Feasibility of cetuximab and chemoradiotherapy combination in Chinese patients with unresectable stage III non-small cell lung cancer: a preliminary report
2015-10-31DiLiuYuXinShenWeiXinZhaoGuoLiangJiangJiaYanChenMinFan
Di Liu, Yu-Xin Shen, Wei-Xin Zhao, Guo-Liang Jiang, Jia-Yan Chen, Min Fan
1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China;2Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210006, China
Correspondence to: Min Fan. Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China. Email: fanming@fudan.edu.cn; Jia-Yan Chen. Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210006, China. Email: chenjiayan2008@126.com.
Feasibility of cetuximab and chemoradiotherapy combination in Chinese patients with unresectable stage III non-small cell lung cancer: a preliminary report
Di Liu1, Yu-Xin Shen1, Wei-Xin Zhao1, Guo-Liang Jiang1, Jia-Yan Chen2, Min Fan1
1Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China;2Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210006, China
Correspondence to: Min Fan. Department of Radiation Oncology, Fudan University Shanghai Cancer Center, Shanghai 200032, China. Email: fanming@fudan.edu.cn; Jia-Yan Chen. Department of Radiation Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing 210006, China. Email: chenjiayan2008@126.com.
Objective: In recent years, the combination of cetuximab and chemoradiotherapy (CRT) has been used to treat stage III non-small cell lung cancer (NSCLC); however, limited data are available for Chinese patients. Herein, we report preliminary data from a phase I/II study testing the combination of cetuximab with inductive chemotherapy, followed by concurrent CRT (CCRT) in Chinese patients with stage III NSCLC. Methods: Eligibility criteria were Zubrod performance status (PS) 0-1, forced expiratory volume in 1 second(FEV1) ≥1.2 L and adequate organ function. Enrolled patients received weekly cetuximab (initial dose of 400 mg/m2on day 1 of week 1 and a maintenance dose of 250 mg/m2on week 2 to the end of CCRT) with cisplatin/vinorelbine (NP) chemotherapy (every 3 weeks for 2 cycles from week 2, followed by two cycles of concomitant NP chemotherapy and intensity-modulated thoracic radiotherapy (TRT) (60-66 Gy/2 Gy). The primary endpoints were toxicity and feasibility. All patients received positron emission tomographycomputerized tomography (PET-CT) scans within the 2 weeks prior to enrollment. Univariate analyses were used to assess the correlation between SUV-T, SUV-N, SUV-TOTAL, gender, age, histology, tumor-nodemetastasis (TNM) stage, PS and smoking status and survival. Survival curves were generated for different populations using the Kaplan-Meier method and compared using a log-rank test.
Results: Seventeen patients were enrolled and 16 completed the full regime. The overall response rate (ORR)was 58.8% and 82.3% after the induction and CCRT phases, respectively. With a median follow-up duration of 27.6 months, the median survival was 27.6 months [95% confidence interval (CI): 11.3-43.9 months] with 1-and 2-year survival rates of 88.2% (95% CI, 60.6-96.9%) and 58.8% (95% CI, 60.6-77.8%), respectively. Three patients remain progression-free to date, and the median progression-free survival (PFS) was 13.5 months (95% CI, 6.8-20.2 months). No treatment-related death occurred; however, 76% of the patients experienced grade 3+ adverse events (AEs), including nausea/vomiting, intestinal obstruction, and esophagitis (<6%), while other AEs were mostly of hematological nature (71%). The cut-off values for SUV-T and SUV-TOTAL were 11 and 20, respectively. Univariate analyses revealed SUV-TOTAL (P=0.027), SUV-T (P=0.025), and PS (P=0.006) as potential survival predictors, with a hazard ratio (HR) of 3.4, 3.7, and 9.9, respectively.
Conclusions: The combination of cetuximab with induction chemotherapy followed by CCRT appears feasible and promising. Local and locoregional maximal SUVs, defined by18F-FDG PET-CT scanning, may represent a prognostic indicator for long-term survival for these patients, which warrants further study.
Cetuximab; induction chemotherapy; concurrent chemoradiotherapy (CRT); positron emission tomography-computerized tomography (PET-CT); locally advanced non-small cell lung cancer (NSCLC)
Introduction
Currently, lung cancer is the most lethal malignancy in China and around the world (1). Locally advanced unresectable non-small cell lung cancer (NSCLC)accounts for approximately 35% of lung cancer diagnoses,for which treatment remains a challenge. Radiotherapy(RT) combined with chemotherapy, ideally concurrent chemoradiation therapy (CCRT), has emerged as the standard of care for these patients (2). However, CCRT is intolerable for some NSCLC patients, and the therapeutic benefits appear to have reached a plateau (3). With proven radiosensitizing properties in head and neck squamous cell carcinoma, based on the increase in survival and locoregional control observed in a randomized phase III trial (4,5), cetuximab treatment appears as an encouraging strategy to safely improve the efficacy of multimodal treatments for intractable stage III NSCLC.
Epidermal growth factor receptor (EGFR) is frequently overexpressed in NSCLC and has been shown to be associated with poor prognosis. Erbitux (C225, cetuximab),a chimeric IgG1 monoclonal antibody directed against the extracellular ligand binding domain of EGFR, could potentiate the response to chemotherapy and RT in NSCLC cell lines and in animal models (6-10). Clinically,the combination of cetuximab with platinum-based chemotherapy, as a first-line treatment for metastatic NSCLC patients, has proven effective and tolerable in both Caucasian (11) and Asian (12) populations. Likewise,the additive effects of cetuximab with either RT (13-15)or CRT (16-18) have been validated by several phase I/II clinical trials in locally advanced NSCLC patients. Notably,the RTOG 0324 trial, testing concurrent consolidative cetuximab treatment with CRT followed by chemotherapy in locally advanced NSCLC patients, reported a remarkable improvement in overall survival (OS) and progression-free survival (PFS) with no increase in grade 3+ toxicities (18). By contrast, the effects of cetuximab used simultaneously with inductive chemotherapy and subsequently followed by CCRT remain uncertain.
At the time our study was planned, the results from the RTOG 0617 trial (19) were still unavailable. Because the full set of data from the RTOG 0617 trial has not yet been published, the possible causes for the failure of this study remain unknown. Given the known synergistic effects of cetuximab with CCRT in NSCLC, we hypothesized that combining cetuximab treatment with induction chemotherapy would ultimately benefit patients with NSCLC. We initiated an open-label, non-randomized phase I/II trial to evaluate the feasibility and efficacy of cetuximab combined with cisplatin/vinorelbine (NP)treatments followed by concomitant chemotherapy plus thoracic intensity-modulated radiotherapy (IMRT) in unresectable stage III NSCLC patients.
Materials and methods
Study population
The study consent form was approved by the institutional review boards and the independent ethics committee. Patients were deemed eligible if they were between 18 and 75 years of age and diagnosed with untreated pathologically or cytologically confirmed unresectable stage IIIA (N2)or IIIB NSCLC without malignant pleural effusion, a weight loss ≤5% over the 3 months before registration, a performance status (PS) 0-1, a forced expiratory volume in 1 second (FEV1) ≥1.2 L, a measurable disease by Response Evaluation Criteria in Solid Tumors (RECIST) (20), and adequate hematologic, hepatic, and renal function. Patients who received previous chemotherapy or RT were excluded. Informed consent was obtained from eligible patients before the screening assessments.
All patients underwent positron emission tomographycomputerized tomography (PET-CT) for staging and contrast enhanced computed tomography (CT) of the chest,electrocardiography (ECG), abdominal ultrasound, bone scan, magnetic resonance imaging scan of the brain and pulmonary function tests within 4 weeks prior to the start of the study. CT scans were used for all subsequent evaluations and for tumor measurements.
Treatment plan
Eligible patients were treated weekly with cetuximab (loading dose of 400 mg/m2on day 1 of week 1, followed by a weekly maintenance dose of 250 mg/m2from week 2 to week 13)and inductive vinorelbine (25 mg/m2on day 1 and day 8)and cisplatin (75 mg/m2on day 1) every 3 weeks for 2 cycles,starting from week 2. Cetuximab was given before the administration of chemotherapy and thoracic radiotherapy(TRT) during the inductive and concurrent phases,respectively. In addition, a regimen of intensity modulated TRT (60-66 Gy/2 Gy) with two synchronous cycles of NP chemotherapy (vinorelbine 12.5 mg/m2days 1 and 8, cisplatin 25 mg/m2days 1 to 3, every 3 weeks) was initiated on week 7.
Radiation therapy
Chest irradiation using 6 MeV photon beams began at week 7,and all patients received standard fractionated IMRT (60-66 Gy). Patients with systemic disease progression after induction chemotherapy were withdrawn from the study,whereas patients showing disease progression within the thoracic cavity were considered eligible for TRT.
Post-chemotherapy lung tumor lesions and lymph nodes measuring ≥1 cm in short-axis diameter (as per CT and/or PET-positive screening) were all included in the gross tumor volume (GTV). The GTV was expanded by 1 cm to achieve the clinical target volume (CTV). The planning target volume (PTV) encompassed the CTV with a margin of 0.5-1 cm for respiratory variation and fixation error. Minimum and maximum PTV doses were, respectively, >95% and <110% of the prescribed dose Tissue heterogeneity factors for bone, soft tissue, and lung were used in the dose calculation. Dose volume histograms (DVHs) for the PTV, normal lung(defined as both lungs minus PTV), spinal cord, heart and esophagus were generated for all patients. The maximum point dose to the spinal cord was limited to 45 Gy, and the volume of the normal lungs receiving 20 Gy or more (V20)was below 28%, with a mean lung dose (MLD) below 15 Gy.
Evaluation and follow-up
Data concerning efficacy, safety, concomitant medications and therapies were collected until the first follow-up visit,which occurred 4 weeks after completion of the treatment and afterwards every 4 weeks, until all study treatmentrelated toxicities resolved, returned to baseline, or were deemed irreversible, whichever was longer. Patient followup visits were scheduled every 3 months for the first 3 years until the date of disease progression, death, or failure to follow-up. Follow-up visits were then scheduled every 6 months until year 5 and annually thereafter.
Adverse events (AEs) were defined by National Cancer Institute Common Terminology Criteria for Adverse Events(NCI-CTCAE) version 3.0 (21). The response rate (determined after induction chemotherapy and 2 months after completion of CCRT) was defined as the proportion of patients who achieved a complete or partial response by RECIST Version 1.1 (20).
Positron emission tomography-computerized tomography(PET-CT) scan
Before imaging, all patients received an intravenous injection of18F-FDG (7.4 MBq/kg) followed by a 60-minute uptake phase. All scans were obtained with a Siemens Biography 16HR PET-CT scanner. The vendor-provided software was used to interpret imaging data. Patients were fasted for at least 6 hours before the PET scan and had a blood glucose level below 7 mmol/L at the time of injection. PET and CT scans were obtained from the base of skull to the hips and were centrally and blindly reviewed by two independent nuclear medicine physicians. Regions of interest (ROIs)were manually drawn on the transaxial images around the focal18F-FDG uptake zone of the primary tumor (SUV-T)and regional lymph node (SUV-N). The maximum SUV(SUVmax) was always used in this study to minimize the partial-volume effects. Abnormal18F-FDG uptake was defined as areas with activity greater than that of the surrounding tissue, avoiding sites with normally increased uptake of tracer, such as the myocardium or the bladder(excretion). A PET-CT scan was interpreted as positive if the SUVmax (T or N) of a study exceeded 2.5. The SUVTOTAL value was defined as the sum of SUV-T and SUV-N.
Statistical methods
The primary endpoints were compliance and safety regarding the addition of cetuximab to induction chemotherapy and concurrent CRT. The secondary endpoints included overall response rate (ORR), PFS and OS. Analyses were performed with an intent-to-treat rationale on registered and treated patients and in the evaluable population. The response rate was evaluated in patients who completed the defined therapeutic regimen. The safety analysis included all patients who received at least one dose of cetuximab.
Survival time was defined as the interval between pathologic diagnosis date until the last scheduled followup date, or until death. Survival was calculated using the Kaplan-Meier method, and groups were compared using log-rank test. Univariate analysis was carried out with the Cox proportional hazards model. The median survival times with a 95% confidence interval (CI) are presented in the tables.
Results
Treatment delivery and compliance
Seventeen patients from our institution were enrolled in the study, and all patient baseline characteristics are listed inTable 1.
All patients completed the inductive cetuximab and chemotherapy per protocol. For the CCRT phase, 16 patients (94%) were treated per protocol. One patient received a TRT of 58 Gy/29 Fx due to pneumothorax. One patient did not receive TRT due to disease progression during the inductive phase and was then withdrawn from the study.
In this study, there was no treatment violation or dose reduction from the planned CRT protocol with the addition of cetuximab.
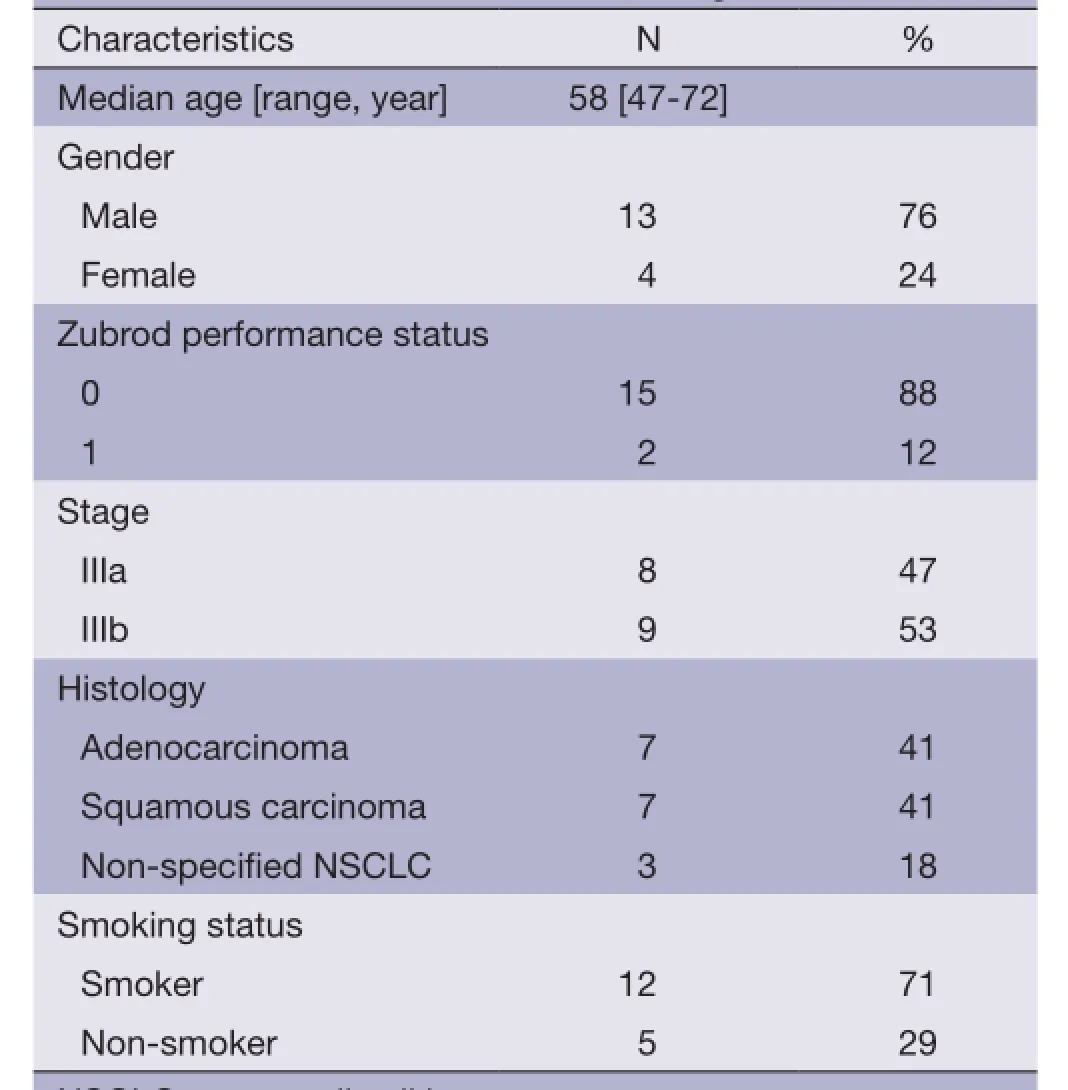
Table 1 Baseline characteristics of enrolled patients (N=17)
Safety
The AEs, outlined in Table 2, are reported as definitely,probably, or possibly related to treatment. All patients were evaluated for safety and efficacy. During the induction chemotherapy phase, hematological toxicity was the main side effect, with 12 patients (71%) and 9 patients(53%) presenting with grade 3 or higher neutropenia and leukopenia, respectively. Febrile neutropenia of grade 3 or higher was reported in 3 patients (19%). Five patients(29%) presented with grade 3 to 4 treatment-related, nonhematologic AEs, which included grade 4 nausea/vomiting(6%), grade 3 hypokalemia (12%), grade 3 intestinal obstruction (6%), and grade 3 hyponatremia (6%).
During the CCRT phase, grade 3 or 4 hematological toxicity occurred in 9 patients (56%) including leukopenia(25%), neutropenia (12%), and anemia (19%). Main nonhematological AEs were grade 2 esophagitis (50%), grade 2 radiation pneumonitis (6%) and grade 2 pneumothorax(6%), the latter condition leading to a TRT dose reduction to 58 Gy in this patient.
Efficacy
With a median follow-up of 27.6 months, the 1- and 2-year survival rates were 88.2% (95% CI, 60.6-96.9%) and 51.8%(95% CI, 32.5-77.8%), respectively. The median survival time (MST) was 27.6 months (95% CI, 11.3-43.9 months). Disease progression was noted in 14 patients. The median PFS was 13.5 months (95% CI, 6.8-20.2 months) with 1- and 2-year PFS rates of 58.8% (95% CI, 32.5-77.8%)and 23.5% (95% CI, 7.3-44.9%), respectively (Figure 1). Differences in tumor stage (IIIA vs. IIIB) or histology did
not influence the survival rates significantly in our patients. Ten patients (58.8%) showed partial response (PR) after 2 cycles of induction chemotherapy, and 1 patient showed signs of disease progression. After CCRT, 14 patients with PR (82.3%) were reported (with an ORR of 82.3%). Two patients (11.8%) presented with stable disease (SD) as best outcome.
Prognostic value of18F-FDG PET-CT
The selected cut-off values for SUV-T, SUV-N and SUVTOTAL were 11, 11 and 20, respectively. Univariate analysis revealed that SUV-T (P=0.025), SUV-TOTAL(P=0.027), and PS (P=0.006) values behaved as possible predictors of survival, with a hazard ratio (HR) of 3.7, 3.4 and 9.9, respectively. The Kaplan-Meier analysis indicated that OS was significantly higher in the lower SUV-T and SUV-TOTAL subgroups than in the corresponding higher subgroups (P<0.05, Figures 2,3). The median OS was 31.7 months (95% CI, 30.6-32.8 months) for the lower SUV-N subgroup and 17.0 months (95% CI, 14.7-19.3 months)for the higher subgroup, although this association was not statistically significant (P=0.067, Figure 4). The 1-, 2- and 3-year survival rates for the lower SUV-T subgroup vs. the higher SUV-T subgroup were 85.7% (95% CI, 33.4-97.9%) vs. 80% (95% CI, 40.9-94.6%), 85.7% (95% CI,33.4-97.9%) vs. 40% (95% CI, 12.3-67%) and 42.9% (95%CI, 9.8-73.4%) vs. 10% (95% CI, 0.5-35.8%), respectively. The 1-, 2- and 3-year survival rates for the lower SUVTOTAL subgroup vs. the SUV-TOTAL higher subgroup were 87.5% (95% CI, 38.7-98.1%) vs. 77.8% (95% CI,36.5-93.9%), 75% (95% CI, 31.5-93.1%) vs. 33.3% (95% CI, 7.8-62.3%), 37.5% (95% CI, 8.7-67.4%) vs. 11.1% (95% CI, 0.6-38.8%), respectively.
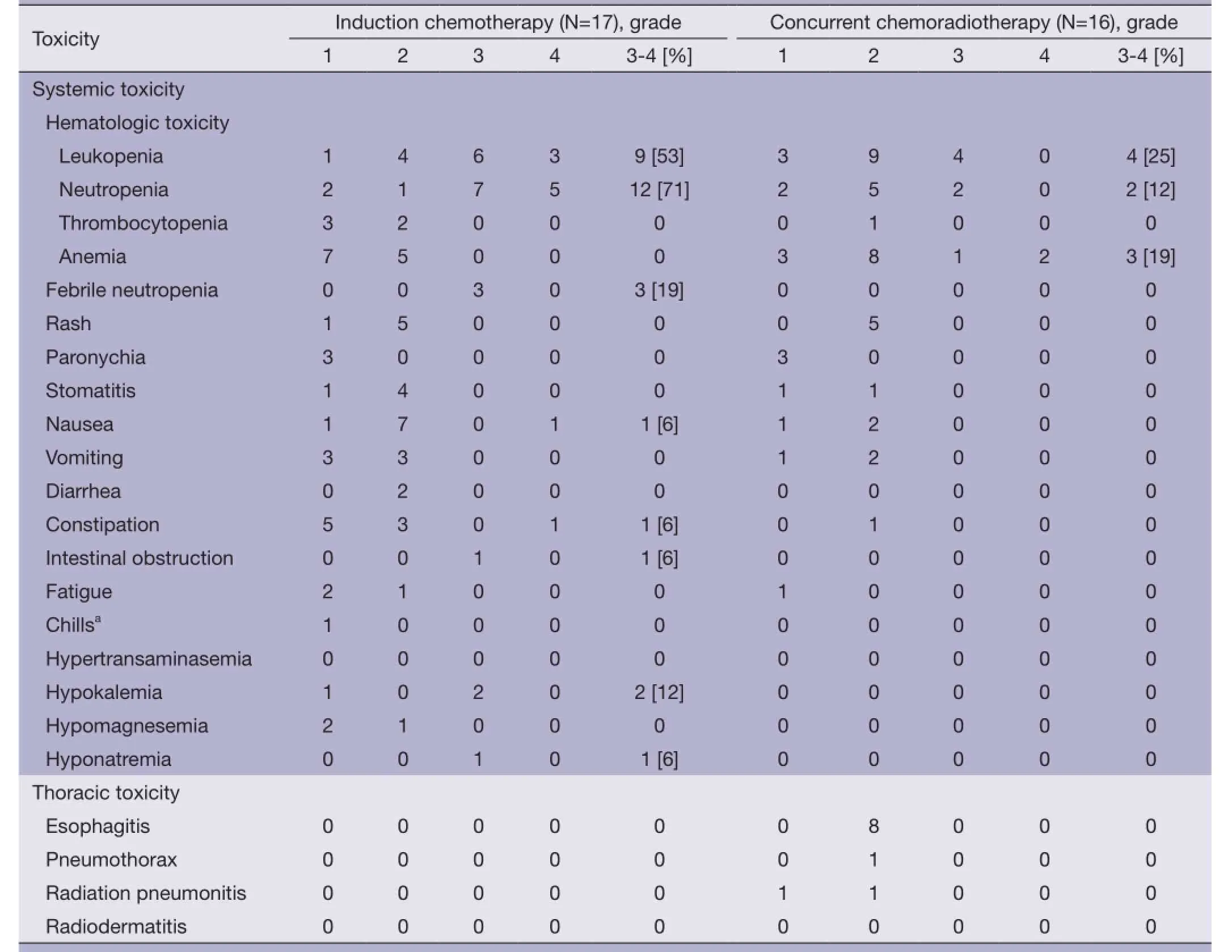
Table 2 Acute toxicities (N=17)
Discussion
Only a few relatively small studies using cetuximab as part of the treatment for stage III NSCLC have been previously published (13-18,22). By contrast, the early reports from the RTOG 0617 phase III trial revealed that cetuximab provided no survival benefit in this disease setting. However,keeping the chemotherapy enhancing potential and the observed radiosensitization in mind, the idea of adding cetuximab to inductive chemotherapy followed by CCRT still appeared promising in a stage III NSCLC setting and led to the initiation of our study.
Our trial combining cetuximab with chemotherapy in the induction phase did show that concurrent cetuximaband chemotherapy was effective and well tolerated. After 2 cycles of inductive chemotherapy, 10 patients (58.8%)achieved PR. The 58.8% ORR was superior to the 37-44% recorded in previous studies using an equivalent NP dose without cetuximab (23). However, toxicity assessment revealed that 65% of the patients displayed AEs ≥ grade 3 during the induction phase. Hematological toxicity was predominant, with 71% of the patients experiencing neutropenia, 53% leukopenia and 19%, febrile neutropenia,all ≥ grade 3. This safety profile was not worse than with most regimens including inductive NP chemotherapy. Severe non-hematological AEs attributable to the therapeutic protocol included 6-12% pulmonary, GI and metabolic/laboratory toxicities. The skin reactions weremostly of grade 1 or 2.
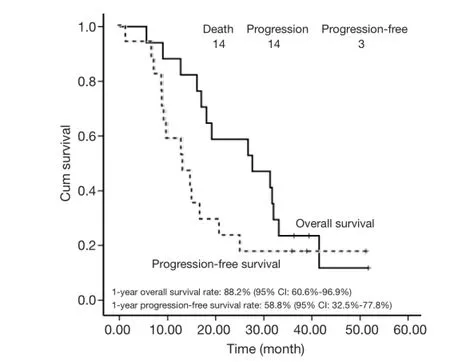
Figure 1 Overall survival and progression-free survival. ITT,intention to treat.
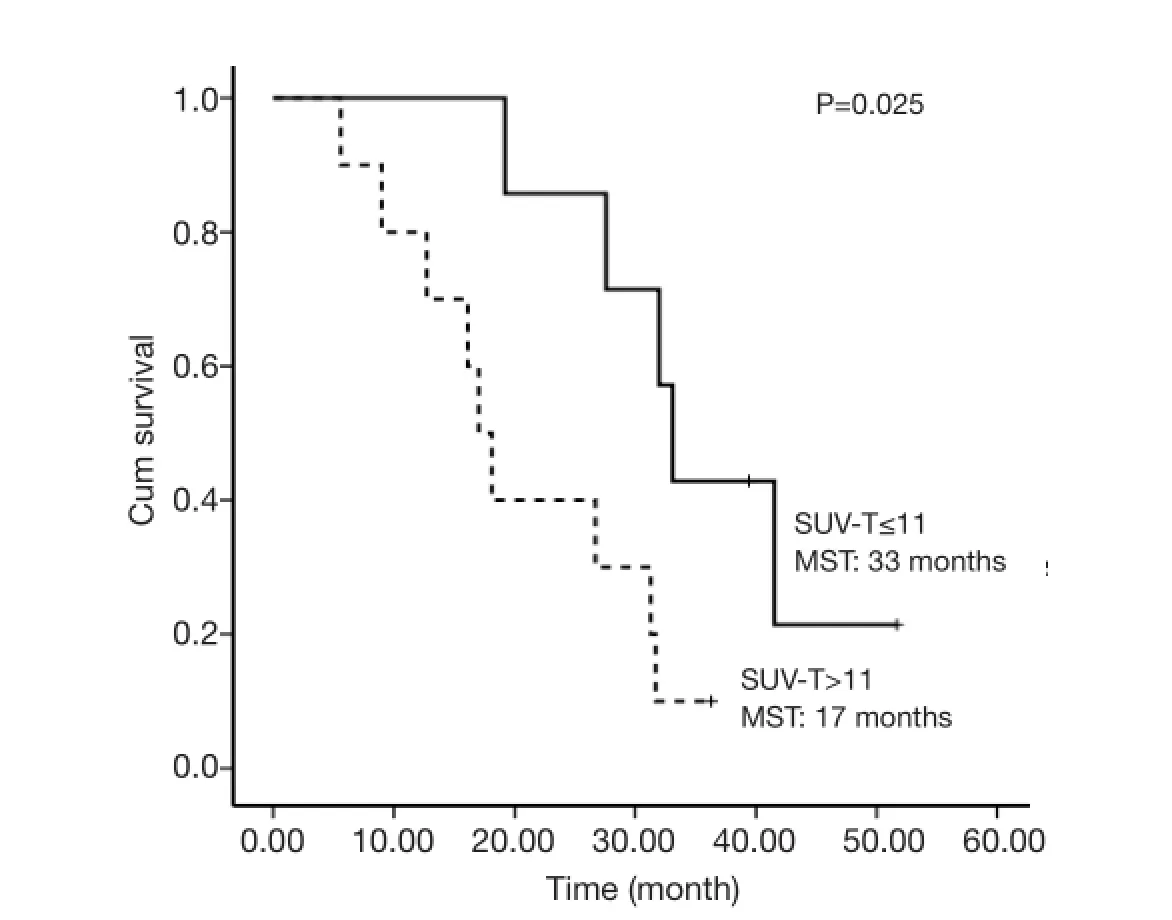
Figure 2 Overall survival of SUV-T ≤11 and SUV-T >11 subgroups. MST, median survival time.
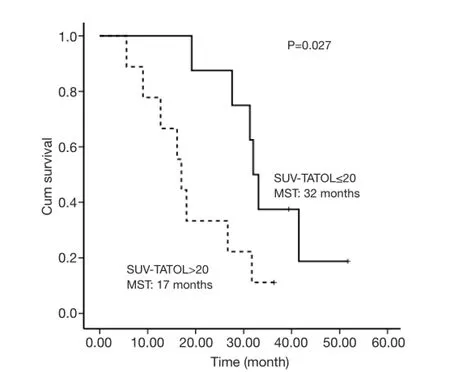
Figure 3 Overall survival of SUV-TOTAL ≤20 and SUV-TOTAL>20 subgroups.
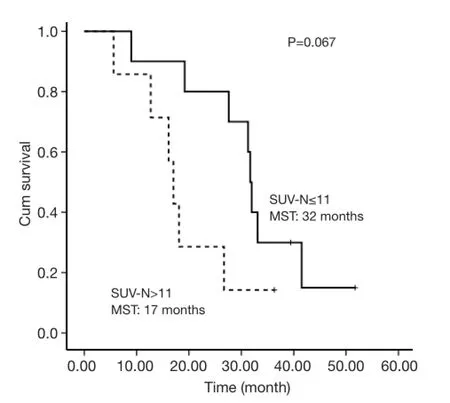
Figure 4 Overall survival of SUV-N ≤11 and SUV-N >11 subgroups.
In view of our final data, the 82.3% ORR and 27.6-month MS significantly exceeded that of similarly designed studies without cetuximab (23,24), with a 1-,2-, 3-year survival rate of 88.2%, 58.8%, and 23.5%,respectively. For PFS, the 13.5-month median time demonstrated a numerical increase superior to the previous two studies. However, this combination was generally well tolerated. The major side effect observed was hematological toxicity. The incidence of grade 3 or 4 neutropenia was 12% in the concomitant phase and 71% during the full course,which is similar to the NP arm in the CALGB 9431 trial (23). No patient experienced ≥ grade 3 esophagitis,which was better than the 26% esophagitis rate in CALGB study. We noted that no grade 3 pneumonitis occurred during this study, which may result from our stringent normal lung allowance dose (V20) of 28%, compared with most CRT studies, which use 35% as the upper limit. Our lower V20 value possibly results from the combination of cetuximab treatment during the inductive chemotherapy phase, which led to smaller GTV in the CCRT phase. In the current trial, the V20 was 25.1% (range, 16.3-27.8%),with MLD being 13.8 Gy (range, 11.3-14.8 Gy). Overall,the increased response activity and survival times with acceptable toxicity could reflect the potential benefits of cetuximab, which still requires further exploration in a larger group of patients to minimize the impact of selection bias or other possible confounding factors.
Unfortunately, at present, no established molecular marker or histological feature of NSCLC has yet been shown to reliably identify patients who are likely to respond best to cetuximab treatments. Pre-treatment PET-CT scan imaging,which is extensively used in the management of NSCLC,has become an important prognostic indicator. A metaanalysis of 13 studies and 1,474 patients demonstrated that high pre-treatment SUVmaxvalues represent a statistically significant indicator of poor prognosis and survival in unresectable NSCLC patients (25). With the increasing use of cetuximab in clinical practice, the prognostic value of PET-CT in patients with stage III NSCLC urgently needs to be explored.
The PET-CT scan, which measures the uptake and trapping of radiolabeled glucose by tissues, is a useful method for the detection of primary tumors as well as of loco-regional and metastatic disease, and it may theoretically be correlated with PFS and OS. The SUVmaxvalues for primary tumor(25,26) or lymph node (27) may help to stratify patients with NSCLC in terms of ultimate prognosis, as demonstrated in previous reports. Liao et al. (28) revealed that the wholebody metabolic tumor burden measurement (including primary tumor, nodal metastasis and distant metastasis)was an independent prognostic factor in patients with unresectable NSCLC. In this study, we found that SUV-T,SUV-TOTAL and PS were the possible predictors of survival. Survival decreased as the SUVmax of locoregional lymph nodes increased, although without reaching statistical significance. Currently, the only recognized independent prognostic factors for phase III NSCLC patients were tumor-node-metastasis (TNM) stage and PS (29). PS was found to correlate with survival in the studied population. However, the prognostic role of stage was not reflected,perhaps due to the small sample size. For NSCLC patients with stage III disease, the locoregional lesions may better represent the whole-body tumor burden, which is probably related to the potential impact of stage on survival. Because of the sample size limitations, however, the prognostic value of SUVmax requires more studies in order to validate its use in the clinic.
Conclusions
In conclusion, a treatment approach involving the addition of cetuximab with NP chemotherapy as induction and followed by CCRT appears feasible and promising in unresectable stage III NSCLC patients, representing a new treatment option for patients with good PS. Local and locoregional maximal SUVs defined by18F-FDG PET-CT scan may have a strong correlation with survival in stage III NSCLC patients and therefore requires further study.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2. Pfister DG, Johnson DH, Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol 2004;22:330-53.
3. Langer CJ, Wakelee H, Schiller J, et al. Cooperative group portfolio in locally advanced non-small-cell lung cancer: are we making progress? Clin Lung Cancer 2008;9:85-91.
4. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 2006;354:567-78.
5. Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol 2010;11:21-8.
6. Milas L, Mason K, Hunter N, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res 2000;6:701-8.
7. Raben D, Helfrich B, Chan DC, et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res 2005;11:795-805.
8. Wang M, Morsbach F, Sander D, et al. EGF receptor inhibition radiosensitizes NSCLC cells by inducing senescence in cells sustaining DNA double-strand breaks. Cancer Res 2011;71:6261-9.
9. Milas L, Fan Z, Andratschke NH, et al. Epidermal growth factor receptor and tumor response to radiation: in vivo preclinical studies. Int J Radiat Oncol Biol Phys 2004;58:966-71.
10. Nasu S, Ang KK, Fan Z, et al. C225 antiepidermal growth factor receptor antibody enhances tumor radiocurability. Int J Radiat Oncol Biol Phys 2001;51:474-7.
11. Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009;373:1525-31.
12. Xia LP, Qiu HJ, Chen XX, et al. Short-term outcomes of cetuximab combined with standard chemotherapy as first line setting for Chinese patients with non-small cell lung cancer: a report of 12 cases. Med Oncol 2011;28:S570-6.
13. Jensen AD, Münter MW, Bischoff HG, et al. Combined treatment of nonsmall cell lung cancer NSCLC stage III with intensity-modulated RT radiotherapy and cetuximab: the NEAR trial. Cancer 2011;117:2986-94.
14. Jatoi A, Schild SE, Foster N, et al. A phase II study of cetuximab and radiation in elderly and/or poor performance status patients with locally advanced non-small-cell lung cancer (N0422). Ann Oncol 2010;21:2040-4.
15. Chen Y, Moon J, Pandya KJ, et al. A Pilot Study (SWOG S0429) of Weekly Cetuximab and Chest Radiotherapy for Poor-Risk Stage III Non-Small Cell Lung Cancer. Front Oncol 2013;3:219.
16. Hughes S, Liong J, Miah A, et al. A brief report on the safety study of induction chemotherapy followed by synchronous radiotherapy and cetuximab in stage III nonsmall cell lung cancer (NSCLC): SCRATCH study. J Thorac Oncol 2008;3:648-51.
17. Hallqvist A, Wagenius G, Rylander H, et al. Concurrent cetuximab and radiotherapy after docetaxel-cisplatin induction chemotherapy in stage III NSCLC: satellite--a phase II study from the Swedish Lung Cancer Study Group. Lung Cancer 2011;71:166-72.
18. Blumenschein GR Jr, Paulus R, Curran WJ, et al. Phase II study of cetuximab in combination with chemoradiation in patients with stage IIIA/B non-small-cell lung cancer: RTOG 0324. J Clin Oncol 2011;29:2312-8.
19. Bradley JD, Masters GA, Hu C. An intergroup randomized phase III comparison of standard-dose (60 Gy)versus high-dose (74 Gy) chemoradiotherapy (CRT) +/-cetuximab (cetux) for stage III non-small cell lung cancer(NSCLC): Results on cetux from RTOG 0617. J Thorac Oncol 2013;8:S3.
20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47.
21. Common Toxicity Criteria classification v3.0. Available online: http://ctep.info.nih.gov/reporting/ctc.html. Accessed on 26 July 2010.
22. Govindan R, Bogart J, Stinchcombe T, et al. Randomized phase II study of pemetrexed, carboplatin, and thoracic radiation with or without cetuximab in patients with locally advanced unresectable non-small-cell lung cancer: Cancer and Leukemia Group B trial 30407. J Clin Oncol 2011;29:3120-5.
23. Vokes EE, Herndon JE 2nd, Crawford J, et al. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-smallcell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol 2002;20:4191-8.
24. Krzakowski M, Provencio M, Utracka-Hutka B, et al. Oral vinorelbine and cisplatin as induction chemotherapy and concomitant chemo-radiotherapy in stage III non-small cell lung cancer: final results of an international phase II trial. J Thorac Oncol 2008;3:994-1002.
25. Berghmans T, Dusart M, Paesmans M, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDGPET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and metaanalysis (MA) by the European Lung Cancer WorkingParty for the IASLC Lung Cancer Staging Project. J Thorac Oncol 2008;3:6-12.
26. Vansteenkiste JF, Stroobants SG, Dupont PJ, et al. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J Clin Oncol 1999;17:3201-6.
27. Okereke IC, Gangadharan SP, Kent MS, et al. Standard uptake value predicts survival in non-small cell lung cancer. Ann Thorac Surg 2009;88:911-5; discussion 915-6.
28. Liao S, Penney BC, Wroblewski K, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2012;39:27-38.
29. Kanters SD, Lammers JW, Voest EE. Molecular and biological factors in the prognosis of non-small cell lung cancer. Eur Respir J 1995;8:1389-97.
Cite this article as: Liu D, Shen YX, Zhao WX, Jiang GL, Chen JY, Fan M. Feasibility of cetuximab and chemoradiotherapy combination in Chinese patients with unresectable stage III non-small cell lung cancer: a preliminary report. Chin J Cancer Res 2015;27(2):172-180. doi: 10.3978/ j.issn.1000-9604.2014.11.05
10.3978/j.issn.1000-9604.2014.11.05
Submitted May 18, 2014. Accepted for publication Oct 19, 2014.
View this article at: http://dx.doi.org/10.3978/j.issn.1000-9604.2014.11.05
杂志排行
Chinese Journal of Cancer Research的其它文章
- A case of small intestinal hemorrhage secondary to metastatic lung cancer in the elderly
- Diffusion-tensor imaging as an adjunct to dynamic contrastenhanced MRI for improved accuracy of differential diagnosis between breast ductal carcinoma in situ and invasive breast carcinoma
- Activity estimation in radioimmunotherapy using magnetic nanoparticles
- Nonintubated uniportal video-assisted thoracoscopic surgery for primary spontaneous pneumothorax
- Nab-paclitaxel (abraxane)-based chemotherapy to treat elderly patients with advanced non-small-cell lung cancer: a single center, randomized and open-label clinical trial
- Activation of Toll-like receptors signaling in non-small cell lung cancer cell line induced by tumor-associated macrophages
