CT-guided high-dose-rate brachytherapy in the interdisciplinary treatment of patients with liver metastases of pancreatic cancer
2015-11-21GeroWienersAlexanderChristianSchippersFedericoCollettiniDirkSchnapauffBerndHammPeterWustHannoRiessandBernhardGebauer
Gero Wieners, Alexander Christian Schippers, Federico Collettini, Dirk Schnapauff, Bernd Hamm, Peter Wust, Hanno Riess and Bernhard Gebauer
Berlin, Germany
CT-guided high-dose-rate brachytherapy in the interdisciplinary treatment of patients with liver metastases of pancreatic cancer
Gero Wieners, Alexander Christian Schippers, Federico Collettini, Dirk Schnapauff, Bernd Hamm, Peter Wust, Hanno Riess and Bernhard Gebauer
Berlin, Germany
BACKGROUND: CT-guided high-dose-rate brachytherapy (CT-HDRBT) is an interventional radiologic technique for local ablation of primary and secondary malignomas applying a radiation source through a brachycatheter percutaneously into the targeted lesion. The aim of this study was to assess local tumor control, safety and efficacy of CT-HDRBT in the treatment of liver metastases of pancreatic cancer.
METHODS: Twenty consecutive patients with 49 unresectable liver metastases of pancreatic cancer were included in this retrospective trial and treated with CT-HDRBT, applied as a single fraction high-dose irradiation (15-20 Gy) using a192Irsource. Primary endpoint was local tumor control and secondary endpoints were complications, progression-free survival and overall survival.
RESULTS: The mean tumor diameter was 29 mm (range 10-73). The mean irradiation time was 20 minutes (range 7-42). The mean coverage of the clinical target volume was 98% (range 88%-100%). The mean D100 was 18.1 Gy and the median D100 was 19.78 Gy. Three major complications occurred with post-interventional abscesses, three of which were seen in 15 patients with biliodigestive anastomosis (20%) and overall 15%. The mean follow-up time was 13.7 months(range 1.4-55.0). The median progression-free survival was 4.9 months (range 1.4-42.9, mean 9.4). Local recurrence occurred in 5 (10%) of 49 metastases treated. The median overall survival after CT-HDRBT was 8.6 months (range 1.5-55.3). Eleven patients received chemotherapy after ablation with a median progression-free survival of 4.9 months (mean 12.9). Nine patients did not receive chemotherapy after intervention with a median progression-free survival of 3.2 months (mean 5.0). The rate of local tumor control was 91% in both groups after 12 months.
CONCLUSION: CT-HDRBT was safe and effective for the treatment of liver metastases of pancreatic cancer.
(Hepatobiliary Pancreat Dis Int 2015;14:530-538)
brachytherapy;
pancreatic cancer;
liver metastases;
CT;
intervention
Introduction
Pancreatic cancer ranks among the 10 most frequently occurring cancers with an incidence of around 14 per 100 000 individuals. It is the fourth leading cause of cancer death.[1]The prognosis of pancreatic cancer remains poor with a 5-year survival rate of 6%. Unfortunately, a considerable number of patients present an advanced disease without any symptoms in the early stage at the moment of diagnosis. And only 10%-15% of such patients are eligible for curative resection.[2,3]Even if no liver metastases can be identified at diagnosis, a large number of patients eventually develop liver metastases after curative surgery. Hence, control of metastatic spread seems to be an important factor for the improvement of survival of patients with liver metastases caused by pancreatic cancer. Liver metastasectomy asa central method fails to show significant effect and cannot be repeatedly used because of patient conditions and impaired liver function.[4,5]
In recent years, interventional radiologists have established minimal invasive image guided therapies including interstitial laser therapy and radiofrequency ablation, which serve as alternatives for the treatment of metastases of other primaries.[6,7]But both of these thermo-ablative techniques have several limitations. They are ineffective in tumor volume larger than 4-5 cm and in highly perfused regions ("heat sink" effect[8]), or cannot be used in areas close to thermosensitive structures, i.e. bile ducts otherwise causing complications such as cholestasis and jaundice.[9]Additionally, many pancreatic cancer patients have a biliodigestive anastomosis (BDA) after resection of the primary tumor (Whipple's resection) or placement of a bile duct stent, causing bacterial colonization of the intrahepatic bile ducts with a higher abscess rate after thermal liver ablation.[10,11]
In the present study, CT-guided high-dose-rate brachytherapy (CT-HDRBT) was introduced to advance the treatment of pancreatic cancer. CT-HDRBT is an afterloading technique using an192Ir-source which is temporarily inserted through catheters placed under CT guidance into the targeted tumor volume. The therapeutic effect of this technique is based upon single application of unfractioned high radiation dosages. The irradiation provides an extensive cytotoxic effect in a circumscribed volume while sparing surrounding tissue through preinterventional 3D-intervention planning and anatomybased 3D optimization of the dose distribution ensuring adequate coverage of the to be treated lesions and therefore leading to an excellent rate of tumor control.
Major advantages of the method relate to the independence of inherent patient motion, i.e. respiration. Moreover, there are no known restrictions to the maximum size or cooling effects of tumor perfusion and, therefore CT-HDRBT can be applied in proximity to thermosensitive structures.[12-14]CT-HDRBT can even be used for local ablation of hepatocellular carcinoma, which has a low radiation-sensitivity to conventional fraction schemes (i.e. external beam radiation therapy of 1.8-2.0 Gy per fraction), providing excellent local tumor control.[15]
CT-HDRBT has been successfully used for the treatment of several secondary and primary liver malignancies in addition to lung malignomas and other extrahepatic and extrapulmonary tumors.[14,16-18]The present study was undertaken to assess the feasibility, safety, and clinical outcome of CT-HDRBT of liver metastases from pancreatic cancer in the palliative setting with the goal of local tumor control.
Methods
Patients
This analysis included 20 consecutive patients with liver metastases caused by pancreatic adenocarcinoma who had been treated with CT-HDRBT from October 2007 until January 2013. Before CT-HDRBT treatment, the institutional interdisciplinary tumor board discussed and considered all appropriate therapy options and judged patients suitable for the intervention. Inclusion criteria for CT-HDRBT were as follows: (1) a liver function status at Child class A or B; (2) prothrombin time (quick)>50%, international normalized ratio >1.5; (3) partial thromboplastin time <50 seconds; (4) platelet count>50×109/L; and (5) total serum bilirubin <2 mg/dL. If necessary, hemostatic function was pre-interventionally corrected (e.g., platelet concentrates). Exclusion criteria included: >5 liver metastases and the evidence of extrahepatic diseases. Stable extrahepatic metastases were not a contraindication for ablation. Related imaging and clinical records were evaluated for all patients. The patients provided written informed consent. The local ethics committee approved this study. Primary endpoint was local tumor control. Secondary endpoints were complications, progression-free survival (PFS) and overall survival (OS). CT-HDRBT was indicated for 16 patients with locally unresectable tumors or bilobar tumors, general surgery contraindicated for 3 patients and surgical resection refused for one patient. All of the patients were heavily pre-treated patients with metachronous disease (i.e., had a tumor-free interval after resection of the primary tumor). In this series of patients, primary tumor had been surgically removed and only 3 patients had no systemic chemotherapy before CT-HDRBT. No patient demonstrated extrahepatic tumor at preoperative staging.
Interventional technique, radiation properties and treatment planning
Pre-interventional contrast-enhanced CT of the abdomen and thorax was performed before actual ablation to attain a complete staging and to assure absence of extrahepatic metastases. The procedure consists roughly of three major steps and its detailed description has been reported elsewhere:[19-21](1) CT-guided catheter implantation; (2) computer-based 3D treatment planning; and (3) subsequent high-dose irradiation in afterloading technique.[22]CT-guided catheter positioning was executed under analgesia and sedation (an initial dose of 75 μg fentanyl and 1 mg midazolam given intravenously) and local anesthesia (lidocaine). Additional pain medication was titrated in increments of 25 μg fentanyl or 0.5 mg midazolam given intravenously as needed. A 17-gaugetrocar puncture needle was inserted under CT fluoroscopy into the tumor and exchanged over a stiff angiographic guide wire (Amplatz Super Stiff Guide Wire, Boston Scientific, Natick, MA, USA) to a flexible 6F sidearm catheter sheath (Radiofocus, Terumo, Tokyo, Japan) performing Seldinger-technique. Malignant lesions were localized through anatomical orientation using the preinterventional acquired CT in comparison with fluoroscopy, and lesions >1 cm in diameter were directly visible under fluoroscopy. Finally, the angiographic guide wire was removed, and a closed-end 6F afterloading catheter (Primed, Halberstadt Medizintechnik GmbH, Halberstadt, Germany) was inserted into the tumor. The arrangement and numbers of catheters depended on the shape and size of the lesion and its local anatomy (i.e. adjacent to the stomach, gut, kidneys or spinal cord). After catheter positioning, a contrast-enhanced CT scan of the liver was performed to confirm exact placement inside the tumor and to plan further treatment. Computer-based 3D treatment planning and irradiation were performed by a physicist, interventional radiologist and radio-oncologist on a dedicated workstation using the treatment planning unit BrachyVision (Varian medical systems, Charlottesville, VA, USA) (Fig. 1).[23]The dosage calculations implemented in BrachyVision conform to the recommendations of the American Association of Physicists in Medicine Task Group Report 43, and BrachyVision performs its volume optimization using the Nelder Mead Simplex optimization method.[24-28]The treatment planning was 3D image-based with anatomyoriented dose optimization to ensure correct conformality and precise prediction and documentation of energy deposition. All afterloading catheters were digitized from the body exit point to the tip. Afterwards, the clinical target volume and all at-risk structures (e.g. bowel, stomach, liver hilum and spinal cord) were carefully outlined. Clinical target volume was defined as the visible tumor borders in contrast-enhanced CT scans including the enhancing rim. To achieve complete coverage of the target volume while sparing critical structures, we semi-auto-matically calculated and optimized source dwell points and times for the192Ir-sources inside the afterloading catheters. All irradiations were executed as single fraction irradiations in afterloading technique using the192Ir radiation source (diameter <1 mm) with a nominal activity of 10 Ci. Dose gradients follow the inverse square law, and for a typical treatment doses fall off to clinical irrelevant numbers in 10-20 mm distance. Intention of radiation treatment planning was a complete (100%) confinement of the clinical target volume with the prescribed target dose. Inside the clinical target volume, a high-dose in homogeneties was regularly seen ranging from minimal applied doses (D100) at the margin of the clinical target volume to maximum doses of several hundred Gy in smallest volumes next to the applicator. D100 was defined as the dose covering the full volume (100%) of a certain structure, meaning that any point in this structure received at least i.e. in this report 15 Gy or 20 Gy. For a typical treatment, doses of 80-150 Gy for the clinical target volume and a V5 of the liver of several hundred mL were seen (V5 was defined as the volume receiving at least 5 Gy). To preserve liver function after irradiation, at least one-third of the liver had to receive less than 5 Gy. Further dose restrictions were applied at risk structures (i.e. stomach or gut <15 Gy, myelon <15 Gy).[29,30]The minimum dose to cover the clinical target volume was 15 Gy. Maximum doses up to 50 Gy were allowed in the tumor center. If exposure of the gastric wall or duodenal mucosa exceeded the critical dose of 10 Gy/mm3of the at-risk organ, proton pump inhibitors were prescribed (pantoprazole 40 mg) for 6 weeks. After irradiation, the brachytherapy catheters were cautiously removed, and the puncture channels were sealed with absorbable thrombogenic material Gelfoam (Gelaspon, Chauvin ankerpharm, Germany) to prevent post-interventional bleeding.
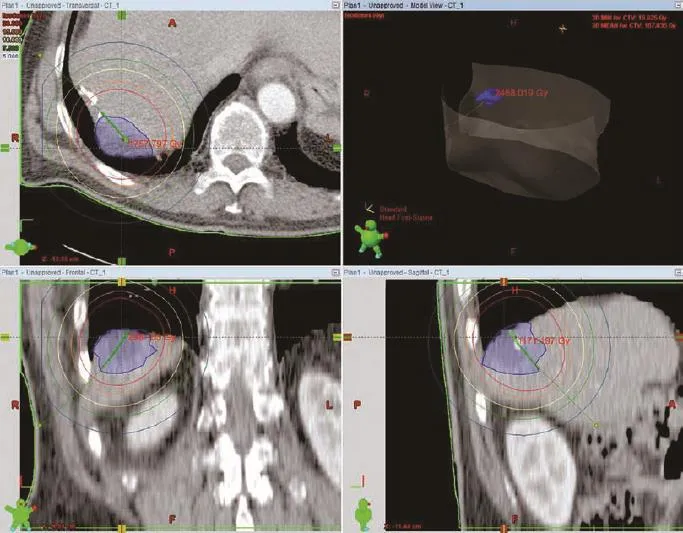
Fig. 1. Irradiation was performed by a physicist, an interventional radiologist and a radio-oncologist using the treatment planning unit BrachyVision (Varian medical systems, Charlottesville, VA, USA). An192Ir-radiation source with an activity of 10 Ci was used, diameter<1 mm. The target dose of 20 Gy enclosing the clinical target volume was applied as a single fraction demonstrated by the red line. In the center of the tumor next to the percutaneously used catheter the dose is around 100 Gy in this case. Dose gradients follow the inverse square law.
Tumor response and follow-up
Post procedure imaging was contrast-enhanced MRI of the liver in all cases. Time was calculated from the day of ablation to the first and consecutive contrastenhanced MRI. MRI was performed every three months after treatment for assessment of the initial result of ablation and identification of residual tumor necessitating additional CT-HDRBT ablation sessions. The MRI parameters were T2-weighted ultra-turbo spin echo (UTSE; TE/TR 90/2100) with and without fat suppression, T1-weighted gradient echo (GRE; TE/TR 5/30, flip angle 30°) plain, dynamic sequences and sequences 20 minutes post intravenous injection of 0.1 mL/kg Gd-EOBDTPA (Primovist, Bayer-Schering Healthcare). Absence of enhancement was considered to represent complete necrosis. Primary effectiveness was defined as the percentage of tumor volume successfully eradicated during the initial course of treatment. Residual tumor enhancement within or at the periphery of the target tumor was considered a viable tumor. Additional contrast-enhanced CT was performed 3 months after treatment and then at 3-month intervals with the timing altered if needed to accommodate management of new disease, especially of the lung. The evolution of the size of ablation zone was monitored with serial MRIs and reported as orthogonal diameter in the axial plane in which the zone of ablation was the largest. Local tumor control after brachytherapy was defined as the absence of tumor progression and/or a loss of tumor volume starting in MRI at 3 months post-treatment. PFS was defined as local tumor control after brachytherapy and the absence of tumor progression elsewhere. Images were reviewed with consensus of two experienced radiologists. Major complications were defined as those necessitating unplanned hospitalization or intensive care unit admission, prolonging hospital stay, or additional surgical or interventional procedures. Minor complications were those managed conservatively but not necessitating or prolonging a hospital stay.
Statistical analysis
For statistical analysis, Wilcoxon's rank-sum test, the Kaplan-Meier method and the log-rank test were used.
Results
Interventional procedure
CT-HDRBT was performed to treat 49 liver metastases of pancreatic cancer in 20 consecutive patients (9 females and 11 males) (Table). Fourteen patients had monolobar and six patients had bilobar tumor manifestations. The mean tumor diameter was 29 mm (range 10-73). The mean number of metastasis treated by brachytherapy in one intervention was 1.8 (range 1-4), the mean amount of catheters employed was 3.1 (range 1-9) depending on the tumor size, configuration and number of metastases. The minimal dose inside the target volume was 15-20 Gy (mean 19).192Ir-source with 10 Ci was used for CTHDRBT. The mean irradiation time was 20 minutes (range 7-42). The mean coverage of the clinical target volume was 98% (range 88%-100%). The mean D100 was 18.1 Gy and the median D100 was 19.78 Gy. Each treatment was applied as a single fraction irradiation (Fig. 2).
Duration of hospitalization and complications
The median hospital stay was 4.0 days (range 4-8). Early minor complications were pain (n=3) and fever 24hours post irradiation (n=1). Three major complications occurred with post-interventional abscesses, 3 abscesses in 15 patients with BDA (20%) with an overall rate of 15%. Most likely these abscesses were due to ascending biliary infection. These three patients had undergone Whipple's or pylorus-preserving resection of the pancreas head with BDA as pre-surgery. However, periinterventional antibiotics were given, starting with 2× 500 mg ciprofloxacin per day (Ciprobay, Bayer-Schering, Germany) at intervention for 14 days. Altogether 15 patients had pre-interventional surgery including BDA. The mean time after intervention to abscess diagnosis was 5.1 months (range 3.3-6.2).
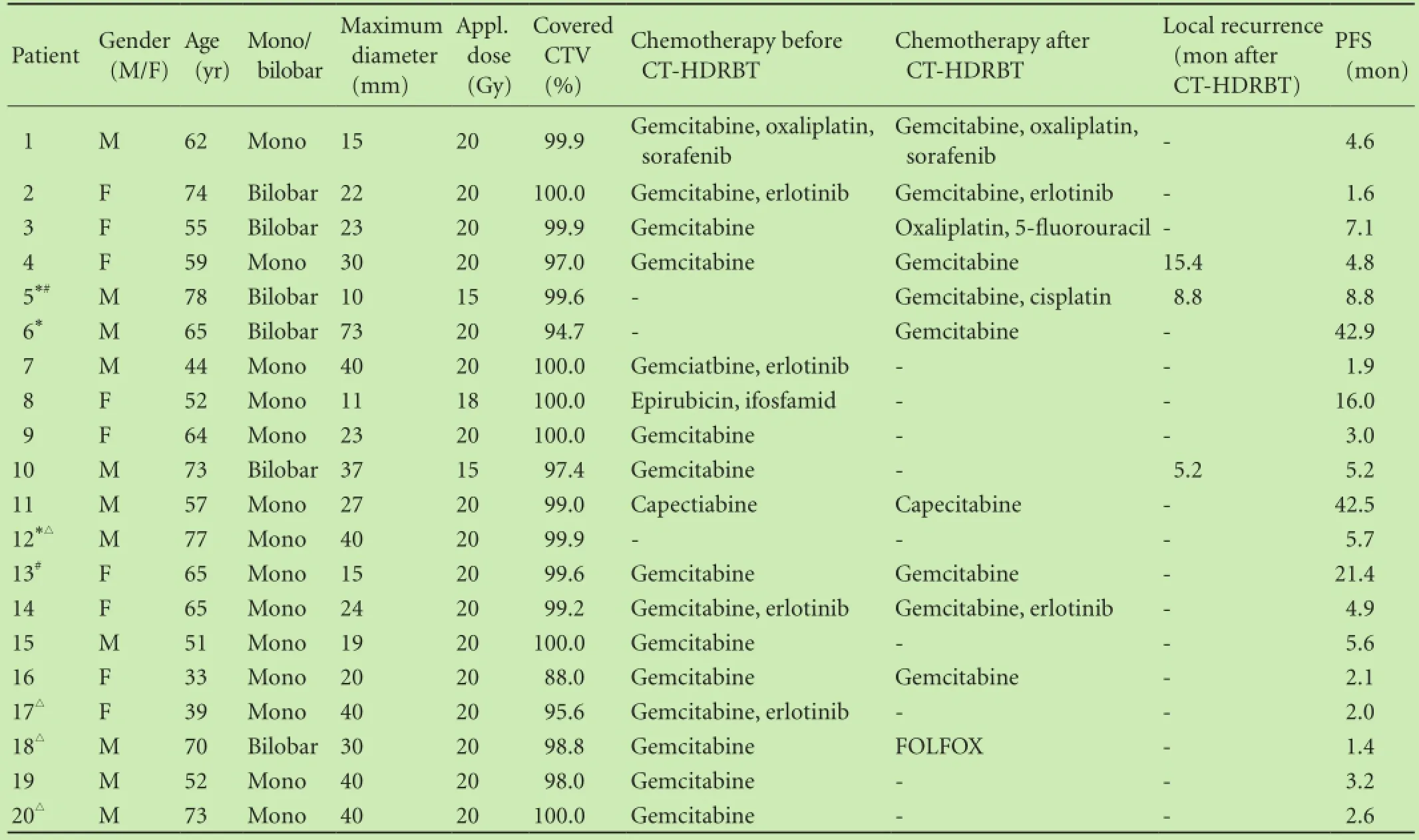
Table. Patients' characteristics
Tumor response
The details of tumor, duration of follow-up and evolution of the zone of ablation are shown in Table. The mean follow-up time was 13.7 months (range 1.4-55.0). The median PFS was 4.9 months (range 1.4-42.9, mean 9.4). Four patients demonstrated no progression (20%). In the first 6 months, eight patients (40%) showed intrahepatic progression, four patients (20%) extrahepatic progression, and four patients (20%) intra- and extrahepatic progression (Fig. 3).
Three patients had local recurrence (5 of 49 metastases). In patients with local recurrence, the local progression-free interval ranged from 5.2 to 15.4 (median 8.8 months). Five patients with intrahepatic progression were treated with CT-HDRBT ablation for a second time and one patient had three consecutive interventions.
The median OS after CT-HDRBT was 8.6 months (range 1.5-55.3). During follow-up, 15 patients (75%) died from disease progression. The OS after 6- and 12-month was 80% and 45%, respectively (Fig. 4).
Comparison of PFS and local tumor control of patients with and without chemotherapy
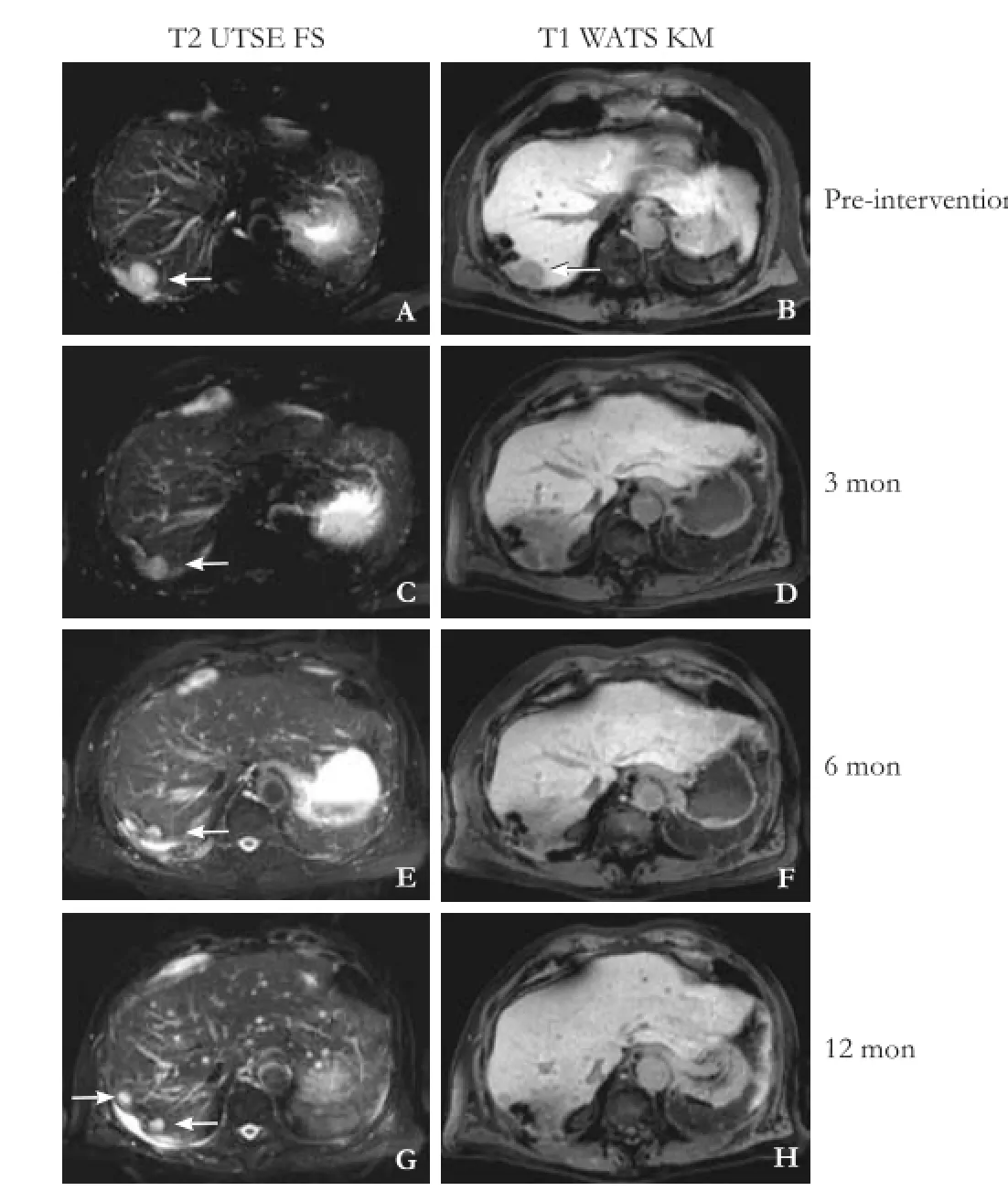
Fig. 2. The same patient as described in Fig. 1, who had liver metastasis from pancreatic cancer in liver segment 7, pre-interventional (A) and (B) and was detected in the follow-up after 3, 6 and 12 months (open arrow). Typical imaging in the follow-up with function loss of the surrounding hepatocytes, equivalent to the 10 Gy isodose (yellow line in Fig. 1) after 3 months (D) shown by reduced uptake of hepatocyte specific contrast agent. No local recurrence took place but diffuse progression in the liver after 12 months with new metastasis.
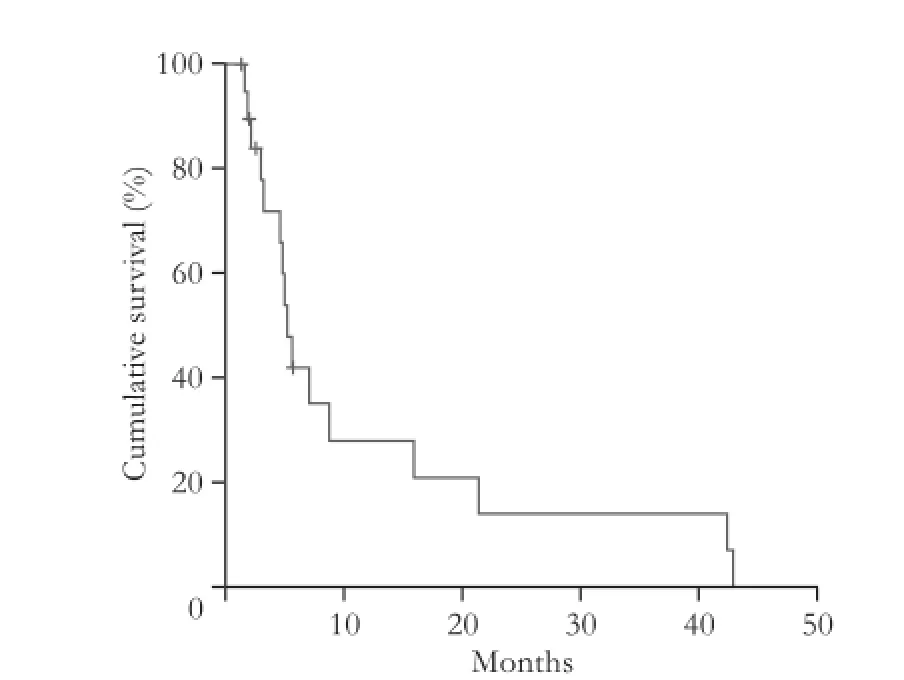
Fig. 3. The progression-free survival after 6 and 12 months was 50% and 40% respectively shown by the Kaplan-Meier method.
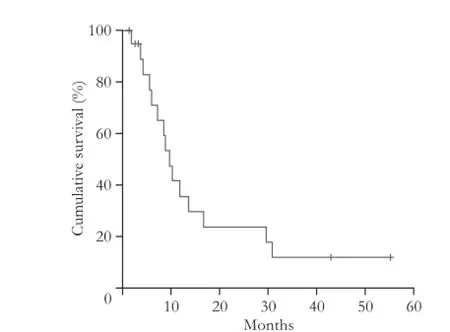
Fig. 4. The overall survival after 6 and 12 months was 80% and 45% respectively shown by the Kaplan-Meier method.
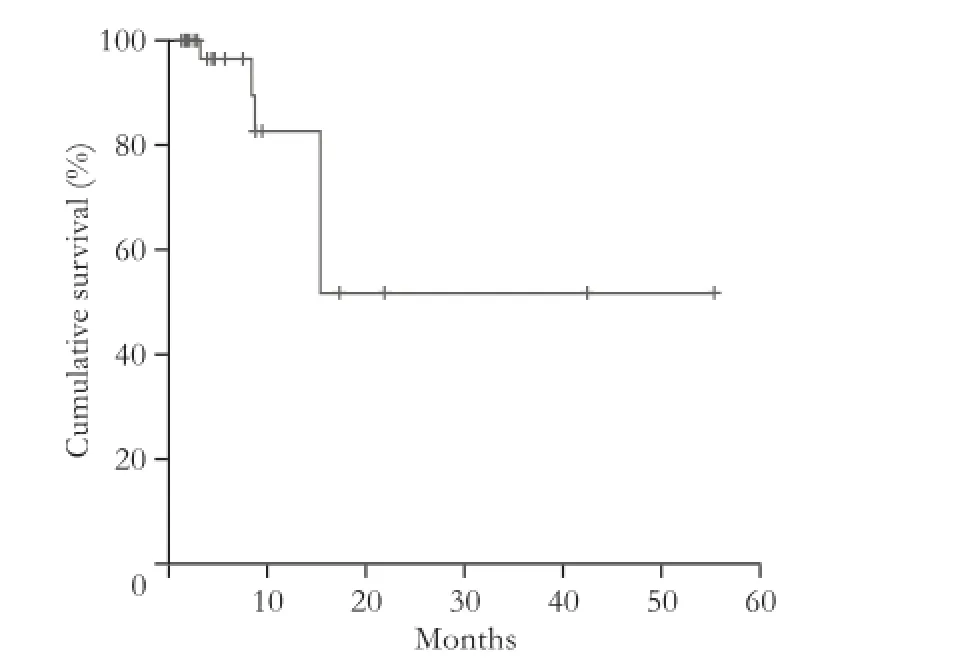
Fig. 5. Local tumor control shown by the Kaplan-Meier method. Five local recurrences (in three patients) occurred in 49 metastases treated with CT-HDRBT.
Discussion
Despite all efforts to improve survival of patients with advanced pancreatic cancer in a metastatic state, the OS remains generally poor. The low survival is due to aggressive metastasis in 65% of the patients with advanced local disease or distant metastases, with the liver being the most common site upon initial investigation.[31]
Therapy options vary from systematic or local chemotherapy to surgical resection of the metastases and radiological interventions. Surgical resection is the method of choice in hepatic metastases of colorectal cancer,[32]but only few studies have discussed the resection of liver metastases of non-colorectal cancer, i.e. pancreatic adenocarcinoma. Klempnauer et al[33]analyzed retrospectively the median survival time and the 1-year survival rate of seven patients after liver resection of liver me-tastases from pancreatic adenocarcinoma. The median survival of patients after metachronous liver resection was 5.8 months and the 1-year survival rate was 40%. In our study, the median survival time was 8.6 months and the 1-year survival rate was 45%. Despite the comparable survival time and rate, the median hospital stay of our patients was 4.0 days (range 4-8). Buell et al[34]investigated the liver resection of colorectal metastasis, primary hepatobiliary cancer and non-colorectal metastasis and found a hospital stay of 7.3±2.5 vs 9.5±5.9 vs 7.5±4.7 days, respectively. Even though they treated different primaries and metastases, hospital stay may be compared showing that CT-HDRBT is able to provide similar survival time and rate compared to surgery while being less stressful for the patient and resulting in a shorter hospital stay. Another advantage of CT-HDRBT compared with surgical resection is that it can be repeatedly applied, while sparing important functioning liver parenchyma through 3D-intervention planning.[35]One patient had three consecutive interventions and five patients had two consecutive interventions, but only one of them had a major complication with post-interventional abscess. This abscess was most likely caused by ascending biliary infection advantaged through BDA, a major risk factor for the development of liver abscesses, after Whipple's resection as pre-interventional surgery. To reduce the risk of abscess, peri-interventional antibiotics were given to all the patients with BDA. Further reduction of complications like abscesses might be achieved through metronomical antibiotics-schemes and are considered for future interventions.
Other studies[36,37]also examined a positive effect of minimal invasive treatments on the survival of patients with liver metastases from pancreatic cancer. Park et al[36]concluded that patients with liver metastases from pancreatic ductal adenocarcinoma with single, smallsized (<2 cm) liver metastasis gain a survival benefit from radiofrequency ablation. However, radiofrequency ablation has limitations compared to CT-HDRBT. These limitations include: maximal tumor diameter above 4 cm, major vessels adjacent to the lesion or strong tumor perfusion and location of the tumor or metastases to the central bile structures.[13,15,18]There are no known restrictions to the maximum size or cooling effects of tumor perfusion, and therefore CT-HDRBT can be applied close to the thermo-sensitive structures and the central bile structures.[12,13]CT-HDRBT can be even utilized for tumors unsuitable for stereotactical body radiation therapy, which has a tumor size limit of 6 cm and lesions>3.5 cm treated by stereotactical body radiation therapy appear to be a predictive factor for radiation-induced liver disease.[38]Thanks to the anatomy-based dose optimization, CT-HDRBT decreases the irradiation of healthy liver tissue and associated radiation-induced liver disease and the high hypofractioned doses ensure biologic dose escalation.
Since 5-fluorouracil was replaced by gemcitabine,[39]many trials tried to improve the impact of the cytosineanalogon,[40-43]which is currently the standard of chemotherapy in metastatic pancreatic carcinoma. In this study, a longer PFS for patients who received postinterventional chemotherapy with a median PFS of 4.9 months (mean 12.9) after ablation compared to a median PFS of 3.2 months (mean 5.0) recommends an adjuvant chemotherapy after CT-HDRBT. The local tumor control was not influenced in both groups with an local tumor control rate of 91% after 12 months. This finding shows that CT-HDRBT could be of valuable benefit for patients with liver metastases from pancreatic cancer in a multimodal treatment setting. Conroy et al[44]reported a significantly higher median OS of 11.1 months (FOLFORINOX) compared to 6.8 months (gemcitabine) and a significantly higher median PFS of 6.4 months in the FOLFIRINOX group vs 3.3 months in the gemcitabine group. Unfortunately, patients treated with FOLFIRINOX experienced a decrease in quality of life and more adverse events compared to patients who received gemcitabine, leading to the conclusion that FOLFIRINOX should be applied in patients with a more suitable or rather good performance status. Another regimen was evaluated by Von Hoff et al.[45]They compared gemcitabine+nab-paclitaxel vs gemcitabine alone and found a median OS of 8.5 months vs 6.7 months (P<0.001), respectively. The 1-year survival rate was 35% and the median PFS was 5.5 months in the combination group and the 1-year survival rate was 22% and the median PFS was 3.7 months in the monotherapy group (P<0.001). But again more adverse events occurred in the combination group. Nevertheless, both new regimens might be of value in combination with CT-HDRBT to prolong PFS and OS in future trials.
As liver metastasis correlates with poor prognosis, CT-HDRBT of local liver metastasis might prolong OS, though further investigation in a prospective design and in comparison to a control group needs to be done to provide solid data. The relative short PFS and OS is considered to be related to the low complication rate, thus CT-HDRBT offers an excellent local tumor control rate, justifying its use in a multimodal treatment setting, such as a useful combination with systemic chemotherapy. In this study, local recurrence occurred only in three patients. Their liver metastases were exposed to at least 15-20 Gy (only 1 lesion received 15 Gy), which is consistent with that reported elsewhere, showing comparablelocal control rates.[46,47]
Despite these promising results, this study has still limited validity because of its retrospective design and relative small patient population. Nevertheless, this study on the use of CT-HDRBT for liver metastatic lesions of pancreatic cancer proved that CT-guided brachytherapy is a safe and promising method to attain an excellent local control of liver metastasis.
Contributors: WG proposed the study. WG and SAC performed research, collected and analyzed the data and wrote the first draft. WG and SAC contributed equally to this article. All authors contributed to the design and interpretation of the study and to further drafts. WG is the guarantor.
Funding: None.
Ethical approval: This study was approved by local ethics committee.
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1 Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30.
2 Riall TS, Cameron JL, Lillemoe KD, Winter JM, Campbell KA, Hruban RH, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery 2006;140:764-772.
3 Schnelldorfer T, Ware AL, Sarr MG, Smyrk TC, Zhang L, Qin R, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg 2008;247:456-462.
4 de Jong MC, Tsai S, Cameron JL, Wolfgang CL, Hirose K, van Vledder MG, et al. Safety and efficacy of curative intent surgery for peri-ampullary liver metastasis. J Surg Oncol 2010;102:256-263.
5 Yamada H, Hirano S, Tanaka E, Shichinohe T, Kondo S. Surgical treatment of liver metastases from pancreatic cancer. HPB (Oxford) 2006;8:85-88.
6 Chen J, Tang Z, Dong X, Gao S, Fang H, Wu D, et al. Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol 2013;39:701-706.
7 Christophi C, Nikfarjam M, Malcontenti-Wilson C, Muralidharan V. Long-term survival of patients with unresectable colorectal liver metastases treated by percutaneous interstitial laser thermotherapy. World J Surg 2004;28:987-994.
8 Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the "heat sink" effect. AJR Am J Roentgenol 2002;178:47-51.
9 Rhim H. Percutaneous radiofrequency ablation therapy for patients with hepatocellular carcinoma during occlusion of hepatic blood flow: comparison with standard percutaneous radiofrequency ablation therapy. Cancer 2003;98:433-435.
10 Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol 2005;184:1860-1867.
11 De Jong MC, Farnell MB, Sclabas G, Cunningham SC, Cameron JL, Geschwind JF, et al. Liver-directed therapy for hepatic metastases in patients undergoing pancreaticoduodenectomy: a dual-center analysis. Ann Surg 2010;252:142-148.
12 Collettini F, Schnapauff D, Poellinger A, Denecke T, Schott E, Berg T, et al. Hepatocellular carcinoma: computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of large (5-7 cm) and very large (>7 cm) tumours. Eur Radiol 2012;22:1101-1109.
13 Collettini F, Singh A, Schnapauff D, Powerski MJ, Denecke T, Wust P, et al. Computed-tomography-guided high-dose-rate brachytherapy (CT-HDRBT) ablation of metastases adjacent to the liver hilum. Eur J Radiol 2013;82:e509-514.
14 Wieners G, Pech M, Rudzinska M, Lehmkuhl L, Wlodarczyk W, Miersch A, et al. CT-guided interstitial brachytherapy in the local treatment of extrahepatic, extrapulmonary secondary malignancies. Eur Radiol 2006;16:2586-2593.
15 Mohnike K, Wieners G, Schwartz F, Seidensticker M, Pech M, Ruehl R, et al. Computed tomography-guided high-dose-rate brachytherapy in hepatocellular carcinoma: safety, efficacy, and effect on survival. Int J Radiat Oncol Biol Phys 2010;78:172-179.
16 Collettini F, Schippers AC, Schnapauff D, Denecke T, Hamm B, Riess H, et al. Percutaneous ablation of lymph node metastases using CT-guided high-dose-rate brachytherapy. Br J Radiol 2013;86:20130088.
17 Collettini F, Schnapauff D, Poellinger A, Denecke T, Banzer J, Golenia MJ, et al. Percutaneous CT-guided high-dose brachytherapy (CT-HDRBT) ablation of primary and metastatic lung tumors in nonsurgical candidates. Rofo 2012;184:316-323.
18 Wieners G, Mohnike K, Peters N, Bischoff J, Kleine-Tebbe A, Seidensticker R, et al. Treatment of hepatic metastases of breast cancer with CT-guided interstitial brachytherapy - a phase II-study. Radiother Oncol 2011;100:314-319.
19 Ricke J, Wust P, Stohlmann A, Beck A, Cho CH, Pech M, et al. CT-guided interstitial brachytherapy of liver malignancies alone or in combination with thermal ablation: phase I-II results of a novel technique. Int J Radiat Oncol Biol Phys 2004;58:1496-1505.
20 Collettini F, Golenia M, Schnapauff D, Poellinger A, Denecke T, Wust P, et al. Percutaneous computed tomography-guided high-dose-rate brachytherapy ablation of breast cancer liver metastases: initial experience with 80 lesions. J Vasc Interv Radiol 2012;23:618-626.
21 Ricke J, Seidensticker M, Lüdemann L, Pech M, Wieners G, Hengst S, et al. In vivo assessment of the tolerance dose of small liver volumes after single-fraction HDR irradiation. Int J Radiat Oncol Biol Phys 2005;62:776-784.
22 Polgár C, Major T, Somogyi A, Takácsi-Nagy Z, Mangel LC, Forrai G, et al. CT-image-based conformal brachytherapy of breast cancer. The significance of semi-3-D and 3-D treatment planning. Strahlenther Onkol 2000;176:118-124.
23 Shwetha B, Ravikumar M, Supe SS, Sathiyan S, Lokesh V, Keshava SL. Dosimetric evaluation of two treatment planning systems for high dose rate brachytherapy applications. Med Dosim 2012;37:71-75.
24 Lliso F, Pérez-Calatayud J, Carmona V, Ballester F, Puchades V, Granero D. Technical note: fitted dosimetric parameters of high dose-rate192Ir sources according to the AAPM TG43 formalism. Med Phys 2003;30:651-654.
25 Lliso F, Pérez-Calatayud J, Carmona V, Ballester F, Lluch JL, Serrano MA, et al. Fitted dosimetric parameters of high doserate192Ir sources according to the AAPM TG43 formalism. Med Phys 2001;28:654-660.
26 Nath R, Anderson LL, Luxton G, Weaver KA, Williamson JF, Meigooni AS. Dosimetry of interstitial brachytherapy sources: recommendations of the AAPM Radiation Therapy Committee Task Group No. 43. American Association of Physicists in Medicine. Med Phys 1995;22:209-234.
27 Lagarias JC, Reeds JA, Wright MH, Wright PE. Convergence properties of the Nelder-Mead simplex method in low dimensions. Siam J Optim 1999;9:112-147.
28 Olsson DM, Nelson LS. The Nelder-Mead Simplex procedure for function minimization. Technometrics 1975;17:45-51.
29 Streitparth F, Pech M, Böhmig M, Ruehl R, Peters N, Wieners G, et al. In vivo assessment of the gastric mucosal tolerance dose after single fraction, small volume irradiation of liver malignancies by computed tomography-guided, high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2006;65:1479-1486.
30 Ricke J, Wust P, Stohlmann A, Beck A, Cho CH, Pech M, et al. CT-Guided brachytherapy. A novel percutaneous technique for interstitial ablation of liver metastases. Strahlenther Onkol 2004;180:274-280.
31 Danet IM, Semelka RC, Nagase LL, Woosely JT, Leonardou P, Armao D. Liver metastases from pancreatic adenocarcinoma: MR imaging characteristics. J Magn Reson Imaging 2003;18: 181-188.
32 Nguyen KT, Laurent A, Dagher I, Geller DA, Steel J, Thomas MT, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg 2009;250:842-848.
33 Klempnauer J, Ridder GJ, Piso P, Pichlmayr R. Is liver resection in metastases of exocrine pancreatic carcinoma justified? Chirurg 1996;67:366-370.
34 Buell JF, Rosen S, Yoshida A, Labow D, Limsrichamrern S, Cronin DC, et al. Hepatic resection: effective treatment for primary and secondary tumors. Surgery 2000;128:686-693.
35 Seidensticker M, Wust P, Rühl R, Mohnike K, Pech M, Wieners G, et al. Safety margin in irradiation of colorectal liver metastases: assessment of the control dose of micrometastases. Radiat Oncol 2010;5:24.
36 Park JB, Kim YH, Kim J, Chang HM, Kim TW, Kim SC, et al. Radiofrequency ablation of liver metastasis in patients with locally controlled pancreatic ductal adenocarcinoma. J Vasc Interv Radiol 2012;23:635-641.
37 Azizi A, Naguib NN, Mbalisike E, Farshid P, Emami AH, Vogl TJ. Liver metastases of pancreatic cancer: role of repetitive transarterial chemoembolization (TACE) on tumor response and survival. Pancreas 2011;40:1271-1275.
38 Janoray G, Chapet S, Ruffier-Loubière A, Bernadou G, Pointreau Y, Calais G. Robotic stereotactic body radiation therapy for tumors of the liver: radiation-induced liver disease, incidence and predictive factors. Cancer Radiother 2014;18: 191-197.
39 Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-2413.
40 Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-5518.
41 Fortune BE, Li X, Kosuri KV, Weatherby LM, Thomas JP, Bekaii-Saab TS. Fixed-dose-rate gemcitabine in combination with oxaliplatin in patients with metastatic pancreatic cancer refractory to standard-dose-rate gemcitabine: a single-institute study. Oncology 2009;76:333-337.
42 Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-3622.
43 Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-1966.
44 Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-1825.
45 Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-1703.
46 Sharma DN, Thulkar S, Sharma S, Gandhi AK, Haresh KP, Gupta S, et al. High-dose-rate interstitial brachytherapy for liver metastases: first study from India. J Contemp Brachytherapy 2013;5:70-75.
47 Tselis N, Chatzikonstantinou G, Kolotas C, Milickovic N, Baltas D, Zamboglou N. Computed tomography-guided interstitial high dose rate brachytherapy for centrally located liver tumours: a single institution study. Eur Radiol 2013;23:2264-2270.
Accepted after revision January 6, 2015
10.1016/S1499-3872(15)60409-X
Author Affiliations: Department of Diagnostic and Interventional Radiology (Wieners G, Schippers AC, Collettini F, Schnapauff D, Hamm B and Gebauer B), Department of Radiation Oncology (Wust P) and Department of Oncology (Riess H), Charité-Universitätsmedizin Berlin, Campus Virchow-Klinikum, Augustenburger Platz 1, 13353 Berlin, Germany
Gero Wieners, MD, Department of Diagnostic and Interventional Radiology, Charité-Universitätsmedizin Berlin, Campus Virchow-Klinikum, Augustenburger Platz 1, 13353 Berlin, Germany (Tel: +49-30-450-557012; Fax: +49-30-450-557901; Email: gero.wieners@ charite.de)
© 2015, Hepatobiliary Pancreat Dis Int. All rights reserved.
Published online July 21, 2015.
Eleven patients chemotherapy after ablation (group A). The median PFS was 4.9 months (range 1.4-42.9, mean 12.9). Two patients demonstrated no progression with a follow-up of 2-4 months. Nine patients did not receive chemotherapy after intervention (group B). The PFS of these patients ranged from range 1.9 to 16.0 months (median 3.2; mean 5.0). Two patients demonstrated no progression after follow-up for 4 and 6 months, respectively. In group A, two local recurrenceswere found after 8.8 and 15.4 months respectively, and the rate of local tumor control was 91% after 12 months and 82% after 18 months. In group B, the rate of local tumor control was also 91% after 12 months and 18 months because of a local recurrence after 5.2 months. An adjuvant chemotherapy had no significant effect on local tumor control (Fig. 5).
August 26, 2014
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Extrahepatic right portal vein ligation allows parenchyma-sparing en bloc resection of segments 7, 8 and 4a for liver tumors engaging the right and middle hepatic veins
- Effects of epidermal growth factor receptor inhibitor on proliferative cholangitis in hepatolithiasis
- 18F-FDG PET/CT in differentiating malignant from benign origins of obstructive jaundice
- Diagnostic and prognostic roles of soluble CD22 in patients with Gram-negative bacterial sepsis
- Congenital extrahepatic portosystemic shunt complicated by the development of hepatocellular carcinoma
- Multidisciplinary management of Mirizzi syndrome with choIecystobiIiary fistuIa: the vaIue of minimaIIy invasive endoscopic surgery
