Separation and Purification of Total Phloroglucinols in Dryopteris crassirhizoma with DM-130 Macroporous Adsorption Resin
2015-11-25JinZheHuangJianpingWangHemengJuhanxunRenSiruiandChangYing
Jin Zhe, Huang Jian-ping, Wang He-meng, Ju han-xun, Ren Si-rui, and Chang Ying
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
Separation and Purification of Total Phloroglucinols in Dryopteris crassirhizoma with DM-130 Macroporous Adsorption Resin
Jin Zhe, Huang Jian-ping, Wang He-meng, Ju han-xun, Ren Si-rui, and Chang Ying*
College of Life Sciences, Northeast Agricultural University, Harbin 150030, China
To improve the purity of the total phloroglucinols from Dryopteris crassirhizoma extracts, the separation and purification conditions of the total phloroglucinols from Dryopteris crassirhizoma were studied with DM-130 macroporous adsorption resin in this study. Adsorption rate, elution rate and purity of the total phloroglucinols were used as indexes to investigate the adsorption and desorption capacity of the total phloroglucinols with DM-130 macroporous adsorption resin. Through the study, the optimum sample concentration of the total phloroglucinols and maximum sample volume were 1.5 mg • mL-1and 7 BV (210 mL), respectively. The optimum desorption conditions were achieved by using 80% ethanol as desorption solvent at elution flow rate of 1.0 mL • min-1. The result showed DM-130 macroporous adsorption resin performed effective adsorption and desorption. After purification, the purity of the total phloroglucinols increased by 11.5-fold.
DM-130, adsorption resin, Dryopteris crassirhizoma, total phloroglucinols, separation, purification
Introduction
Dryopteris crassirhizoma is a deciduous perennial herb belonging to the family Dryopteridaceae and is widely distributed in Heilongjiang, Liaoning and Hebei Provinces in China (Sekiya et al., 2004). Maoer Mountain (Heilongjiang Province, China) is one of the major distribution areas. D. crassirhizoma mainly grows at mountain slope wetlands. D. crassirhizoma could be used as rhizome medicine, functioning in detoxification, deworming and hemostasis (Bruder et al., 1994). It is also widely used in clinical treatments of common cold, heat rash, vomiting, bloody diarrhea and uterine bleeding (Ling, 1984). It was also shown that phloroglucinol derivative extracts from D. crassirhizoma had antitumor activities (Kapadia et al., 1996; Ito et al., 2000).
In recent years, the pharmacological value of D. crassirhizoma is being increasingly realized. As the chemical constituents of D. crassirhizoma are very complex, efforts have been made to isolate the active components. Currently, the variety of the chemicals have been isolated. It is found that flavonoids, phloroglucinol and terpenoids are responsible for the major biological activities (Widen et al., 2001). Among these components, phloroglucinol was found performing major activities in anticancer, antioxidation, antiinflammatory, inhibiting bacteria and relieving pains (Patama and Widen, 1991). In view of these beneficial effects, the application of lowcost methods to obtain the phloroglucinol compounds from D. crassirhizomais indispensable for in-depth pharmacological researches and clinical trials.
In preparative enrichment of active components, various physical and chemical techniques have been successfully applied, such as liquid-liquid extraction (Kumar et al., 2008, Wang et al., 2011), precipitation (Byrne et al., 2002), membrane filtration (Priyananda and Chen, 2006) and adsorption (Liu et al., 2010). Among those methods, resin column adsorption seems to be suitable in economy and is also more environmentfriendly, due to its excellent characteristics, such as high mechanical strength, good acid and alkali resistance, various surface functional groups, porous availability, high surface area and easy regeneration. Moreover, resin is endowed with selective adsorption properties through electrostatic force, hydrogen bonding interaction, complexation and size sieving action, etc. Nowadays, resin column adsorption has been widely used in separation and enrichment of bioactive substances from plant materials, including flavonoids (Liu et al., 2010), polyphenols (Ai et al., 2007), alkaloids (Huang et al., 2003) and saponins (Kong et al., 2010). DM-130 macroporous adsorption resin has weak polarity, and its functional group belongs to polystyrene. It has been shown that DM-130 macroporous adsorption resin had good separation and enrichment capabilities in phloroglucinol compounds (Wang et al., 2010). Researches on D. crassirhizoma mainly focused on pharmacognosy characteristics, physiology and chemical composition. Little work had been done on the extraction and purification of phloroglucinol. In this study, the separation and purification conditions of the total phloroglucinols from Dryopteris crassirhizoma were studied using DM-130 macroporous adsorption resin.
Materials and Methods
Reagent and instrument
D. crassirhizoma was collected from Maoer Mountain (N45°18'26",E127°34'40"),andauthenticatedby Professor Chang Ying from College of Life Sciences, Northeast Agricultural University, Harbin, China. Deionized water was used in all the experiments. Dryocrassin ABBA (Purity 98%, Forever Biotechnology, Shang-hai, China) was used as the standard for the total phloroglucinols. The main instruments used included disintegrator (Tianjin City Test Instrument Co., Ltd., China), ultrasonic bath (Ningbo Xinzhi Biological Polytron Technologies Inc., China), circulating water vacuum pump (SHZ-DⅢ, Gongyiyingyu Yuhua Instrument Factory, China), and rotary evaporator device (RE-52B, Gongyiyingyu Yuhua Instrument Factory, China).
Sample preparation
Dried D. crassirhizoma was powdered by disintegrator and then sieved through a 40 mesh. Total 200 g of the powder was added into 2 000 mL of the ethanol. The volume fraction of the ethyl alcohol was 80%. Then, ultrasonic extraction (800 W) was applied thrice under the same conditions (30 min under 30℃). With n-hexane extraction, took the upper liquid. The filtered solutions were gathered and concentrated to dryness by removing the ethanol solvent using a rotary evaporator device at 45℃. The total phloroglucinols in extracts were 8.10 mg • g-1. The extracts obtained were diluted with 20% ethanol to an appropriate concentration.
Plotting standard curve
D. crassirhizoma had a high content of Dryocrassine ABBA which was a phloroglucinol derivative. In this experiment, Dryocrassine ABBA was used as the standard to measure the contents of the total phloroglucinols in D. crassirhizoma. Weighed 3 mg Dryocrassine ABBA, and dissolved it in 95% ethanol to make a 0.03 mg • mL-1solution. Dryocrassin ABBA was quantified at a wavelength of 476 nm. Eight volumes (0, 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 mL) of Dryocrassine ABBA standard solution (0.03 mg • mL-1) was added to volumetric flask, respectively, and diluted with 95% ethanol to 2 mL. Five mL of Fast Blue B salt solution was added, mixed thoroughly and placed under room temperature for 20 min. The regressionline for Dryocrassin ABBA was Y=21.022X+0.0133, r=0.9996. Where, Y was the absorbance of Dryocrassin ABBA; and X was the concentration of analyte (mg).
Static adsorption test
Five g of pretreated macroporous resin and 20 mL of the crude sample solution (1.5 mg • mL-1) were added into a 250 mL conical flask. The flask was shaken on anoscillator (130 r • min-1) at 25℃ for 12 h. One mL of the solution was taken per hour and used for the quantitation of the total phloroglucinols. The calculation was conducted as the following.

Where, W was the adsorption capacity at adsorption equilibrium (mg • g-1resin); C0was the initial concentration of Dryocrassine ABBA in the solution (mg • mL-1); C1was the equilibrium concentration of the solute in the solution (mg • mL-1); V1was the volume of the sample solution; and M was the weight of the dry resin.

R was the adsorption rate at adsorption equilibrium.
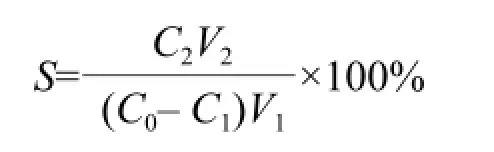
S was the desorption ratio (%); C2was the concentration of the solute in the desorption solution (mg • mL-1); and V2was the volume of the desorption solution (mL).
Adsorbent
As shown in Table 1, the three types (brands) of the macroporous resins, AB-8, ADS-7 and DM-130 had different physical and chemical properties. They had different adsorptions and desorption effects on the total phloroglucinols.

Table 1 Physical and chemical properties of macroporous resins
After selecting the most suitable resin, the resins were pre-treated to remove the impurities trapped inside the pores during the synthesis process. Before the adsorption experiments, the weighed resins were soaked in ethanol for 24 h, and subsequently washed by deionized water until the ethanol was thoroughly replaced by deionized water.
Dynamic adsorption and desorption test
Pretreated DM-130 resins were packed in a column, followed with the loading of the total phloroglucinols. After adsorption, the resins were desorbed with 30 mL of deionized water, and then eluted with different volume fractions of ethanol. By measuring the contents of the total phloroglucinols in eluent, we detected sample concentration, sample volume, ethanol concentration, and eluent flow rate effect of DM-130 macroporous resin. Finally, the optimal elution condition was determined.
Resin column adsorption
The separation and enrichment of the total phloroglucinols by resin column adsorption were compared with those of the crude products before purification. Dried extract (2 g) obtained in sample preparation was dissolved with 20% ethanol and then distributed to the whole resin column. Elution was performed under optimal conditions of the resin column adsorption. Concentrations of the total phloroglucinols in the eluents were calculated by the standard curve.
Results
Resin selection
As shown in Table 2, DM-130 resins had higher adsorption and desorption capacity than ADS-7 and AB-8 resins. Therefore, DM-130 resin was selected for separating and enriching the total phloroglucinols in the following tests.
Static adsorption
DM-130 resins had good adsorption and desorption capabilities. Its average pore diameters and surface areas were suitable for adsorption and desorption of the total phloroglucinols.
Effects of sample concentrations on resin column adsorption
30 mL of pre-weighed DM-130 resins were added into resin column. Different concentrations of the total phloroglucinols solution flowed through macroporous resin column, conducting dynamic adsorption. Detected outflowing liquid until phloroglucinol content of the sample solution in the effluent liquid reached five percentage points, stopped injection and calculated adsorption of the total phloroglucinols. The results are shown in Table 3.
As shown in Table 4, with the increasing concentration of sample solution, the adsorption rate of DM-130 resins increased. Therefore, in the adsorption operation, in the premise of maintaining the sample solution clarified, injected high concentration of the solution into the column. However, when the concentration of the sample solution exceeded 1.5 mg • mL-1, solution turbidity increased gradually, which meant that the sample concentration should not be increased anymore. Consequently, 1.5 mg • mL-1was selected as the most suitable sample concentration for DM-130 resins adsorption.

Table 2 Adsorption and desorption properties of three types of resins for total phloroglucinols

Table 3 Static adsorption capacity, adsorption rate and desorption ratio of DM-130

Table 4 Effects of sample concentrations on its adsorption capacity
Effects of sample volume on resin column adsorption
Thirty mL (1 BV) of DM-130 resins were added into a resin column. Sample solutions (1.5 mg • mL-1) flowed through macroporous resin column, conducting dynamic adsorption. Inspected the phloroglucinol con-centration in sample solution per 1 BV, and drewadsorption curve. The results are shown in Fig. 1. When the sample volume exceeded 6 BV, the content of the phloroglucinol in effluent liquid increased sharply. Therefore, 7 BV was determined as the maximum sample volume.

Fig. 1 The maximum sample volume
Effects of ethanol concentrations on resin column adsorption
As shown in the previous results, the most suitable sample concentration was 1.5 mg • mL-1, and the maximum sample volume was 7 BV. Under these conditions, the sample solution was loaded, and deionized water was used to wash the soluble impurities. Different concentrations of the ethanol (50%, 60%, 70%, 80%, and 90% ethanol) were used to desorb the target compounds, successively. The elution volume for each concentration of the ethanol was 5 BV.
The desorption ratio increased with the growth of the ethanol concentrations (Fig. 2). The highest desorption ratio was found under 80% and no more increase was observed afterwards. There was not significantly difference between 80% and 90% ethanol for desorption ratio. To reduce test cost, 80% ethanol was selected as the eluent.
Effects of eluent flow rate on resin column adsorption
The sample solution was packed into column on the basis of the above conditions. After carrying on the resin adsorption equilibrium, 80% ethanol was applied to the eluent. Different elution rates (0.5, 1.0, and 1.5 mL • min-1) were used to desorb the target compounds, successively. By measuring the content of the total phloroglucinols in eluent, the impact of the eluent flow rate on dynamic desorption ratio was examined. The results are shown in Fig. 3.

Fig. 2 Effects of ethanol concentration on elution result

Fig. 3 Effects of the eluent flow rate on elution result
Fig. 3 showed that when eluent flow rate was 0.5 mL • min-1or 1.0 mL • min-1, the elution effect had little difference. However, when the elution flow rate was 1.5 mL • min-1, the elution effect was poorer. It might be that too fast elution flow rate resulted in inadequate elution, thus the eluent was lost out. However, low elution flow rate would extend production cycle. To sum up, 1.0 mL • min-1elution flow rate was used in the further experiment.
Effects of operating factors on resin column adsorption
The optimal separation conditions for adsorption of the total phloroglucinols on DM-130 resin column were determined as the followings: the concentration of the sample was 1.5 mg • mL-1, and the maximum sample volume was 7 BV. Resin column with 2 g sample was eluted by 5 BV 80% ethanol to wash the total phloroglucinols. The elution flow rate was 1.0 mL • min-1, and the results are listed in Table 5.
The corresponding adsorption of the samples after separation is shown in Table 5, the relative contents of the total phloroglucinols were improved distinctly. After dynamic separation from DM-130 resin column adsorption, the content of the total phloroglucinols in the product was 55.9 mg • g-1, which was 11.5-fold increases.

Table 5 Optimization method for separation of total phloroglucinols from DM-130 resin column
Discussion
Taking adsorption and desorption performance among the tested resins into account, DM-130 resin showed higher adsorption capacity and desorption ratio than the other two resins, AB-8 and ADS-7. In dynamic adsorption test, 1.5 mg • mL-1was selected as the most suitable concentration, and the maximum sample volume was 7 BV. Then, in dynamic desorption test, 80% ethanol was selected as the eluent, and 1.0 mL• min-1elution flow rate was selected as the most suitable flow rate. After purification, the purity of the total phloroglucinols was 11.5-fold increases.
This study substantiated that the separation efficiency of DM-130 macroporous adsorption resin for the total phloroglucinols was superior. However, there were still many deficiencies. First, we only compared three types of the resin of different physical and chemical properties. If conditions permitted, more types of the resin should be chosen for comparison. Second, Dryocrassin ABBA was used as control to plot standard curve for measuring the concentration of the total phloroglucinols in different samples of the solution. Although this method was simple and effective, it still lacked accuracy. Third, in this study, separation and purification of the total phloroglucinols from D. crassirhizoma were preliminary studied, and the resulting sample was mixtures. In order to perform further isolation, purification and identification, we should choose Silica Gel Thin Layer Chromatography and High Performance Liquid Chromatography (HPLC) for the separation and purification of the active ingredients. Finally, structural analysis by Ultraviolet Spectroscopy (UV), Infrared Spectroscopy (IR) and Nuclear Magnetic Resonance (1H-NMR, 13C-NMR) could be done to determine the exact structures of the compounds. Additionally, antibacterial activity, antioxidative activity, anti-cancer activity and other biological activities of the phloroglucinols from D. crassirhizoma also needed to be studied for further in-depth functional analysis.
Conclusions
In this study, resin column adsorption was successfullyapplied to preparative separation and enrichment of the total phloroglucinols from D. crassirhizoma. The adsorption feature of phloroglucinols onto three macroporous resins of different chemical and physical properties were investigated and DM-130 resin exhibited higher separation efficiency than other two resins. The operating parameters of the resin column adsorption were optimized for maximizing multi-target product yield. The concentration of the sample was 1.5 mg • mL-1, and the maximum sample volume was 7 BV. Resin column with 2.0 g sample was eluted by 5 BV 80% ethanol to wash the total phloroglucinols. The elution flow rate was 1.0 mL• min-1. Under the optimal conditions, the content of the total phloroglucinols in the product was 11.5-fold increases. This study substantiated that the separation efficiency of the resin column adsorption for the total phloroglucinols was superior. Recently, more and more new structure modified macroporous resins come forth, and they can be used in this manner for the preparative separation of the natural compounds in the future researches.
References
Ai Z L, Wang Y H, Wang H, et al. 2007. Absorption of polyphenols from apple pomace by macroporous absorbent resins. Trans Chin Soc Agric Eng, 23: 245-248.
Bruder S P, Fink D J, Caplan A L, et al. 1994. Mesenchymal stem cells in bone development, bone repair and skeletal regeneration theropy. Cell Biochem, 56(3): 283.
Byrne E P, Fitzpatrick J J. 2002. Investigation of how agitation during precipitation, and subsequent processing affects the particle size distribution and separation of α-lactalbumin enriched whey protein precipitates. Biochem Eng J, 10: 17-25.
Huang J M, Guo J X, Chen W S, et al. 2003. Adsorption properties and application of macroporous resin for purification of alkaloids in Aconitum kusnezoffii. Fudan Univ J Med Sci, 30: 267-269.
Ito H, Muranaka T, Mori K, et al. 2000. Ichthyotoxic phloroglucinol derivatives from Dryopteris fragrans and their anti-tumor promoting activity. Chem Pharm Bull (Tokyo), 48(8): 1190.
Kapadia G J, Tokuda H, Konoshima T, et al. 1996. Anti-tumor promoting activity of Dryopteris phlorophenone derivatives. Cancer Lett, 105(2): 161.
Kong Y, Yan M M, Liu W, et al. 2010. Preparative enrichment and separation of astragalosides from Radix Astragali extracts using macroporous resins. J Sep Sci, 33: 2278-2286.
Kumar J R, Lee H I, Lee J Y, et al. 2008. Comparison of liquid-liquid extraction studies on platinum (IV) from acidic solutions using bis (2, 4, 4-trimethylpentyl) monothiophosphinic acid. Sep Purif Technol, 63: 184-190.
Ling Y K. 1984. Chinese herbology. Shanghai Science and Technology Press, Shanghai. pp. 137.
Liu W, Zhang S, Zu Y G, et al. 2010. Preliminary enrichment and separation of genistein and apigenin from extracts of pigeon pea roots by macroporous resins. Bioresour Technol, 101: 4667-4675.
Liu Y F, Liu J X, Chen X F, et al. 2010. Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem, 123: 1027-1034.
Patama T T, Widen C J. 1991. Phloroglucinol derivatives from Dryopteris fuscoatra and D. hawaiiensis. Phytochemistry, 30(10): 3305-3310.
Priyananda P, Chen V. 2006. Separation of residual fatty acids from aqueous solutions using an agitated solution of protein and membrane filtration. Sep Purif Technol, 48: 113-120.
Sekiya I, Larson B L, Vuoristo J T, et al. 2004. Adipogenic differentiation of human adult stem cells from bone marrow stroma (BMMSCs). J Bone Miner Res, 19(2): 256-264.
Wang H N, Xie Y Z, Fan F Y, et al. 2010. Process of Dryocrassine macroporous resin purification. Chinese Traditional Patent Medicine, 30(10): 1539-1542.
Wang L H, Mei Y H, Wang F, et al. 2011. A novel and efficient method combining SFE and liquid-liquid extraction for separation of coumarins from Angelica dahurica. Sep Purif Technol, 77: 397-401.
Widen C J, Fraser-Jenkins C R, Reichstin T, et al. 2001. A survey of phenolic compounds in Dryopteris and related fern genera. Annales Botanici Fennici, 38(2): 99-138.
Q81 Document code: A Article ID: 1006-8104(2015)-02-0008-07
11 December 2014
Supported by the National Natural Science Foundation of China (31070291)
Jin Zhe (1987-), male, Master, engaged in the research of antitumor activity of the phloroglucinol derivative extracted from ferns. E-mail: swkx082jz@126.com
. Chang Ying, professor, supervisor of Ph. D student, engaged in the research of plant resource. E-mail: changying@neau.edu.cn
杂志排行
Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Rice Yield and Quality Across Accumulated Temperature Zone Planting in Cold Area
- Characterization and Expression of Outer Membrane Protein A I Gene of Aeromonas veronii
- Construction and Expression of Methionine-rich and Lysine-rich Fusion Gene in Bacillus natto
- Isolation and Pathogenicity Analyses on Yersinia enterocolitica from Pelteobagrus vachelli
- Effects of Three Different Diluents on Quality of Boar Semen Stored at 17℃
- Effects of Sub-chronic Aluminum Exposure on Renal Structure in Rats
