Congenital extrahepatic portosystemic shunt complicated by the development of hepatocellular carcinoma
2015-11-21RuchiSharmaAbidSuddleAlbertoQuagliaPraveenPedduJohnKaraniThomasSatyadasandNigelHeaton
Ruchi Sharma, Abid Suddle, Alberto Quaglia, Praveen Peddu, John Karani, Thomas Satyadas and Nigel Heaton
London, United Kingdom
Congenital extrahepatic portosystemic shunt complicated by the development of hepatocellular carcinoma
Ruchi Sharma, Abid Suddle, Alberto Quaglia, Praveen Peddu, John Karani, Thomas Satyadas and Nigel Heaton
London, United Kingdom
Congenital extrahepatic portosystemic shunt, also known as Abernethy malformation, is a rare congenital malformation. It causes shunting of blood through a communication between the portal and systemic veins such as a patent ductus venous. We report 3 cases of Abernethy malformation complicated by the development of hepatocellular carcinoma. Additionally, we comprehensively reviewed all previously reported cases and highlighted common features that may help in early diagnosis and appropriate management. Patients with Abernethy malformation may have an increased propensity to develop hepatocellular carcinoma. All 5 previously reported cases, plus the three of our patients, have a type 1 (complete) shunt suggesting a role for absent portal blood flow in the pathogenesis of hepatocellular carcinoma. Congenital extrahepatic portosystemic shunt should be sought for in cases with raised serum ammonia, hepatic encephalopathy or hepatocellular carcinoma in the absence of cirrhosis.
(Hepatobiliary Pancreat Dis Int 2015;14:552-557)
Abernethy malformation; congenital extrahepatic portosystemic shunt;
congenital absence of portal vein;
patent ductus venosus;
hepatocellular carcinoma
Introduction
Abernethy malformation, also known as congenital extrahepatic portosystemic shunt (CEPS), is a rare malformation. Portal venous blood bypasses the liver via CEPS to drain directly into the systemic veins such as the inferior vena cava, azygous vein or the right heart.[1]It was first described in 1793 by John Abernethy,[2]a surgeon, and is being diagnosed more frequently with progress in cross sectional imaging. CEPS can be classified into two types based on its anatomical features centered on the presence or absence of portal flow through the hepatic parenchyma.[1]In type 1 shunts the portal blood is entirely diverted to the inferior vena cava and the extrahepatic portal vein is absent with no portal flow through the liver. Hence it is also known as congenital absence of the portal vein. Type 1 shunts are further sub-classified into type 1a in which the splenic vein and superior mesenteric vein join the inferior vena cava separately or type 1b in which they join the inferior vena cava as a confluence. Type 2 shunts are partial side to side shunts in which partial portal flow to the liver is maintained via a hypoplastic portal vein. In both types, liver function is usually well preserved and cirrhosis is not a common finding. Both are associated with other anomalies involving the cardiovascular, gastrointestinal, skeletal and biliary systems as well as benign and malignant liver tumors. To the best of our knowledge, only 5 cases of Abernethy malformation complicated by hepatocellular carcinoma (HCC) have been reported.[3-7]HCC in CEPS is typically found in the absence of underlying cirrhosis.
The diagnosis of Abernethy malformation is often missed on initial presentation due to low level of suspicion and wide variability in clinical presentation. Although more common in children CEPS is not un-commonly diagnosed in adulthood. Patients maybe asymptomatic or have mildly deranged liver function. Other presentations include hyperammonemia secondary to portosystemic shunting, hepatic encephalopathy,[8]hepatopulmonary syndrome and pulmonary hypertension.[4]Patients may present with a hepatic mass due to nodular regenerative hyperplasia (NRH),[9]focal nodular hyperplasia (FNH),[10]adenoma, HCC or hepatoblastoma.[11]NRH is probably a reaction to uneven perfusion due to absent or reduced portal flow and compensatory increase in hepatic arterial flow resulting in atrophy of ischemic areas and regenerative nodule formation in well perfused areas.[9]Liver biopsy of patients with CEPS commonly show complete or near complete absence of the portal venules along with hypertrophy of hepatic artery branches with or without NRH. Radiology usually demonstrates an enlarged hepatic artery along with venous shunt features. Magnetic resonance imaging of the brain may reveal features of brain injury due to chronic portosystemic shunting in the form of white matter atrophy[12]or a hyperintense globus pallidus on T1 weighted images.[13]
Treatment of CEPS has evolved over time. Surgical treatment is required in patients with refractory hyperammonemia or portosystemic encephalopathy, hepatopulmonary syndrome or clinically significant or malignant liver tumors.[14]Most type 2 shunts can be treated by ligating or banding the shunt with carefully monitoring for the development of portal hypertension.[15]This was not thought to be an option for patients with type 1 shunts who have no other outflow and do not develop collateral circulation like patients with cirrhosis. It was feared that shunt ligation in these patients could result in significant splanchnic congestion and mesenteric edema. However, recent reports have demonstrated that the portal system has considerable plasticity. This allows revascularization of the portal system after shunt closure without the development of portal hypertension even in type 1 shunts where no extrahepatic portal vein or intrahepatic vasculature was identified on initial imaging. Moreover, most benign but not malignant tumors disappear or regress upon shunt closure. Thus, shunt closure is recommended for all patients especially in view of the serious long-term complications.[16,17]
Tolerance to shunt ligation should be tested by monitoring portal pressure response to shunt occlusion by a balloon catheter (balloon occlusion test). Shunt ligation can be attempted in patients who tolerate this test well. Raised portal pressure during balloon occlusion may mandate the patient to undergo a two-stage procedure. The shunt is banded to allow the development of collaterals in the first stage and shunt ligation is carried out in the second stage. Patients require careful long-term follow up to monitor complications of the shunt and the emergence of new previously undisclosed shunts.
However, these guidelines are based on reports in pediatric patients. They may not always be suitable for adults, such as those included in our study, who present with complications.
Case reports
Case 1
A 31-year-old woman was investigated for multiple liver lesions diagnosed as FNH on liver biopsy. She was a type 1 diabetic with no other significant medical history. Physical examination showed nothing abnormal.
Liver function tests showed good synthetic function but significant cholestasis: serum bilirubin 16 μmol/L, albumin 38 g/L, aspartate aminotransferase 64 IU/L, alkaline phosphatase 1125 IU/L, and gamma glutamyl transferase 586 IU/L. Viral hepatitis screen hepatitis was negative, alpha-fetoprotein (AFP) was 2 ng/mL (normal<7), alpha-1-antitrypsin was normal phenotype, and serum copper and ferritin levels were in normal range. Autoimmune antibody and immunoglobulin screens were negative.
Ultrasound showed multiple bilobar focal liver lesions with increased reflectivity in keeping with multiple adenomas and FNH. Initial ultrasound missed the Abernethy malformation and reported portal flow to be present. There was no portal hypertension. Triphasic computed tomography (CT) scan showed mild hepatomegaly and steatosis with multiple bilobar ill-defined areas of abnormal attenuation. The lesions enhanced heterogeneously in the arterial phase and were isodense with the rest of the liver on venous phase scans. The largest lesion measured 13 cm maximally and occupied segments V, VII and VIII (Fig. 1A). There was a patent portocaval communication in the form of a direct shunt, 15 mm in diameter, between the inferior vena cava and the mesosplenic confluence. The intrahepatic portal vein was absent and the hepatic artery was hypertrophic. These findings were confirmed by angiography (Fig. 1C) and were consistent with type 1b CEPS. Magnetic resonance imaging with hepatocyte specific contrast agent confirmed the lesions to be hepatocellular adenomas (Fig. 1B).
Portal pressure studies showed a hepatic venous pressure gradient of 3 mmHg (>5 mmHg implies portal hypertension). Transthoracic echocardiogram was normal with no evidence of pulmonary hypertension.
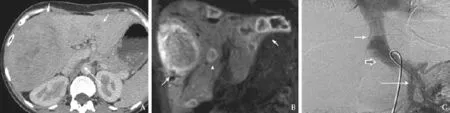
Fig. 1. Post contrast CT (A) and MR (B) of case 1: CT and MR at initial presentation demonstrating multiple lesions in both lobes of the liver (arrows). CT also depicts the shunt between meso-splenic confluence and the inferior vena cava (long arrow). Aortoportogram of case 1 (C): A trans-femoral catheter placed in the superior mesenteric vein and delayed image after injection of contrast depicting the shunt (block arrow), between the SMV inferiorly and the inferior vena cava superiorly (arrows). Note the absence of the intrahepatic portal vein.
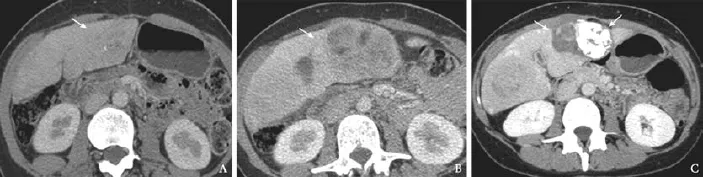
Fig. 2. Comparative CT of case 1: CT at presentation (A) and 2 years later (B) demonstrating a significant increase in size of the segment IV lesion consistent with transformation into HCC (arrows). Final CT (C) demonstrating effective response following combination therapy with lipiodol embolization and subsequent microwave ablation (arrows).
Biopsy from the dominant right liver lesion showed bland, often steatotic hepatocytes with individual intervening arteries but no identifiable portal tracts. There were no features of HCC. On immunohistochemistry, aberrant cytokeratin 7 expression was observed in hepatocytes and focally in ductules. There was mild and focal staining for serum amyloid A. Glutamine synthetase expression was not present. Fatty acid protein binding capacity appeared to be preserved. β-catenin staining showed a patchy membranous but no nuclear expression. The appearance was in favor of an inflammatory variant of hepatocellular adenoma.
As the patient was asymptomatic and liver lesions were bilobar and appeared benign it was decided to follow up with repeat liver function tests and imaging.
Two years later, her AFP was 822 ng/mL. CT scan showed changes consistent with an HCC in a lesion in segment IV which had a significant increase in size from 28 mm (Fig. 2A) on initial scans to 8 cm (Fig. 2B). Biopsy revealed a moderately and focally poorly differentiated HCC. This was treated with selective transcatheter arterial embolization with a combination of lipiodol and spherical particles (500 μm to 750 μm). Post procedure, the patient experienced significant pain, fever and abdominal tenderness which settled over one week.
Follow-up scans revealed viable tumor in the inferolateral margin of the previously embolized lesion and she underwent two further embolizations with good response (Fig. 2C). She finally underwent microwave ablation and a repeat CT scan after one month showed stable disease. Her AFP was 8 ng/mL at discharge and she remains on close surveillance.
Case 2
A 49-year-old man presented with two months of right upper quadrant abdominal pain, weight loss, anorexia and pruritis. He had a history of "delayed milestones" as a child and had been left with severe learning disabilities after an episode of 'viral encephalitis' at the age of 4 years. On examination he was cachectic with hepatomegaly. His ECOG (Eastern Cooperative Oncology Group) performance status was 2-3.
He had an elevated AFP (380 ng/mL) and liver function tests showed bilirubin 33 μmol/L with mild cholestasis (alkaline phosphatase 249 IU/L). Serum ammonia was 59 μmol/L (normal 12-50). CT scan (Fig. 3) revealed a type 1b CEPS. The hepatic artery, celiac axis and upper abdominal aorta were hypertrophied. There was a large tumor measuring 14 cm in diameter in the right lobe of the liver with a central stellate scar. The ra-diological diagnosis was HCC or FNH though the clinical presentation and raised AFP favored HCC. The core needle biopsy specimen from the lesion revealed a well differentiated HCC. Non lesional liver biopsy showed hepatocellular plate disarray, NRH, sinusoidal dilatation, mild fibrosis and mild patchy steatosis. Portal tracts were small with attenuated portal vein radicals and prominent arterial branches.
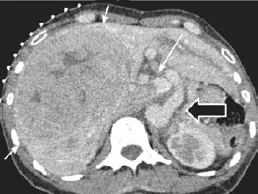
Fig. 3. Case 2: CT demonstrating large HCC in the right lobe of the liver with central scar (arrows inferior vena cava), portosystemic shunt between the extrahepatic portal vein and inferior vena cava (long arrow) and hypertrophied coeliac axis (black arrow).

Fig. 4. Case 3: Left: CT scan depicting a large exophytic HCC in the right lobe of the liver with peri-capsular hematoma (arrows), portocaval shunt (long arrow) and hypertrophied coelic axis (black arrow). Right: CT post embolization demonstrating necrosis within the tumor.
Due to his poor performance status he was not considered fit for any treatment and was started on dexamethasone to combat his pain and improve his appetite. His appetite improved as a result of this and his performance status improved to ECOG 1-2. He is currently on systemic treatment with sorafenib (Nexavar®, Bayer).
Case 3
A 29-year-old man presented to the emergency department with severe right upper quadrant abdominal pain. His medical history was unremarkable. On physical examination he was thin with palpable hepatomegaly. Laboratory studies showed a normal AFP but cancer antigen-125 was elevated: 113 U/mL (normal <35). Bilirubin was normal. CT (Fig. 4) and magnetic resonance imaging scan revealed a large 21 cm tumor with features of an HCC arising exophytically from the right lobe of the liver and extending into the caudate lobe with satellite lesions in segments II, III and IV. The large tumor showed features of rupture with capsular discontinuity and a peri-capsular hematoma. CT scan also showed features of type 1b CEPS. On laparoscopic biopsy the large mass was extensively necrotic with some residual viable satellite nodules of moderately differentiated HCC. Non lesional tissue showed a heterogenous pattern with areas of bridging fibrosis and hepatocellular plate disarray with sinusoidal dilatation. Portal tracts, where present, showed attenuated portal veins.
The size and segmental distribution precluded the patient from being treated with surgical resection or liver transplantation. He was treated twice with transcatheter arterial embolization with good response. However, he too experienced significant pain post embolization. He has been started on chemotherapy with sorafenib.
Discussion
We report 3 cases of type 1 CEPS.[1]This is a rare malformation with a wide range of clinical presentations[8]and is often missed on initial imaging. Suspicion should be raised in cases where hepatic encephalopathy, raised serum ammonia or HCC exist in the absence of cirrhosis.
Embryologically, the right and left vitelline veins normally form an inter-communicating network around the gut which drains directly into the primitive sinus venosus. As the liver bud develops around the vitelline veins, it incorporates the vitelline veins to form the hepatic sinusoids. Selective involution of the peri-intestinal right and left vitelline veins between weeks 4-10 of embryological development results in the formation of a single portal vein. Excessive involution of these veins results in Abernethy malformation with an absent or attenuated extrahepatic portal vein.
A genetic basis has been proposed for Abernethy malformation[18]and suggests that the patent shunt, HCC and other manifestations associated with CEPS may well be a part of a genetic syndrome rather than have any causal relationship with each other.
The exact etiology for HCC and other liver tumors in these patients is not clear. A study has shown well differentiated HCCs to have lower attenuation on CT arteriography and iso-attenuation on CT arterioportography due to a combination of hepatic arteriolar degeneration and preserved portal venous blood supply as seen on corresponding histopathological specimens. Moderate to poorly differentiated HCCs on the other hand showed high attenuation on CT arteriography with lowattenuation on CT arterio-portography due to abnormal neoplastic angiogenesis with obliteration of the portal veins.[19]Thus an association has been drawn between the development of HCCs and a combination of high arterial blood flow and reduced portal flow. It may be speculated that the compensatory increase in hepatic arterial flow, as observed in Abernethy malformation, creates conditions that cause the hepatic parenchymal cells to de-differentiate resulting in the occurrence of HCC. However, this remains to be proven.
Interestingly, all reported cases of CEPS who went on to develop an HCC, including the 3 cases we report, have a type 1 shunt (Table) further supporting the role of absent portal blood flow as an important factor in the development of this tumor.
Why hyperammonemia is not a more consistent finding in these patients, given the large amount of portosystemic shunting, is a matter of speculation. Modification of the gut bacterial profile has been proposed as a reason for the absence of hyperammonemia.[20]
Although the presence of CEPS has been viewed as a potential contraindication to the use of loco-regional therapy such as embolization due to lack of portal inflow and the risk of precipitating hepatic failure, two of our patients underwent embolization procedures. However, both patients experienced significant pain, and inflammatory response and tissue necrosis were much more prominent than in patients with cirrhosis and HCC. Chemo-embolization was avoided because of the perceived higher risk of complications. These patients may have a good response to transcatheter arterial embolization as the hepatic artery is the only blood supply.
Of note, the second patient presented with an HCC but had radiological features more in favor of an FNH. Although FNH is not classically known to progress to an HCC, there has been a case report demonstrating progression of an FNH to HCC in a patient with Abernethy malformation.[7]This suggests a need for close surveillance for patients with FNH in the presence of Abernethy malformation.
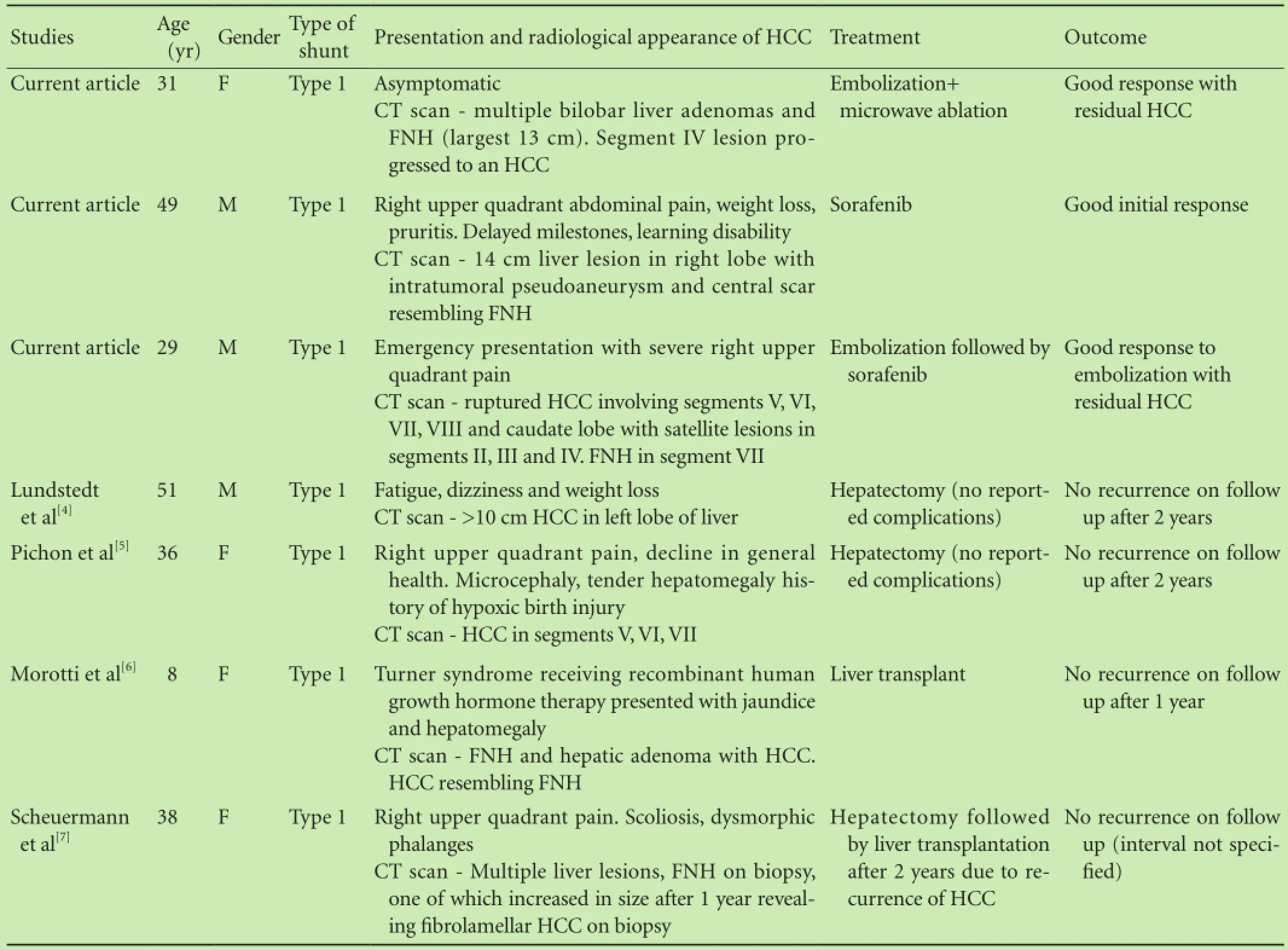
Table. Summary of cases with Abernethy malformation complicated by HCC
Taken together, these three cases demonstrate the variability in the clinical presentation of Abernethy malformation and the fact that clinical investigation is initiated more often for complications associated with the shunt rather than manifestations of the shunt itself. Consistently seen in these cases is the presence of HCC in the absence of cirrhosis or chronic liver disease, the radiological appearance of the shunt on imaging along with a hypertrophied hepatic artery and the absence of portal venules on biopsy specimens.
Liver regeneration post-hepatectomy has been believed to be dependent on hepatotropic factors transported from the gut via the portal vein. However, patients with congenital absence of the portal vein have been reported to undergo hepatectomy with uncomplicated post-operative recovery.[4,5]
These 3 patients and the others reported in the literature suggest that HCC is a common complication of this disorder. The patients had type 1 shunts, mild liver dysfunction and multiple liver nodules. This group of patients need close surveillance and selection criteria for liver transplantation need to be developed to offer treatment at an appropriate time.
Contributors: HN proposed the study. SR performed literature review and studied the case and wrote the paper. PP and KJ reviewed the radiological images. QA reviewed histology. SA provided insight into medical and chemotherapeutic management and performed portal pressure studies. ST provided information and follow up for the third case in the series. HN was the key supervisor and coordinator providing guidance throughout and for formatting and approving the final draft of the paper. All authors contributed to the design and interpretation of the study and to further drafts. SR is the guarantor.
Funding: None.
Ethical approval: Not needed.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1 Morgan G, Superina R. Congenital absence of the portal vein: two cases and a proposed classification system for portasystemic vascular anomalies. J Pediatr Surg 1994;29:1239-1241.
2 Abernethy J, Banks J. Account of two instances of uncommon formation, in the Viscera of the human body. PRS. Philos Trans R Soc Lond 1793;83:59-66.
3 Taïeb J, Castera L, Boige V, Frouge C, Bedossa P, Bernard O, et al. Hepatocellular carcinoma complicating agenesis of the portal vein. Gastroenterol Clin Biol 1998;22:246-247.
4 Lundstedt C, Lindell G, Tranberg KG, Svartholm E. Congenital absence of the intrahepatic portion of the portal vein in an adult male resected for hepatocellular carcinoma. Eur Radiol 2001;11:2228-2231.
5 Pichon N, Maisonnette F, Pichon-Lefièvre F, Valleix D, Pillegand B. Hepatocarcinoma with congenital agenesis of the portal vein. Jpn J Clin Oncol 2003;33:314-316.
6 Morotti RA, Killackey M, Shneider BL, Repucci A, Emre S, Thung SN. Hepatocellular carcinoma and congenital absence of the portal vein in a child receiving growth hormone therapy for turner syndrome. Semin Liver Dis 2007;27:427-431.
7 Scheuermann U, Foltys D, Otto G. Focal nodular hyperplasia precedes hepatocellular carcinoma in an adult with congenital absence of the portal vein. Transpl Int 2012;25:e67-68.
8 Witters P, Maleux G, George C, Delcroix M, Hoffman I, Gewillig M, et al. Congenital veno-venous malformations of the liver: widely variable clinical presentations. J Gastroenterol Hepatol 2008;23:e390-394.
9 Grazioli L, Alberti D, Olivetti L, Rigamonti W, Codazzi F, Matricardi L, et al. Congenital absence of portal vein with nodular regenerative hyperplasia of the liver. Eur Radiol 2000;10:820-825.
10 De Gaetano AM, Gui B, Macis G, Manfredi R, Di Stasi C. Congenital absence of the portal vein associated with focal nodular hyperplasia in the liver in an adult woman: imaging and review of the literature. Abdom Imaging 2004;29:455-459.
11 Barton JW 3rd, Keller MS. Liver transplantation for hepatoblastoma in a child with congenital absence of the portal vein. Pediatr Radiol 1989;20:113-114.
12 Wakamoto H, Manabe K, Kobayashi H, Hayashi M. Subclinical portal-systemic encephalopathy in a child with congenital absence of the portal vein. Brain Dev 1999;21:425-428.
13 Pujol A, Pujol J, Graus F, Rimola A, Peri J, Mercader JM, et al. Hyperintense globus pallidus on T1-weighted MRI in cirrhotic patients is associated with severity of liver failure. Neurology 1993;43:65-69.
14 Sanada Y, Mizuta K, Kawano Y, Egami S, Hayashida M, Wakiya T, et al. Living donor liver transplantation for congenital absence of the portal vein. Transplant Proc 2009;41:4214-4219.
15 Tercier S, Delarue A, Rouault F, Roman C, Bréaud J, Petit P. Congenital portocaval fistula associated with hepatopulmonary syndrome: ligation vs liver transplantation. J Pediatr Surg 2006;41:e1-3.
16 Bernard O, Franchi-Abella S, Branchereau S, Pariente D, Gauthier F, Jacquemin E. Congenital portosystemic shunts in children: recognition, evaluation, and management. Semin Liver Dis 2012;32:273-287.
17 Franchi-Abella S, Branchereau S, Lambert V, Fabre M, Steimberg C, Losay J, et al. Complications of congenital portosystemic shunts in children: therapeutic options and outcomes. J Pediatr Gastroenterol Nutr 2010;51:322-330.
18 Jacob S, Farr G, De Vun D, Takiff H, Mason A. Hepatic manifestations of familial patent ductus venosus in adults. Gut 1999;45:442-445.
19 Honda H, Tajima T, Taguchi K, Kuroiwa T, Yoshimitsu K, Irie H, et al. Recent developments in imaging diagnostics for HCC: CT arteriography and CT arterioportography evaluation of vascular changes in premalignant and malignant hepatic nodules. J Hepatobiliary Pancreat Surg 2000;7:245-251.
20 Komatsu S, Nagino M, Hayakawa N, Yamamoto H, Nimura Y. Congenital absence of portal venous system associated with a large inferior mesenteric-caval shunt: a case report. Hepatogastroenterology 1995;42:286-290.Announcements for this section should be submitted in the correct format at least 3 months before the required date of publication. This list is provided as a service to readers; inclusion does not imply endorsement by the Hepatobiliary & Pancreatic Diseases International.
Accepted after revision June 1, 2015
Section editor
Shui-Ying Lei
Email: hbpdint@126.com
November, 2015
Advances in pediatric cancer research: from mechanisms and models to treatment and survivorship
November 9-12, 2015; Florida, USA
This special conference will address the unique issues and challenges in investigating the biologic basis of childhood cancers and translating recent findings into new treatment approaches. Presentations will examine the most recent research on novel strategies for modeling pediatric cancers, basic and applied genomics, and the most difficult to treat pediatric cancers. Strategies for translating basic science findings into treatments with a clinical impact will be featured, including the most up-to-date findings on targeted therapeutics, resistance, and immunotherapy. Advances in our understanding of the early and late effects of treatment will also be featured. This conference will bring together basic, clinical, and translational researchers in the field of pediatric cancer with expertise ranging from the most recent advances in the basic mechanisms of childhood cancers to the application of these findings in the clinical setting. For more information, please visit: http://www.aacr.org/ Meetings/Pages/MeetingDetail.aspx?EventItemID=41#. VZ3bZ_nvPGg.
New horizons in cancer research conference: bringing cancer discoveries to patients
November 12-15, 2015; Shanghai Marriott Parkview Hotel, Shanghai, China
Conference co-chairpersons are Lewis C. Cantley from Sandra and Edward Meyer Cancer Center at Weill Cornell Medical College, New York, New York; and Carlos L. Arteaga from Vanderbilt-Ingram Cancer Center at Vanderbilt University, Nashville, Tennessee. The meeting will serve as a dynamic platform for the advancement of cancer science and medicine, and for the establishment of new collaborations on an international scale. The highly engaging and interactive four-day multidisciplinary program will cover the entire cancer research spectrum--from basic, to translational, to clinical--drawing on the latest findings presented at the 106th annual meeting of the AACR to be held in Philadelphia, April 18-22, 2015. The meeting will feature talks from an outstanding roster of international speakers and local experts. For more information, please visit: http://aacr. com.cn/.
The liver meeting 2015®
November 13-17, 2015; San Francisco, CA, USA
The 2015 meeting programming has been refined as part of a major initiative of AASLD in order to reflect the rapid scientific advances in the field of hepatology, and improve the overall attendee experience. This meeting is to exchange the latest liver diseases research, discuss treatment outcomes, and interact with colleagues at the annual must-attend even in the science and practice of hepatology. For more information, please visit: http:// www.aasld.org/events-professional-development/livermeeting-2015#sthash.n6bpBw6B.dpuf.
December
CSHA/AACR joint meeting: big data, computation and systems biology in cancer
December 1-5, 2015; Suzhou Dushu Lake Conference Center, Suzhou, China
The conference will include eight oral sessions and one poster session covering the latest findings across many topics in big data, computation, and systems biology in cancer research. Many talks will be selected from the openly submitted abstracts on the basis of scientific merit and relevance. Social events throughout the conference provide ample opportunity for informal interactions. Major topics include: systems biology, computational biology, functional screening and drug discovery, tumor heterogeneity, models and networks, regulation and signaling, and cancer genomics and bioinformatics. For more information, please visit: http://www.csh-asia. org/2015meetings/AACR.html.
EASL Masterclass 2015
December 3-5, 2015; Mezzana Bigli - Pavia, Italia
This year's Masterclass will include 1) scientific sessions on: viral hepatitis; metabolic and cholestatic liver disease; pro & cons discussions: a) Should betablockers be stopped in patients with advanced cirrhosis? b) Treating the HCV decompensated cirrhotic patient: before or after liver transplantation? 2) Special lectures and workshops: The Story of EASL; The story of my Life by Roger Williams; Hepatology in the future by Didier Samuel;Presentation Skills workshop; 3) Case based discussions on: abnormal liver test; complications of liver cirrhosis; viral hepatitis and co-infections; dedicated parallel session on basic science. For more information, please visit: http://www.easl.eu/discover/events/detail/easl-masterclass-2015.
January, 2016
The function of tumor microenvironment in cancer progression
January 7-10, 2016; Hard Rock Hotel, San Diego, California, USA
Conference Co-Chairpersons are Raghu Kalluri, The University of Texas MD Anderson Cancer Center, Houston, Texas; Robert A. Weinberg, MIT Whitehead Institute for Biomedical Research, Cambridge, Massachusetts; Douglas Hanahan, Swiss Institute for Experimental Cancer Research (ISREC), Lausanne, Switzerland; and Morag Park, Goodman Cancer Research Centre, McGill University, Montréal, Quebec, Canada. This AACR special conference will focus on discussing the emerging concepts in stromal cell and ECM heterogeneity, stromal cell metabolism, early events in carcinogenesis involving contributions from tumor cells and their microenvironment, stress responses to oxygen and nutrient gradients, translational impact of targeting the microenvironment, tumor immunity and immunotherapy. For more information, please visit: http://www.aacr.org/Meetings/Pages/ MeetingDetail.aspx?EventItemID=73#.VfD6ifnvPGg.
February
Patient-derived cancer models: present and future applications from basic science to the clinic
February 11-14, 2016; Hyatt Regency New Orleans, New Orleans, Louisiana, USA
This multilayered program includes leaders from academia, pharmaceutical industry, biotech companies, and regulatory agencies that will discuss technical and methodological issues regarding PDX development; disease-oriented collections; newer approaches to PDX development; applications in translational cancer research; personalized medicine opportunities; and US and EU-based network initiatives. Continuing Medical Education Activity AMA PRA Category 1 CreditsTM is available. For more information, please visit: http://www.aacr.org/Meetings/Pages/MeetingDetail. aspx?EventItemID=75#.VfE10fnvPGg.
April
AACR annual meeting 2016
April 16-20, 2016; Ernest N. Morial Convention Center, New Orleans, Louisiana, USA
The AACR annual meeting highlights the best cancer science and medicine from institutions all over the world. Attendees are invited to stretch their boundaries, form collaborations, attend sessions outside their own areas of expertise, and learn how to apply exciting new concepts, tools, and techniques to their own research. Program Committee chairperson is Scott A. Armstrong, Memorial Sloan Kettering Cancer Center, New York, New York, USA. For more information, please visit: http://www.aacr.org/Meetings/Pages/MeetingDetail. aspx?EventItemID=63#.VfFHZ_nvPGg.
May
ILTS workshop: liver transplantation in HCV positive recipients
May 19-20, 2016; San Francisco, CA, USA
The ILTS workshop and consensus conference will take place the 2 days prior to digestive diseases week (DDW) being held in San Diego, CA, so any delegates would be able to attend this workshop and conference as well as DDW. For further information, please contact: Norah Terrault, MD, MPH, Consensus Co-Chair, Professor of Medicine and Surgery, Director, Viral Hepatitis Center, Division of Gastroenterology, University of California San Francisco, San Francisco, California, Norah. Email: Terrault@ucsf.edu; Or Lisa D. Pedicone, PhD, Consensus Committee Consultant, Executive Vice President, Clinical Affairs, Focus Medical Communications, Parsippany, New Jersey 07054, USA. Tel: +973 520-1822; Email: lpedicone@focusmeded.com.
10.1016/S1499-3872(15)60418-0
10.1016/S1499-3872(15)60023-6)
Author Affiliations: Institute of Liver Studies (Sharma R, Suddle A, Quaglia A and Heaton N); Department of Radiology (Peddu P and Karani J), King's College Hospital, London SE5 9RS; Central Manchester University Hospital, Manchester Royal Infirmary, Manchester M13 9WL, United Kingdom (Satyadas T)
Ruchi Sharma, MBBS, MS (Gen Surg), MRCS, Department of Hepatopancreaticobiliary Surgery, Institute of Liver Studies, King's College Hospital, Denmark Hill, London SE5 9RS, United Kingdom (Tel: +447702348683; Email: drruchisharma@ymail.com)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
Published online September 17, 2015.
August 22, 2014
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Multidisciplinary management of Mirizzi syndrome with choIecystobiIiary fistuIa: the vaIue of minimaIIy invasive endoscopic surgery
- The "meso" of the rectum and the "meso" of the pancreas: similar terms but distinctconcepts in surgical oncology
- Extrahepatic right portal vein ligation allows parenchyma-sparing en bloc resection of segments 7, 8 and 4a for liver tumors engaging the right and middle hepatic veins
- CT-guided high-dose-rate brachytherapy in the interdisciplinary treatment of patients with liver metastases of pancreatic cancer
- Diagnostic and prognostic roles of soluble CD22 in patients with Gram-negative bacterial sepsis
- 18F-FDG PET/CT in differentiating malignant from benign origins of obstructive jaundice
