植物激素调控籽粒大小的研究进展
2015-11-02董庆坤刘慧丽
董庆坤, 刘慧丽
(华南农业大学生命科学学院,广州 510642)
植物激素调控籽粒大小的研究进展
董庆坤, 刘慧丽*
(华南农业大学生命科学学院,广州 510642)
植物种子的大小和品质是影响农作物产量的主要因素之一,研究控制种子籽粒大小发育的相关因素,对提高农作物产量具有重要意义.近年通过分析种子发育缺陷突变体或QTL等分子遗传学的方法,发现许多控制种子发育的重要基因影响着种子的大小和产量.对模式植物拟南芥和水稻的研究发现,许多调控种子发育的功能基因通过整合到植物激素的代谢或信号转导途径起作用,说明植物激素在调控籽粒发育中发挥重要作用,但有关作用的分子机理及其遗传调控网络待阐明.该文以模式植物拟南芥和水稻种子发育研究为例,着重介绍植物激素调控种子籽粒大小调控的研究进展.
水稻; 种子发育; 种子大小; 植物激素
近年来粮食产量进入了一个停滞发展期.在粮食作物中,种子发育是生命周期的重要一环.颗粒大的种子能为早期发育提供更多的营养物质,并且提高种子对环境的抗逆性以及作物产量.因而,研究调控种子大小发育的分子机理,发掘调控籽粒大小的新基因不仅对种子发育的理论研究有重要意义,也为农作物育种提供基因资源.
1 籽粒大小发育规律
高等植物的种子由胚胎、胚乳和种皮3部分组成,共同协调发育,最终决定种子的大小.种子的发育起始于双受精过程[1],成熟双子叶植物的种子只有一层胚乳细胞,营养主要储存在子叶中[2].由于受精后胚囊和胚乳的发育比胚发育快,珠被和胚乳细胞分裂比胚胎细胞的分裂启动早,在胚胎还没有膨大前,珠被细胞就已快速分裂形成膨大的胚囊腔,同时胚乳细胞也快速分裂和分化,随后胚的体积才迅速增大并逐渐吸收胚乳长到完整大小[2].拟南芥在种子发育早期,种皮和胚乳的发育为胚的生长铺路,胚的体积增大前种子已经达到最终的大小,种子的大小是由胚乳、胚珠和胚协同调控的[3](图1).
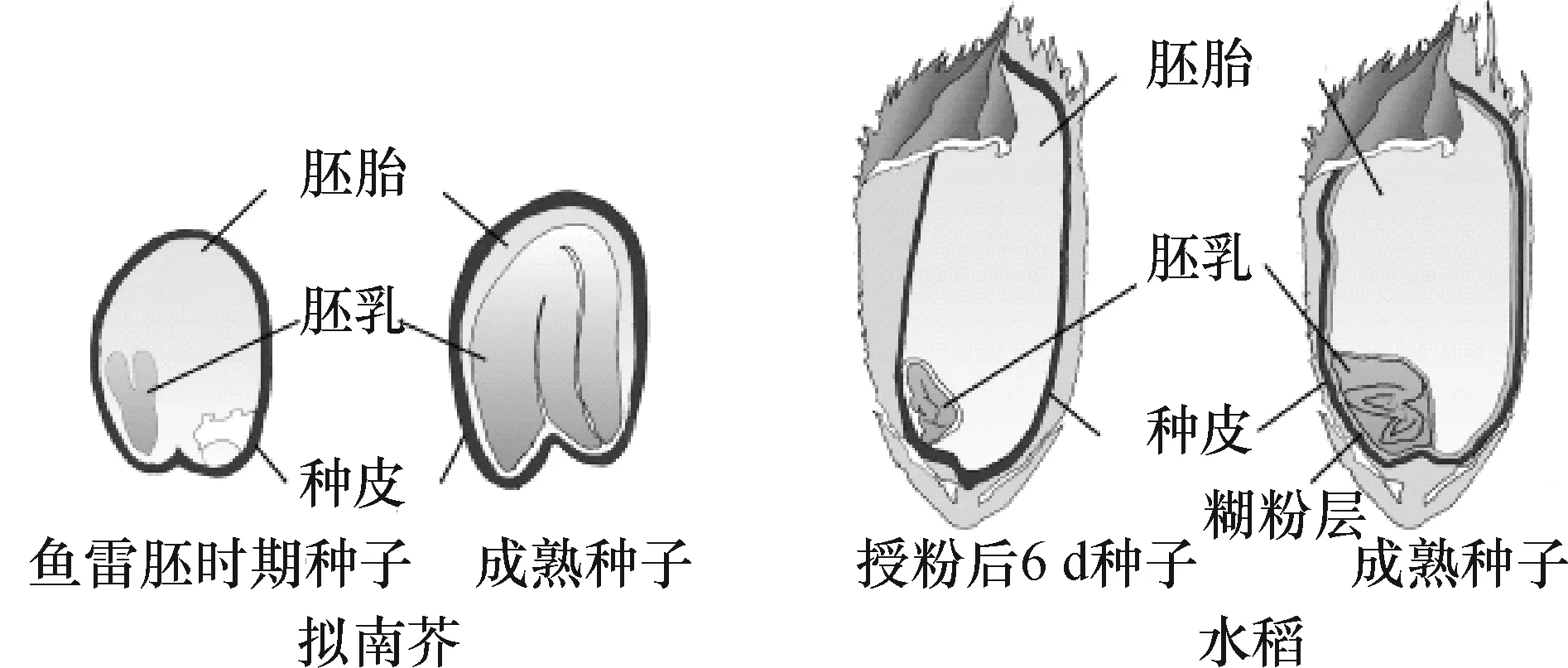
图1 拟南芥与水稻种子的发育[4]
在单子叶植物水稻中,种子的营养主要储存在胚乳中,籽粒的大小很大程度上取决于胚乳的发育状况,并影响水稻的产量和品质.同拟南芥一样,水稻胚乳的发育先于胚的发育,受精后几小时内胚乳核就开始分裂,标志胚乳发生的开始,在胚囊腔周围形成游离的胚乳核,随后游离胚乳核开始进入细胞化时期,胚乳细胞的增殖是由位于胚囊周边的细胞分裂引起的[5].授粉后10 d左右胚乳细胞快速分裂结束,胚乳细胞总数就此决定,细胞分化开始,表面一至数层细胞分化为糊粉层,内部细胞开始形成质体积累淀粉等营养物质,构成胚乳的主体部分,这部分细胞后来经历从中央向周边的程序化细胞死亡过程.种子的大小是由胚胎、胚乳、珠被三者共同决定的, 影响到这3个发育过程的因素,都会最终影响籽粒的大小.
近年来通过遗传学及分子生物学的方法,发现很多内在因素调控种子的发育.如由表观遗传途径HAIKUMINI3调控拟南芥胚乳的发育[6-7],一些重要的转录因子如WRKYTRANSPARENT TESTAGLABRA2( TTG2)/EOD3/CYP78A6[8-10]和AP2[9,11-12]与珠被发育相关;泛素途径相关的作用蛋白影响种子的发育,如由GW2编码的RING蛋白,具有E3泛素链接酶活性,通过抑制细胞分裂负调控种子大小[13],DA1、DA2所在的泛素途径也对种子大小有重要影响[14-15];作物母体组织对谷物颖果大小也有重要影响,其分子和代谢机制仍不明确[16]. 本实验室也开展了植物激素信号转导、基因表达与调控、植物生长发育的分子机理的研究,重点关注生长素与细胞分裂素的相互作用以及在调控胚胎和种子发育中的功能与分子机制,并在一些植物激素信号转导调控网络中有新发现,如拟南芥RAC/ROP小G蛋白的活化因子RopGEF7参与生长素介导的胚胎发育过程[17],并进一步证明受生长素诱导的小G蛋白ROP3通过特异性控制生长素输出载体PIN蛋白在细胞膜的极性定位影响胚胎发育[18],说明植物激素在胚胎跟种子发育过程中具有重要的调控作用.
2 影响籽粒发育的植物激素
植物激素是影响种子生长发育的重要因素之一[19],已经鉴定到很多与植物激素相关的基因(表1)在调控植物籽粒发育的过程中发挥关键作用.
近年发现细胞分裂素、油菜素内酯对调控种子大小起到了至关重要的作用.而生长素、脱落酸、赤霉素在一定程度上对种子发育也有调控作用.不同植物激素信号途径之间的相互作用,例如油菜素内酯与G蛋白,对种子大小起到了必要的作用[20-21].
2.1细胞分裂素对籽粒发育的调控
细胞分裂素对调控拟南芥种子籽粒大小和质量起到关键的作用.在拟南芥大孢子细胞起始发生时期,细胞分裂素受体AHK2、AHK3及CRE1/AHK4的三突变体ahk2-2ahk3-3cre1-12珠被起始发育严重缺陷,珠被内层的细胞形态不正常,最终形成指状的不正常胚珠结构[22].细胞周期marker基因cyclinB:GUS在该三突变体的胚珠及珠被组织中表达增强,暗示突变体珠被的异常是由于细胞分裂增强所致,说明细胞分裂素负调控来自母体胚珠组织的细胞分裂[22],与已报道细胞分裂素缺陷突变体能够形成较大种子的结果吻合,说明细胞分裂素途径在种子受精前的早期发育时期负调控胚珠组织细胞分裂,进而影响种子的最终大小.细胞分裂素合成途径或信号途径的基因突变都导致种子增大,胚细胞数量增加,细胞增大、胚的增大主要是源于胚乳发育的改变所致[23].当过表达2个细胞分裂素氧化酶(CKX,促进细胞分裂素降解)基因AtCKX1和AtCKX3,种子结实率下降,但是种子增大,种子质量是野生型的2倍[23].细胞分裂素信号转导途径的基因单突变或双突变的表型不明显,但多突变后的籽粒增大,如细胞分裂素受体的三突变体ahk2ahk3cre1 种子显著增大,是野生型的2倍以上[23-24];同样,磷酸转移蛋白的ahp2,3,5三突变体和五突变体ahp1ahp2ahp3ahp4ahp5[25]的种子体积也显著增大;细胞分裂素正调控因子B-型RR基因的三突变体rr1arr10arr12也产生大的种子[26].最近发现,调控种子胚乳发育的HAIKU(IKU)途径和表观遗传调控途径同时通过靶向调控CKX2的表达,协同控制种子的发育[27],一方面,IKU途径的转录因子WRKY10/MINI3能直接结合到CKX2的启动子上激活其表达,影响细胞分裂素在胚乳中的分布模式,从而影响种子大小;另一方面,CKX2的表达受多梳蛋白复合物PRC2的甲基化抑制.因此CKX2控制的胚乳生长就成为整合IKU途径和表观遗传调控途径的共同节点.研究还发现细胞分裂素在胚乳发育过程中呈特定的分布模式,在IKU途径的突变体中,这种分布模式发生改变.这些结果说明细胞分裂素对调控胚乳发育,最终影响种子大小起到重要作用.
2003年有报道指出,在水稻胚乳发育早期,用细胞分裂素处理根或喷施地上部分能增加胚乳细胞数,促进灌浆,使粒质量增加[28].胚乳内的细胞分裂素水平与胚乳细胞分裂正相关,但其分子调控机制仍不清楚.近年来的研究表明,水稻中影响细胞分裂素的生物合成会显著影响穗粒数.水稻中一个主要控制穗粒数的QTL-GRAINNUMBER1 (Gn1a),编码细胞分裂素氧化酶OsCKX2,Gn1a突变体的籽粒数显著增加[29].在水稻生殖期,OsCKX2在花序分生组织的表达量降低,促进细胞分裂素的积累,进而促进生殖器官的发育,增加产量而不影响水稻生长,相反,水稻细胞分裂素合成缺陷突变体lonelyguy(log) 则导致穗子变小[30-31].锌指转录因子DROUGHT AND SALT TOLERANCE (DST) 正调控Gn1a/OsCKX2的表达,DSTreg1突变体的花序中细胞分裂素水平升高,植株的穗粒数明显增加,千粒质量也有所增加,而过表达野生型DST的转基因植株穗粒数减少[31].另一个编码F-box蛋白的LARGEPANICLE(LP) 突变体中OsCKX2的表达量降低,籽粒数和籽粒大小都相应地增加[32].由于DST、LP和OsCKX2都在花中表达,推测它们很可能作用于母体的胚乳组织调控种子发育[33].最近的报道发现水稻MADS29过表达植株中细胞分裂素的含量升高.而MADS29下调表达影响质体的分化和胚乳淀粉粒的形态,由于细胞分裂素能促进质体的分化,MADS29可能影响了细胞分裂素的动态平衡从而调控了胚乳的发育[34].OsMADS29的表达受生长素诱导,生长素可能在调控早期水稻种子发育尤其是母体组织的降解过程中起重要作用[35],暗示MADS29可能通过影响生长素和细胞分裂素在细胞内的动态平衡而调控了胚乳的发育[34].水稻中一个CYTOKININRESPONSIVEGATATRANSCRIPTIONFACTOR1 (Cga1)的表达受细胞分裂素诱导,该基因调控叶绿体的发育并影响胚乳内淀粉合成及籽粒灌浆,Cga1过表达植株的籽粒变小,粒质量下降,籽粒形状也不规则,下调表达不影响粒质量,但籽粒变得细长[36].这些研究结果也说明细胞分裂素能调控籽粒的大小,并影响产量.
2.2生长素对籽粒发育的调控
生长素也在种子发育中发挥着关键作用,授粉后子房中生长素的含量迅速增加,暗示生长素可能在受精后促进子房的发育[28,37].用2,4-D处理体外培养的水稻子房,会发生单性结实现象,形成不含胚乳和胚的膨大的子房,说明生长素能诱导类似于种子受精后的胚囊发育过程[38],这些结果表明生长素在启动种子的早期发育过程中具有重要的作用.拟南芥mnt突变体种子体积和质量都比野生型显著增大,MNT编码生长素响应因子ARF2,是生长素信号途径的重要转录因子,其表达受到生长素的调控.mnt突变体中由于珠被细胞额外的分裂导致胚囊腔显著增大,最终形成较大的种子.mnt/arf2突变体的表型也说明生长素信号途径通过调控珠被细胞分裂调控种子的发育,并对决定种子的最终大小具有重要作用[39].最近报道指出MADS29在种子受精或用生长素处理后表达量增加,说明生长素信号途径在早期胚乳的发育过程中具有重要的作用.水稻MADS29是MADS box基因家族成员,主要在水稻珠心和珠心突起处表达,调控珠心组织的PCD降解过程,影响受精后胚乳发育过程,是水稻种子发育早期的一个重要调节子[35].OsMADS29下调表达导致珠心组织PCD降解过程受抑制,影响了胚囊及随后的胚乳发育过程,如胚乳淀粉积累不足造成种子干瘪等.OsMADS29的表达受生长素诱导,说明生长素可能在调控早期水稻种子发育尤其是母体组织的降解过程中起重要作用.
2.3油菜素内酯BRs对籽粒发育的调控
油菜素内酯BRs能够促进籽粒的发育.拟南芥中BR生物合成缺陷突变体det2和BR不敏感突变体bri1-5的种子均比野生型短小[40-42].det2的胚细胞数目减少,珠被细胞长度比野生型短,而外施BR能恢复det2表型,并能提高野生型种子的质量,说明BRs正调控籽粒的大小发育过程[42].进一步研究发现BR信号途径的转录因子BZR1能直接结合到多个调控种子大小的基因启动子上,促进种子发育.BZR1能够激活对胚乳和胚发育起促进作用的SHB1-MINI3-IKU2途径,同时也抑制对珠被发育起负调控作用的转录因子AP2和ARF2的表达[42].水稻中已报道的BR生物合成途径的突变体如d1、d2、d11、brd1和brd2,或影响BR信号途径的突变体如Osbri1、d61(d61也是水稻OsBRI1功能缺失的突变体)均导致水稻籽粒变小,籽粒形状变得短圆[43-46].相反水稻中过表达BR生物合成基因AtDWF4/ZmCYP则促进同化物积累,促进灌浆,增加籽粒大小,显著提高产量[47];过表达BR信号途径的正调控因子BRASSINOSTEROID UPREGULATED1使得水稻籽粒增大[48].这些说明BRs在水稻的籽粒发育中起重要的调控作用,但有关BRs调控种子大小的分子机制不清楚,一般认为BRs可能影响细胞分裂、伸长或分化,或者也可能是影响种子灌浆[49].
2.4脱落酸对籽粒发育的调控
脱落酸调控种子发育的许多过程,例如:胚胎营养物质的合成、种子休眠、抑制种子萌发等.在种子发育过程中,脱落酸的积累存在2个峰值,在种子成熟早期,脱落酸主要来源于成熟组织并达到第一个表达峰值[50].随后,脱落酸开始在胚胎合成,其表达水平在种子成熟后期达到第二个表达峰值.早期研究发现,在种子发育过程中,有许多涉及脱落酸生物合成的相关基因表达[51],说明脱落酸ABA对调控种子大小可能也有重要作用.最近的报道表明拟南芥中ABA生物合成途径的基因如ABA2、ABA1及NCED6及ABA信号途径的转录因子ABI5突变后种子均比野生型大[52],aba2-1突变体种子发育早期胚乳细胞化过程延迟,其种子增大的表型可以被外源ABA抑制.ABA可能通过负调控胚乳细胞分裂,促进早期胚乳的细胞化过程对种子的大小起负调控作用.拟南芥中DA1所在的泛素途径对决定种子大小有重要作用,da1-1突变体的种子体积和质量均显著增加[15].DA1编码一个泛素受体蛋白,其表达受ABA诱导,da1突变体的器官生长对ABA的敏感性降低,说明ABA对于决定器官及种子的最终大小起重要作用.拟南芥中DA2编码泛素途径的E3泛素连接酶,da2突变体的种子也增大,DA2与DA1互作,协同调控珠被细胞的增殖,影响种子的大小[14].DA2在水稻中的同源基因GW2突变后能增加颖壳宽度,加速籽粒灌浆,导致种子宽度增加,提高产量[13].水稻中GW2是否与ABA信号途径相关尚不清楚.转录组学分析表明,水稻中28个与种子发育相关的基因受ABA诱导而在胚乳中大量表达,说明ABA在种子胚乳发育期间也起到重要作用[53].
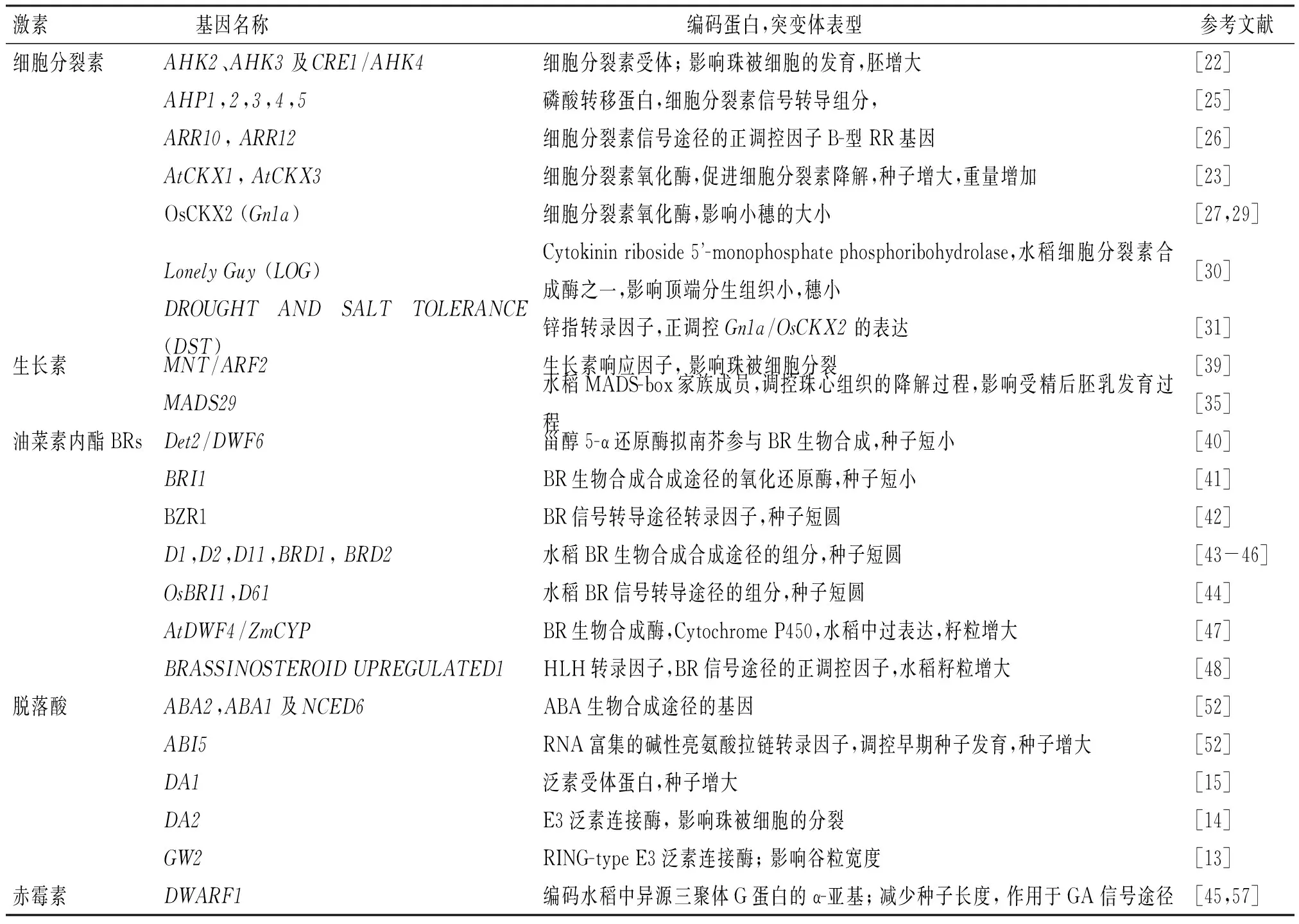
表1 与植物激素相关的调控种子大小的基因
2.5赤霉素对籽粒发育的调控
已有的研究表明,赤霉素对植物种子萌发、打破种子休眠以及植物的花和种子的发育具有重要作用.赤霉素通过形成GA-GID1-DELLA三聚体,经过SCF (SKP1-CUL1-F-box)聚合体标记,诱导泛素26S蛋白酶体途径,降解DELLA蛋白,从而产生赤霉素效应[54-56].赤霉素可能也调控种子大小.BRs途径的突变体dwarf1种子变小,dwarf1最初被鉴定为GA信号途径突变体,表现出GA缺乏的表型.DWARF1编码水稻中异源三聚体G蛋白的α-亚基,G蛋白被认为在GA信号途径中传递信号,负责调控水稻节间和种子的正常发育,因此GA信号可能也影响种子籽粒的大小[45,57].
3 展望
植物激素在植物整个生命周期中都发挥重要的作用,近几年发现许多调控种子发育的基因,通过植物激素途径起作用.这些功能基因通过调控植物激素的生物合成,运输或信号转导广泛参与调控胚、胚乳、种皮的发育过程,影响作物种子的大小和产量.大量的研究有助于了解这些基因在控制种子大小方面的潜在机理.但是目前,这些功能基所介导的植物激素调控种子发育的分子机理方面的研究还有待深入,例如有些功能基因在激素信号途径调控网络的具体位置尚不清楚,它们的上下游是什么?是否存在特殊的配体或调节因子,他们在种子发育过程中存在哪些相互作用?这些功能基因在不同物种间是否具有同效性?不同的激素调控网络之间存在哪些交叉?植物种子大小是一个复杂的农艺性状,尽管现在已经完成了大多数重要经济作物的基因组测序,但在细胞学和分子水平对调控种子大小的机理还有待更深入的研究.
[1]Kesavan M, Song J T, Seo H S. Seed size: A priority trait in cereal crops[J]. Physiologia Plantarum, 2013, 147(2): 113-120.
[2]Sun X, Shantharaj D, Kang X, et al. Transcriptional and hormonal signaling control of Arabidopsis seed development[J]. Current Opinion in Plant Biology, 2010, 13(5): 611-620.
[3]Sundaresan V. Control of seed size in plants[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(50): 17887-17888.
[4]Zhou S R, Yin L L, Xue H W. Functional genomics based understanding of rice endosperm development[J]. Current Opinion in Plant Biology, 2013, 16(2): 236-246.
[5]Zhou S R, Yin L L, Xue H W. Functional genomics based understanding of rice endosperm development[J]. Current Opinion in Plant Biology, 2013, 16(2): 236-246.
[6]Wang A, Garcia D, Zhang H, et al. The VQ motif protein IKU1 regulates endosperm growth and seed size in Arabidopsis[J]. The Plant Journal, 2010, 63(4): 670-679.
[7]Luo M, Dennis E S, Berger F, et al. MINISEED3 (MINI3), a WRKY family gene, and HAIKU2 (IKU2), a leucine-rich repeat (LRR) KINASE gene, are regulators of seed size in Arabidopsis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(48): 17531-17536.
[8]Fang W, Wang Z, Cui R, et al. Maternal control of seed size by EOD3/CYP78A6 in Arabidopsis thaliana[J]. The Plant Journal, 2012, 70(6): 929-939.
[9]Ohto M A, Floyd S K, Fischer R L, et al. Effects of APETALA2 on embryo, endosperm, and seed coat development determine seed size in Arabidopsis[J]. Sexual Plant Reproduction, 2009, 22(4): 277-289.
[10]Garcia D, Fitz G J, Berger F. Maternal control of integument cell elongation and zygotic control of endosperm growth are coordinated to determine seed size in Arabidopsis[J]. The Plant Cell, 2005, 17(1): 52-60.
[11]Jofuku K D, Omidyar P K, Gee Z, et al. Control of seed mass and seed yield by the floral homeotic gene APETALA2[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(8): 3117-3122.
[12]Ohto M A, Fischer R L, Goldberg R B, et al. Control of seed mass by APETALA2[J]. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(8): 3123-3128.
[13]Song X J, Huang W, Shi M, et al. A QTL for rice grain width and weight encodes a previously unknown RING-type E3 ubiquitin ligase[J]. Nature Genetics, 2007, 39(5): 623-630.
[14]Xia T, Li N, Dumenil J, et al. The ubiquitin receptor DA1 interacts with the E3 ubiquitin ligase DA2 to regulate seed and organ size in Arabidopsis[J]. The Plant Cell, 2013, 25(9): 3347-3359.
[15]Li Y, Zheng L, Corke F, et al. Control of final seed and organ size by the DA1 gene family in Arabidopsis thaliana[J]. Genes & Development, 2008, 22(10): 1331-1336.
[16]Huang R, Jiang L, Zheng J, et al. Genetic bases of rice grain shape: So many genes, so little known[J]. Trends in Plant Science, 2013, 18(4): 218-226.
[17]Chen M, Liu H, Kong J, et al. RopGEF7 regulates PLETHORA-dependent maintenance of the root stem cell niche in Arabidopsis[J]. The Plant Cell, 2011, 23(8): 2880-2894.
[18]Huang J B, Liu H, Chen M, et al. ROP3 GTPase contributes to polar auxin transport and auxin responses and is important for embryogenesis and seedling growth in Arabidopsis[J]. The Plant Cell, 2014, 26(9): 3501-3518.
[19]Kesavan M, Song J T, Seo H S. Seed size: A priority trait in cereal crops[J]. Physiologia Plantarum, 2013, 147(2): 113-120.
[20]Ashikari M, Wu J, Yano M, et al. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the alpha-subunit of GTP-binding protein[J]. Proceeding of the National Academy of Sciences of the United States of America, 1999, 96(18): 10284-10289.
[21]Fujisawa Y, Kato T, Ohki S, et al. Suppression of the heterotrimeric G protein causes abnormal morphology, including dwarfism, in rice[J]. Proceeding of the National Academy of Sciences of the United States of America, 1999, 96(13): 7575-7580.
[22]Cheng C Y, Kieber J J. The role of cytokinin in ovule development in Arabidopsis[J]. Plant Signaling & Behavior, 2013, 8(3): e23393.
[23]Werner T, Motyka V, Laucou V, et al. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity[J]. The Plant Cell, 2003, 15(11): 2532-2550.
[24]Riefler M, Novak O, Strnad M, et al. Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism[J]. The Plant Cell, 2006, 18(1): 40-54.
[25]Hutchison C E, Li J, Argueso C, et al. The Arabidopsis histidine phosphotransfer proteins are redundant positive regulators of cytokinin signaling[J]. The Plant Cell, 2006, 18(11): 3073-3087.
[26]Argyros R D, Mathews D E, Chiang Y H, et al. Type B response regulators of Arabidopsis play key roles in cytokinin signaling and plant development[J]. The Plant Cell, 2008, 20(8): 2102-2116.
[27]Li J, Nie X, Tan J L, et al. Integration of epigenetic and genetic controls of seed size by cytokinin in Arabidopsis[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(38): 15479-15484.
[28]Yang J, Zhang J, Wang Z, et al. Hormones in the grains in relation to sink strength and postanthesis development of spikelets in rice[J]. Plant Growth Regulation, 2003, 41(3): 185-195.
[29]Ashikari M, Sakakibara H, Lin S, et al. Cytokinin oxidase regulates rice grain production[J]. Science, 2005, 309(5735): 741-745.
[30]Kurakawa T, Ueda N, Maekawa M, et al. Direct control of shoot meristem activity by a cytokinin-activating enzyme[J]. Nature, 2007, 445(7128): 652-655.
[31]Li S, Zhao B, Yuan D, et al. Rice zinc finger protein DST enhances grain production through controlling Gn1a/OsCKX2 expression[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(8): 3167-3172.
[32]Li M, Tang D, Wang K, et al. Mutations in the F-box gene LARGER PANICLE improve the panicle architecture and enhance the grain yield in rice[J]. Plant Biotechnology Journal, 2011, 9(9): 1002-1013.
[33]Li N, Li Y. Ubiquitin-mediated control of seed size in plants[J]. Frontiers in Plant Science, 2014, 5: 332.
[34]Nayar S, Sharma R, Tyagi A K, et al. Functional delineation of rice MADS29 reveals its role in embryo and endosperm development by affecting hormone homeostasis[J]. Journal of Experimental Botany, 2013, 64(14): 4239-4253.
[35]Yin L L, Xue H W. The MADS29 transcription factor regulates the degradation of the nucellus and the nucellar projection during rice seed development[J]. The Plant Cell, 2012, 24(3): 1049-1065.
[36]Hudson D, Guevara D R, Hand A J, et al. Rice cytokinin GATA transcription Factor1 regulates chloroplast development and plant architecture[J]. Plant Physiology, 2013, 162(1): 132-144.
[37]Zhang H, Tan G, Yang L, et al. Hormones in the grains and roots in relation to post-anthesis development of inferior and superior spikelets in japonica/indica hybrid rice[J]. Plant Physiology and Biochemistry, 2009, 47(3): 195-204.
[38]Uchiumi T, Okamoto T. Rice fruit development is associated with an increased IAA content in pollinated ovaries[J]. Planta, 2010, 232(3): 579-592.
[39]Schruff M C, Spielman M, Tiwari S, et al. The AUXIN RESPONSE FACTOR 2 gene of Arabidopsis links auxin signalling, cell division, and the size of seeds and other organs[J]. Development, 2006, 133(2): 251-261.
[40]Fujioka S, Li J, Choi Y H, et al. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis[J]. The Plant Cell, 1997, 9(11): 1951-1962.
[41]Yuan T, Fujioka S, Takatsuto S, et al. BEN1, a gene encoding a dihydroflavonol 4-reductase (DFR)-like protein, regulates the levels of brassinosteroids in Arabidopsis thaliana[J]. The Plant Journal, 2007, 51(2): 220-233.
[42]Jiang W B, Huang H Y, Hu Y W, et al. Brassinosteroid regulates seed size and shape in Arabidopsis[J]. Plant Physiology, 2013, 162(4): 1965-1977.
[43]Oki K, Inaba N, Kitagawa K, et al. Function of the alpha subunit of rice heterotrimeric G protein in brassinosteroid signaling[J]. Plant and Cell Physiology, 2009, 50(1): 161-172.
[44]Morinaka Y, Sakamoto T, Inukai Y, et al. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice[J]. Plant Physiology, 2006, 141(3): 924-931.
[45]Hong Z, Ueguchi-Tanaka M, Fujioka S, et al. The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone[J]. The Plant Cell, 2005, 17(8): 2243-2254.
[46]Tanabe S, Ashikari M, Fujioka S, et al. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length[J]. The Plant Cell, 2005, 17(3): 776-790.
[47]Wu C Y, Trieu A, Radhakrishnan P, et al. Brassinosteroids regulate grain filling in rice[J]. The Plant Cell, 2008, 20(8): 2130-2145.
[48]Tanaka A, Nakagawa H, Tomita C, et al. BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice[J]. Plant Physiology, 2009, 151(2): 669-680.
[49]Vriet C, Russinova E, Reuzeau C. Boosting crop yields with plant steroids[J]. The Plant Cell, 2012, 24(3): 842-857.
[50]Finkelstein R R, Gampala S S L, Rock C D. Abscisic acid signaling in seeds and seedlings[J]. The Plant Cell, 2002, 14 suppl(1): S15-S45.
[51]Audran C, Liotenberg S, Gonneau M, et al. Localisation and expression of zeaxanthin epoxidase mRNA in Arabidopsis in response to drought stress and during seed development[J]. Functional Plant Biology, 2001, 28(12): 1161-1173.
[52]Cheng Z J, Zhao X Y, Shao X X, et al. Abscisic acid regulates early seed development in Arabidopsis by ABI5-mediated transcription of SHORT HYPOCOTYL UNDER BLUE1[J]. The Plant Cell, 2014, 26(3): 1053-1068.
[53]Xue L J, Zhang J J, Xue H W. Genome-wide analysis of the complex transcriptional networks of rice developing seeds[J]. PLoS One, 2012, 7(2): e31081.
[54]Sasaki A, Itoh H, Gomi K, et al. Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant[J]. Science, 2003, 299(5614): 1896-1898.
[55]Mcginnis K M, Thomas S G, Soule J D, et al. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase[J]. The Plant Cell, 2003, 15(5): 1120-1130.
[56]Vierstra R D. The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins[J]. Trends in Plant Science, 2003, 8(3): 135-142.
[57]Temple B R, Jones A M. The plant heterotrimeric G-protein complex[J]. Annual Review of Plant Biology, 2007, 58: 249-266.
【中文责编:成文英文责编:李海航】
Progress on the Plant Hormone Regulation Related to Seed Development
Dong Qingkun, Liu Huili*
(School of Life Sciences, South China Agricultrural University, Guangzhou 510642)
The seed is produced after double fertilization as a reproductive organ of higher plants. The size and quality of the seed serve as two main components that influence crop yield. In flowering plants, the seed comprises three major anatomical components, the embryo, the endosperm and the seed coat. Seed size is coordinately determined by the growth of the embryo, endosperm and maternal tissue. Exploring relevant factors that control seed development is crucial for improving crop yield. Recently, some mutants with seed developmental defects and QTL functions have been dissected by genetic and molecular biology methods. Related genes were found to regulate the development of embryo, endosperm and integument thus determining seed final size and yield. Most of these work were carried out inArabidopsisand rice. Some reported work showed these genes function by integrating to the hormone metabolism or signaling pathway indicating plant hormone playing key roles during seed development, but the underlying molecular mechanism and gene regulatory network is still unknown. This article mainly focuses on the model plants of rice andArabidopsis, reviewing recent progress in hormone-dependent regulation of seed development.
rice; seed development; seed size; plant hormone
2015-06-20《华南师范大学学报(自然科学版)》网址:http://journal.scnu.edu.cn/n
国家自然科学基金项目(31200218)
刘慧丽,助理研究员,Email:liuhuili@scau.edu.cn.
Q945.4
A
1000-5463(2015)06-0072-07
