Watson,1990).Dilutedpowderthatcould increasethevolumeofsementomakereasonable andeffectiveuseofsemencouldprovidespermwith metabolicnutrientsneededforthesurvivaltoextend thespermsurvivaltimeandimprovethefertilization abilityofsperm.Diluentcommonlycontainsnutrients, bufferingagent,antioxidants,enzymes,essentialmetalionsandantibacterialsubstancesandsoon.Diluent composition,eachcompositionconcentrationand theratioamongthecompositionshadveryimportant influencesonsemenpreservationeffect.Forexample, thecombinationofamides(3%)andglycerol(2%) wasmoreefficientatcryo-protectionthanglycerol aloneforsemenfreezingfromboarsofthePiaubreed (Pinhoet al.,2014).Someresearchershadaddeda certainamountofenergysubstancesindilutesolution suchasglucose,fructose,lactose,sucrose,etc.They couldimprovespermmotilityandextendthesperm tostoragetime.Glucosewasacommondiluent nutrients,whichcouldprovidetherequiredenergy forthesurvivalofspermandadjustsemenosmotic pressure.Bovineserumalbumin(BSA)couldstabilize thestructureofspermcellsandneutralizesperm metabolitestoprolongthesurvivaltime,maintaining spermmotilityandreducespermagglutination.K+couldmaintainthetransportofNa+-K+pumpthat couldproduceATPtomaintainthesurvivaland movementofspermonspermmembrane.Inaddition, bothVAandVBthatwereaddedinthediluentcould protectthespermfromdifferentfunction.
Itwaswelldocumentedthattheeffectofstorage timeonsemenqualityvariedamongboars(Waberski et al.,1994;Kommisrudet al.,2002;Waterhouse et al.,2004).Thisvariationhadalsobeenobservedby Martín-Hidalgoet al.(2013)whoreportedthatsemen toleranceduringpreservationwasmoreinfluenced bythediluentsinIberianthaninDurocbreed.Inthe presentstudy,thediluentsandbreedshadnoeffects andinteractioneffectsonsurvivalindex,butthere weresignificantinteractioneffectsbetweendiluents andbreedsonspermmotilityandsurvivaltime.The preservationeffectofYorkshireboarsementhat wasdilutedwithdiluentⅢwhichspermmotility in5daysremainedat0.54andsurvivaltimewas higherthanthatwithdiluentⅡ,wasthebest.The effectofLandraceboarsementhatwasdilutedwith diluentⅡthatspermmotilityandsurvivaltime werehigherthanthosewithdiluentⅢafter3days (P<0.05)wasthebest.Buttheeffectsofthreediluents onDurocboarsemenwerenotsignificantlydifferent. Comparingwithotherbreeds,thisresultindicatedthat thesemenofDurocwaslessersensitivetodiluents andfurtherstudyareneeded.Cometothetrialresults, ontheonehand,maybeduetodifferentproportions ofspermandseminalplasmaindifferentbreedsemen thatcouldleadtodifferencesaftersemendilution. Studiesfoundthatsemenvolumehadsignificant differencethatLandrace,YorkshireandDurocreduced inturnbetweendifferentbreeds.Sincethespermof smallvolume,thedifferencesbetweensemenvolume mainlyreflectedonthecontentoftheseminalplasma. Theelectrolytecompositioninseminalplasmacould promotespermprematureaging.Atthesametime, theseminalplasmacontainsvariousionsthatcould maintainspermsurvival.Inaddition,theseminal plasmahadantioxidants,proteinandotherbeneficial ingredientsthatcouldmaintainmembranestabilityand fertility.Forexample,themeanpercentageofsperm motilityofHampshireboarswassignificantlylowerin washedthaninunwashedseminalplasmairrespective ofholdingduringpreservation(Chutiaet al.,2014). Extendingcryopreservedboarspermin50%seminal plasmawouldsignificantlyimprovespermmotility andviability,withthepossibleneteffectofpositively impactingsowfertilityandextendingthesperm's lifespan(Garciaet al.,2010).Ontheotherhand,these threediluentswereimportedproducts,anddidn't indicatethespecificcomponentsofthediluents. Thisresearchwasbasedontheinstructiontodiluteandpreserveboarsemen;eachdiluentmightcontain thesamecomponents,suchasglucose,EDTA, sodiumcitrate,etc.,buttheconcentrationofeach componentmightbedifferent.Atthesametime, eachdilutionmightalsoaddothersubstances,such asBAS,antibiotics,etc.,toprotectthespermbytheir ownresearches.Sotheresultsofthistestmightbe causedbyonereasonalone,mightalsobecausedby combinationoftworeasons.Thereasonneedstobe carefullyanalyzedinfuturestudies.
Undernormalphysiologicalconditions,ROSproducedbyrespiratorymetabolismofspermwasalways inadynamicequilibriumwhichproducedconstantly andclearedbyantioxidantenzymesatthesametime in the body (Strzeżek et al.,2012).However,after ejaculation,spermthatwereinevitablyexposedtoair, contactthelargeamountsofoxygenanddestroythe redoxsystemsproducedalargenumberofROSand wereaffectedseverely;then,sucheffectscouldplaya majorroleonspermmembrane(Vascoet al.,2007). CATthatcouldinduceH2O2todecomposeintowater andmolecularoxygencouldcleanH2O2in vivoand avoidthatthatcellswerepoisonedbyH2O2(Huet al.,2009).ThelowerCATcontent,thelowercellular antioxidantcapacity.T-AOClevelcouldreflectthe organismdefenseoxidativedamageabilitystrong orweak.WhenT-AOCcontentwasstronger,thecontentofbiologicalantioxidantsinthebodywashigher andtheabilitytoresisttheoxidativedamageis stronger.Duringthesemenextending,T-AOClevel andantioxidantcapacityofboarsemendecreased (Brezezinska-Slevbodzinskaet al.,1995).Thelevelof MDAthatwastheendproductoflipidperoxidation metabolismrepresentedthedegreeofspermoxidative damage.ThehigherMDAcontent,thedeeperthe degreeofspermlipidperoxidation(Kumaresanet al., 2009).Inthepresentstudy,duringtheprocessof preservation,CATandT-AOClevelsofspermatozoa decreasedsignificantlywhileMDAlevelincreased significantly.Inearlystageofthepreservationprocess, thespermhadstrongantioxidantcapacityandthe lowlevelofoxidativedamage.Withtheextensionof storagetime,unsaturatedfattyacidsoccurredlipid peroxidationinthespermthatdamagedorinhibited theactivityofantioxidantenzymesanddestroyed thespermprotein,whichcausedspermstructure damage,brokeoriginalequilibriumstateandproduced moreoftheperoxide,whichledtotheexacerbation ofthedegreeofoxidativedamageofspermfurther, thedeclineofthetotalantioxidantcapacityandthe increaseofMDAlevelsgradually.
Inthepresentstudy,thereweredifferenteffects ofdifferentdiluentsonthelevelsofCAT,T-AOC andMDAinYorkshireandLandraceboarsemen. ButInDurocboarsemen,therewerenosignificant effectsofdiluentonCAT,T-AOCandMDAlevels. Thetrialresults,ontheonehand,mightbeduetothe differencesamongdifferentvarietiesofpigsperm,on theotherhand,mightbebecausethesethreediluents haddifferencesinantioxidantsandtheirproportion.
Reactiveoxygenspeciesweregenerallyconsidered tobecytotoxicagentsduetotheirabilitytoinduce lipidperoxidationwithinthespermplasmamembrane andmodifyDNAstructures(Sanockaet al.,2005).It waswellestablishedthatROScoulddamagesperm plasmamembraneandhencereducespermmotility (Guthrie&Welch,2005;Chanapiwatet al.,2009). AsthearisingofROSformedthroughtheunivalent reductionofoxygen,whichledtotheimpairmentof spermmotility,functionalmembraneintegrityand fertilitythroughoxidativestressandtheproduction ofcytotoxicaldehydes(AlvarezandStorey,1989).In thisstudy,thefactthatbothCATandT-AOClevels werepositivecorrelatedwithspermmotilityinall threeboarbreedswhileMDAlevelwassignificant negativecorrelatedwiththespermmotilityindicated thatduringtheprolongingpreservationofsperm,the decreaseofspermactivitypartlycausedbythedecline ofantioxidantcapacity.
Conclusions
Inconclusion,thediluentshouldadapttoeach particularsemenbreed.DiluentⅢwastheoptimalcommercialdiluentforthestorageofYorkshireboar semen.ButDiluentⅡwastheoptimalcommercial diluentforLandraceboarsemenstoredat17℃. Withtheextensionofstoragetime,thefactthat CAT,T-AOCcontentdecreased,butMDAcontent increasedwasbecausethatspermweredestroyedby ROS,whichcausedthedecreaseofthelevelsofthe antioxidantenzymesandthetotalantioxidantcapacity andtheincreaseoftheoxidativedamage.Inaddition, thedecreaseofspermactivityduringthestoragepartly dependedonthedeclineoftheantioxidantcapacity.
References
AitkenRJ,ClarksonJS.1988.Significanceofreactiveoxygenspecies andantioxidantsindefiningtheefficacyofspermpreparation techniques. J Androl,9:367-376.
AitkenRJ,GordonE,HarkissD, et al.1998.Relativeimpactof oxidativestressonthefunctionalcompetenceandgenomicintegrity ofhumanspermatozoa. Biol Reprod,59:1037-1046.
AlthouseGC,WilsonME,KusterC, et al.1998.Characterization oflowertemperaturestoragelimitationsoffresh-extendedporcine semen. Theriogenology,50:535-543.
AlvarezJG,StoreyBT.1989.Roleofglutathioneperoxidasein protectingmammalianspermatozoafromlossofmotilitycausedby spontaneouslipidperoxidation.Gamete Res,23:77-90.
BilodeauJF,BlanchetteS,GagnonC,et al.2001.Thiolsprevent H2O2-mediatedlossofspermmotilityincryopreservedbullsemen. Theriogenology,56:275-286.
BilodeauJF,ChatterjeeS,SirardMA,et al.2000.Levelsofantioxidant defensesaredecreasedinbovinespermatozoaafteracycleof freezingandthawing.Mol Reprod Dev,55:282-288.
Brezezinska-SlevbodzinskaE,SlebodzinskiAB,PrietasB, et al.1995. AntioxidanteffectofvitaminEandglutathioneonlipidperoxidation inboarseminalplasma. Biol Trace Elem Res,47:69-74.
CastellanoCA,AudetI,BaileyJL,et al.2010.Dietaryomega-3fatty acids(fishoils)havelimitedeffectsonboarsemenstoredat17℃ or cryopreserved.Theriogenology,74:1482-1490.
ChanapiwatP,KaeoketK,TummarukP. 2009.EffectsofDHA-enrichedheneggyolkandLcysteinesupplementationonqualityof cryopreservedboarsemen. Asia J Androl,11:600-608.
ChutiaT,BiswasRK,TamuliMK,et al.2014.Effectofholdingof semenandwashingofseminalplasmaonqualityandfertilityof Hampshireboarsemenpreservedatliquidstate.Animal Reproduction Science,145:141-149.
ClarkeRN,JohnsonLA. 1987.Effectofliquidstorageand cryopreservationofboarspermatozoaonacrosomalintegrityand thepenetrationofzona-freehamsterova in vitro. Gamete Res,16: 193-204.
EstienneMJ,HarperAF,DayJL. 2007.Characteristicsofsperm motilityinboarsemendilutedindifferentextendersandstoredfor sevendaysat18degrees.Reprod Biol,7:221-231.
FooteRH,BrockettCC,KaprothMT.2002. Motilityandfertilityof bullsperminwholemilkextendercontainingantioxidants. Anim. Reprod Sci,71:13-23.
FraczekM,SzkutnikD,SanochaD, et al.2001.Peroxidation componentsofspermlipidmembranesinmaleinfertility.Ginekol Pol, 72:73-79.
GadeaJ.2003.Review:semenextendersusedintheartificial inseminationofswine.Span J Agric Res,1:17-27.
GarciaJC,DominguezJC,PenaFJ,et al.2010.Thawingboarsemen inthepresenceofseminalplasma:effectsonspermqualityand fertility.Animal Reproduction Science,119:160-165.
GothL.1991.Asimplemethodfordeterminationofserumcatalase activityandrevisionofreferencerange.Clin Chim Acta,196: 143-151.
GuthrieHD,WelchGR.2005.Impactofstoragepriortocryopreservationonplasmamembranefunctionandfertilityofboarsperm. Theriogenology,63:396-410.
GuthrieHD,WelchGR.2010.Using fluorescence-activated flow cytometry to determine reactive oxygen species formation and membrane lipid peroxidation in viable boar spermatozoa.Advanced protocols in oxidative stressII.HumanaPress.pp.163-171.
HuJH,LiQW,ZhangT,et al.2009.Effectofgynostemmapentaphyllumpolysaccharideonboarspermatozoaqualityfollowing freezing-thawing.Cryobiology,59:244-249.
KommisrudE,PaulenzH,SehestedE,et al.2002.Influenceofboarand semenparametersonmotilityandacrosomeintegrityinliquidboar semenstoredforfivedays.Acta Vet Scand,43:49-55.
KumaresanA,KadirvelG,BujarbaruahKM,et al.2009.Preservation ofboarsemenat18℃induceslipidperoxidationandapoptosislike changesinspermatozoa.Animal Reproduction Science,110:162-171.
KusterCE,AlthouseGC.1999.Thefecundityofporcinesemenstored for2to6daysinAndrohepandX-CELLextenders.Theriogenology, 52:365-376.
LevisDG.2000.Liquid boar semen production: current extender technology for long-term storage and where do we go from here.In: JohnsonLA,GuthrieHD.SemenboarpreservationIV.AllenPress Inc.,LawrenceKS,UnitedStates.pp.121-128.
Martín-HidalgoD,BarónFJ,RobinaA,et al.2013.Inter-andintrabreedcomparativestudyofspermmotilityandviabilityinIberian andDurocboarsemenduringlong-termstorageinMR-AandXCell extenders.Anim Reprod Sci,139:1477-1488.
Perez-LlanoB,SalaR,RegueraG,et al.2009.Changesinsub-populationsofboarspermdefinedaccordingtoviabilityandplasma andacrosomemembranestatusobservedduringstorageat15℃. Theriogenology,71:311-317.
PinhoRO,LimaDMA,ShiomiHH,et al.2014.Effectofdifferentcryo-protectantsontheviabilityoffrozen/thawedsemenfrom boarsofthePiaubreed.Animal Reproduction Science,146:187-192.
Sanocka D, Ciupińska M, Kurpisz M. 2005. Bacterial infection and semenquality.J Reprod Immunol,67:51-56.
SilvaCG,CunhaER,BlumeGR,et al.2015.Cryopreservationofboar spermcomparingdifferentcryoprotectantsassociatedinmediabased onpowderedcoconutwater,lactoseandtrehalose.Cryobiology,70: 90-94.
StoreyBT.1997.Biochemistryoftheinductionandpreventionof lipoperoxidativedamageinhumanspermatozoa.Mol Hum Reprod,3: 203-213.
Strzeżek R, Koziorowska-Gilun M, Stawiszyńska M. 2012. Cryopreservationofcaninesemen:theeffectoftwoextendervariantson thequalityandantioxidantpropertiesofspermatozoa.Polish Journal of Veterinary Sciences,15:721-726.
VascoD,HernandezM,VazquezJM,et al.2007.Generation of reactive oxygen species(ROS)by frozen-thawed boar spermatozoa. In:GarsingtonRD.Peproductionindomesticanimals.Blackwell Publishing,England.pp.89-90.
VytP,MaesD,DejonckheereE,et al.2004.Comparativestudyon fivedifferentcommercialextendersforboarsemen.Reprod Domest Anim,39:8-12.
VytP,MaesD,SysSU,et al.2007.AircontactinfluencesthepHof extendedporcinesemen.Reprod Domest Anim,42:218-220.
WaberskiD,MedingS,DirksenG,et al.1994.Fertilityoflong-termstoredboarsemen:influenceofextender(AndrohepandKiev), storagetimeandplasmadropletsinthesemen.Anim Reprod Sci, 36: 145-151.
WangAW,ZhangH,IkemotoI,et al.1997.Reactiveoxygenspecies generationbyseminalcellsduringcryopreservation.Urology,49: 921-925.
WaterhouseKE,DeAngelisPM,HauganT,et al.2004.Effectsof in vitrostoragetimeandsemen-extenderonmembranequalityof boarspermassessedbyflowcytometry.Theriogenology,62: 1638-1651.
WatsonPF.1990.Artificial insemination and the preservation of semen.In:LammingG.Marshall'sphysiologyofreproduction.4th ed.ChurchillLivingstone,Edinburgh,England.pp.747-869.
WeitzeKF.1991.Long-termstorageofextendedboarsemen.Reprod Dom Anim,Suppl 1:231-253.
ZhangW,YiK,ChenC,et al.2012.Applicationofantioxidantsand centrifugationforcryopreservationofboarspermatozoa.Anim Reprod Sci,132:123-128.
ZouCX,YangZM.2000.Evaluationonspermqualityoffreshly ejaculatedboarsemenduringin vitrostorageunderdifferent temperatures.Theriogenology,53:1477-1488.
S814.8 Document code: A Article ID: 1006-8104(2015)-02-0036-11
1April2015
SupportedbytheNationalSwineIndustryTechnologySystem(CARS-36);theScientifcandTechnologicalProjectofYanglingDemonstrationZone (2014NY-22)
HuShan(1992-),female,engagedintheresearchofanimalreproductivephysiology.E-mail:1243860951@qq.com
*.HuJian-hong,professor,engagedintheresearchofanimalreproductivephysiology.E-mail:hjh19732008@126.com
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Rice Yield and Quality Across Accumulated Temperature Zone Planting in Cold Area
- Separation and Purification of Total Phloroglucinols in Dryopteris crassirhizoma with DM-130 Macroporous Adsorption Resin
- Characterization and Expression of Outer Membrane Protein A I Gene of Aeromonas veronii
- Construction and Expression of Methionine-rich and Lysine-rich Fusion Gene in Bacillus natto
- Isolation and Pathogenicity Analyses on Yersinia enterocolitica from Pelteobagrus vachelli
- Effects of Sub-chronic Aluminum Exposure on Renal Structure in Rats
