Chemical weathering and CO2consumption of a high-erosion-rate karstic river:a case study of the Sanchahe River,southwest China
2015-10-25YanlingAnYiliangHouQixinWuLinQingLongboLi
Yanling An·Yiliang Hou·Qixin Wu,2·Lin Qing·Longbo Li
Chemical weathering and CO2consumption of a high-erosion-rate karstic river:a case study of the Sanchahe River,southwest China
Yanling An1·Yiliang Hou1·Qixin Wu1,2·Lin Qing1·Longbo Li3
The Sanchahe River in southwest China is a tributary of the Wujiang River and experiences high erosion rates.Geochemical analysis was conducted on Sanchahe River basin samples collected in the wet and dry seasons of 2014 in order to better understand local chemical weathering processes,anthropogenic influences,and associated CO2consumption.The samples'total dissolved solid concentrations were found to be significantly higher than that of the global river average.Ca2+was the dominant cation in the samples and accounted for 64%and 73%of the total cations in the dry and wet seasons,respectively.HCO3-and SO42-were the dominant anions,accounting for 92%of the total anions.Stoichiometry analyses of the river waters suggested that the water chemistry is controlled by carbonate dissolution by both carbonic and sulfuric acid.The chemical weathering rates of carbonate and silicate evaporites in the Sanchahe River basin were estimated to be approximately 109.2 and 11.0 t/(km2a),respectively,much higher than both the global mean values and the Wujiang River,a typical karstic river. The CO2consumption by carbonate and silicate weathering are estimated to be 597.4×103and 325.5×103mol/(km2a),which are much higher than corresponding values in the Wujiang River,indicating a high erosion rate in the Sanchahe River basin.
Sanchahe River·Water chemistry·
1 Introduction
Chemical characteristics of water can be used to trace the sources of solutes in rivers,and they also help understand chemical weathering rates,geologic background,and CO2consumption of watersheds(Hu et al.1982;Gaillardet et al. 1999;Han and Liu 2004;Hren et al.2007;Chetelat et al. 2008;Liu et al.2008a;Raymond and Oh 2009;Moosdorf et al.2011).Carbon sinks associated with the processes of rock weathering(including carbonates and silicates)can affect the global carbon cycle(Meybeck 1987;Liu et al. 2011;Moosdorf et al.2011).On a geologic time scale,only silicate weathering produces a net impact on atmospheric CO2content(silicates:CaSiO3+H2CO3→CaCO3+ SiO2+H2O;carbonates:CaCO3+H2CO3→CaCO3+ CO2+H2O)(White et al.1999).In the shorter term,however,from several decades to thousands of years,the influence of carbonate weathering can be more significant than the impact of silicate weathering due to carbonates' much higher weathering rate(Blum et al.1998;Kump et al. 2000;Jacobson et al.2002;Liu et al.2010).Chemical weathering rate is affected by several factors,such as geologic background,tectonism,weather,and land use(Gibbs 1970;Raymond and Cole 2003;Das et al.2005;von Blanckenburg 2005;Williams et al.2005).This study contributes to a more complete understanding of regionalweathering processes and to the broader knowledge base around the theory and mechanism of carbon sinks in the context of rock weathering.
With about 500,000 km2of karst terrain,southwest China is one of the largest karst-covered areas in the world(Han and Liu 2004).Due to the fragile ecological environment of the area,weathering is very intense and water and soil loss are significant.The Sanchahe River is the headwaters river of the Wujiang River,which is the largest river of Southwest China.The Sanchahe River catchment has one of the highest rates of soil erosion in the Wujiang River watershed(Liu et al.2015).There are a number of coal mining enterprises in the Sanchahe River catchment as it is the thermal power base of Guizhou Province,and SO2deposition is significant.The Sanchahe River is a higherosion karst river watershed,and is strongly influenced by human activities.
2 Study area
The Sanchahe River(104°18′—106°18′E and 26°10′—27°00′N)originates in Weining County,at the east side of Wumeng Mountain,western Guizhou(Jiao et al.2013). The total length of the river is 325.6 km.The watershed area is about 7264 km2,including 80%in mountainous regions,15%plateau and hilly land,and 5%flat ground. The vertical drop of the Sanchahe basin is more than 1300 m from west to east,and the average gradient is approximately 4%.The Sanchahe basin consists of various parent rocks,such as carbonatite and coal-bearing rock strata,as well as basalt(Han and Liu 2004).The western portion of the watershed has a temperate climate,with the remainder falling in the subtropical monsoon climate zone. According to meteorological data from the past 3 years,the annual average temperature in the catchment varies between 12 and 16°C,with annual average precipitation of 546.9 mm.More than 75%of the precipitation falls between May and October.Precipitation is at a minimum in winter(December to February),which accounts for less than 5%of annual precipitation.The mean runoff volume of the Sanchahe basin is about 49.5×108m3.Being related to precipitation,the runoff is distributed unevenly during the hydrologic year,with 80%of the annual runoff occurring in the wet season.
3 Sampling and analytical methods
30 wet season water samples and 28 dry season samples were collected from the main river and the tributaries of the Sanchahe(Fig.1).Temperature(T),pH,dissolved oxygen(DO),and electrical conductivity(EC)were determined in situ using multi-parameter water probe meters(WTW-3420).HCO3-was measured on site by acid titration using hydrochloric acid.The HCO3-of each sample was measured at least twice,and the titration error was controlled within 5%.All the water samples were filtered with 0.45 μm mixed cellulose membrane filters(Millipore)on the day they were sampled.After filtration,a fraction of the samples were stored in clean High Density Polyethylene(HDPE)bottles.All the bottles were pre-washed with ultrapurified(double-distilled)HCl and rinsed with Milli-Q 18.2 MΩ water.The samples for cation determination were acidized(pH<2)with HCl.All cations and anions were measured on an ion chromatograph(DIONEX,ICS-1100),with IonPac CS-12A column and IonPac AG-19 column separately,and SiO2was measured by colorimetry.
4 Results and discussion
4.1 Composition and temporal variation of the major ions
Table 1 shows the results of water sample analyses from the Sanchahe River basin in both dry and wet seasons. Samples were generally alkaline.The pH ranged from 7.9 to 10.2 in the dry period,averaging 8.3;while pH values ranged from 6.9 to 7.9 in the wet period,with an average of 7.4.The high pH values might have been impacted by the dissolution of limestone and dolomite in river waters.The pH values in the wet season were lower than those in the dry season,which could be due to the concentrated and heavy acid rainfall in the wet seasons,which would tend to lower pH.The total soluble cations(TZ+=2Ca2++2 Mg2++Na++K+)in river waters during the dry period ranged from 3.2 to 9.3 meq/L,averaging 5.2 meq/L,while those in the wet period ranged between 3.3 and 6.6 meq/L,with an average of 4.4 meq/L—all much higher than the global average value(1.25 meq/L)(Meybeck 1981).The average inorganic charge balance(NICB=(TZ+-TZ-)/ TZ+)of river water samples was 6%and 8%in the dry and wet seasons,respectively.
Thetotaldissolvedsolid(TDS=Ca2++Mg2+Na++K++HCO3-+SO42-+Cl-+NO3-+SiO2)range in the dry season was 247.8—628.4 mg/L,averaging 367.5 mg/L;while the variation in the wet seasons was 241.3—427.5 mg/L,with an average of 311.4 mg/L.These values are much higher than the global average TDS(100 mg/L)(Gaillardet et al.1999).In comparison with the famous rivers of the world,the Sanchahe River basin displayed higher TDS than the Amazon and Mississippi,but lower than the Nile and Rhine(Table 2).Furthermore,compared with large rivers in China,the Sanchahe River exhibitedhigherTDSthantheYangtzeRiver(TDS=220 mg/L)(Chetelat et al.2008)and the Xijiang River(TDS=241 mg/L)(Xu and Liu 2010),and lower than the Yellow River(TDS=460 mg/L)(Gaillardet et al. 1999).

Fig.1 Sample locations in the Sanchahe River Basin of dry season and rain season(in the brackets)

Table 1 Major ionic compositions of the river water samples from Sanchahe River
The main cation in river waters during the sampling campaign was Ca2+(Fig.2).For the dry season samples,Ca2+accountedfor64%ofallthecations,with concentrations varying between 2.2 and 5.2 meq/L,averaging 3.4 meq/L;while for the wet period samples,Ca2+comprised 73%of all the cations,with concentrations ranging from 2.4 to 4.1 meq/L,and averaging 3.2 meq/L. The second most prevalent cations were Mg2+and Na+,with less Mg2+than Na+in the dry season and more Mg2+than Na+in the wet season.Mg2+and Na+together comprised 15%of the total cations.K+accounted for only about 5%of all cations.During the sampling period,HCO3-and SO42-were the major anions in river water samples,accounting for 48%and 44%of the total anions,respectively.Their concentration variations in the dry season were 1.7—2.9 and 0.8—5.6 meq/L,respectively,with average values of 2.3 and 2.1 meq/L.In the wet season,HCO3-and SO42-comprised 50%and 42%of the total anions,respectively,with concentration ranges 1.1—3.0 and 0.9—4.4 meq/L,and average values of 2.0 and 1.7 meq/L. NO3-and Cl-,together accounted for 7%of the total anions,with higher Cl-than NO3-in the dry period and lower Cl-than NO3-in the wet period.
Across the basin,all the equivalent concentration ratio data points of(Ca2++Mg2+)/HCO3-fall beneath the slope of 1,while the equivalent concentration ratio data points of(Ca2++Mg2+)/(HCO3-+SO42-)are on either side of the slope 1.Most points plot along the line of 1:1,indicatingagoodbalanceof(Ca2++Mg2+)and(HCO3-+SO42-).This may be caused by watershed erosion and rock weathering combined with SO42-inputfrom oxidation of sulfide minerals and acid precipitation. Previous studies suggest that sulfuric acid in watershed erosion and rock weathering processes of the southwest karst rivers(Li et al.2008)contributes significantly to the chemical composition of river water.The Sanchahe River basin possesses abundant coal resources.Large-scale mining of high-sulfur coal and numerous coal-fired power plants in this area produce and emit SO2into the atmosphere,whichcouldthenformsulfuricacidin precipitation,contributing to rock weathering and erosion in the watershed(Fig.3).

Table 2 Major ionic compositions of Sanchahe River Basin and other rivers

Fig.2 Piper diagram of the river water samples from Sanchahe River Basin
4.2 Source of dissolved load
4.2.1 Atmospheric input
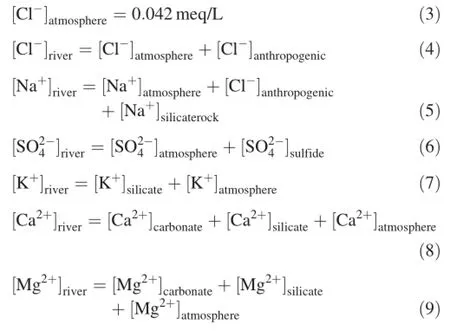
Chlorideisthemostusefulreferencetoevaluateatmospheric inputstoriversinmanystudiesbecauseitisconservativeandis not involved in biogeochemical cycling(Gaillardet et al. 1997;Viersetal.2001;Liuetal.2013).Theconcentrationof Cl-in river water is assumed to be entirely derived from the atmosphere;the contribution of evaporites is negligible(Negrel et al.1993).By using the Cl--normalized ratios of rainwater,concentrations of other elements can be corrected with regard to the contribution of atmospheric deposition. AccordingtoLarssenetal.(1999),theaveragelevelofCl-is 0.01 meq/L in rural Guizhou,and about 0.021 meq/L in Guiyang City(Xiao et al.2013).We did not analyze rainwater during the period of study.Consequently,values of atmospheric[Cl]concentrations calculated with evapotranspiration factors were used in the correction of atmospheric inputs for the mainstem.
The atmospheric contribution of element X(X=Ca2+,Mg2+,Na+,K+,and SO42-)to river water can be derived from the following equation:

where Xris the contribution of element X from rain to rivers;fetis the evapo-transpiration factor;and(X/Cl-)rainis the molar ratio of element X over Cl-in rainwater. Fet=P/(P-E),where P is annual precipitation(mm)and E is annual mean evaporation(mm).The X/Cl-ratios of volume-weighted mean concentrations of rainwater in Puding reported by Wu et al.(2012)were used as(X/Cl)rain in the calculation;Cl-=0.014 meq/L,Ca2+=0.16-meq/L,Mg2+=0.004 meq/L,Na+=0.011 meq/L,K+=0.009 meq/L,and SO42-=0.15 meq/L.
The calculation results show the respective contributions of Cl-,Ca2+,Mg2+,Na+,K+,and SO42-from rain to rivers to be 0.042,0.47,0.012,0.033,0.028,and 0.46 meq/ L,explaining only a fraction of the water chemistry of river water.
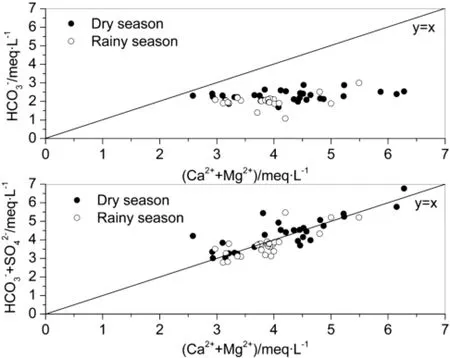
Fig.3 Equivalent charge balance of Ca2++Mg2+versus HCO3-and versus HCO3-+SO42-of the Sanchahe River Basin
4.2.2 Anthropogenic input
The Sanchahe River basin is characterized by high SO42-content,withtheSO42-/Na+valuemuchlargerthaninother rivers globally.SO42-in the river originates from several sources,such as hydatogenic rock(e.g.,gypsum)dissolution,sulfideoxidation,andatmosphericaciddeposition.The molar ratios of SO42-/Na+and NO3-/Na+can also be used to track the sources of SO42-(Fig.4).SO42-and NO3-of the Sanchahe River basin display a linear relationship,indicating that SO42-and NO3-may have derived from the same source;this source is likely to be anthropogenic,as NO3-is generally considered an anthropogenic emission.
Rainwater at Guiyang and Puding contains a high concentration of SO42-(Wu et al.2012;Han et al.2011). Therefore,the high content of SO42-in the rainwater of the Sanchahe River basin implies that the influence of acid rain on water chemistry in the study area is significant.At least some of the SO42-in the river waters is likely due to acid rain input related to extensive and intensive use of S-enriched coal and power production via coal combustion in the research area,one of the most heavily acid rain-polluted areas in China for many years(Larssen et al.1999,2006;Aas et al.2007).
Concentrations of SO42-and NO3-increased from upstream to downstream(Fig.5),which is consistent with the fact that human activity increases upstream to downstream.The headwaters of the Sanchahe River basin are dominated by agricultural activities,while the lower portions contain the significant industrial base of Liupanshui.
In addition,δ34S values in river waters and in industrial emissions,coal,and soil in Guizhou Province were reported to be from 2‰ to 8‰ by Hong et al.(1993),while Jiang et al.(2007)reported δ34S of dissolved SO42-from the Sanchahe River at about-7.3‰,indicating SO42-was mainly derived from the oxidation of sulfide.It is therefore suggested that the dissolved SO42-in the Sanchahe River is primarily derived from sulfide oxidation and acid rain deposition.
4.3 Chemical budget and chemical weathering rate estimation
4.3.1 Chemical budget
As was discussed in Sect.4.2,the solutes in river water have several sources,expressed as the following:

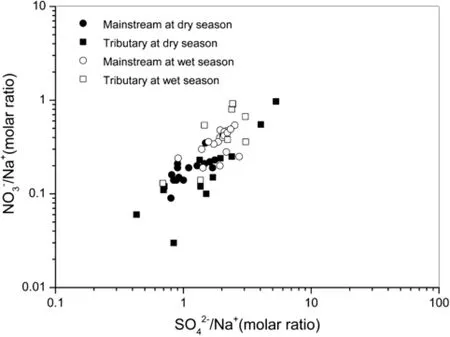
Fig.4 Variations of SO42-/Na+with NO3-/Na+molar ratios of the Sanchahe River waters
Some hypotheses were needed to calculate the exact sources of each element.First,we assumed that the atmosphere and human activities are the main Cl-contributors.According to the analysis in Sect.4.2.1,Clderived from the atmosphere is about 0.042 meq/L;additional Cl-originates from human activities,and the balance with Na+.Second,that SO42-may stem from precipitation and oxidation of sulfide minerals,with all of the additional SO42-(more than[SO42-]atmospheric)derived from sulfide mineral oxidation.Third,that the cation(Ca2+,Mg2+,Na+,and K+)contribution from human activities to river water is negligible.Based on the above assumptions,we reduced the equations as below:
Previous studies(Galy and France-Lanord 1999;Han and Liu 2004;Liu et al.2013)demonstrate that it's difficult to distinguish Ca2+and Mg2+produced by carbonate weathering from that produced by silicate weathering. Generally,K+originates from the atmosphere and silicate weathering,and it's challenging to estimate the Mg2+/K+value of silicate weathering because carbonate rock is the bedrock of Sanchahe River region.Galy and France-Lanord(1999)and Han and Liu(2004)propose that for silicate weathering,Mg2+/K+=0.5 and Ca2+/Na+=0.2. Based on this,Ca2+and Mg2+from carbonate weathering was estimated,allowing for the further simplification of Eqs.(8)and(9):

Fig.5 Spatial distribution of NO3-and SO42-of the Sanchahe River mainstream

where the ratio of silicate weathering and the total rock weathering could be represented by the ratio of dissolved cations in silicate weathering and that in the total rock weathering,resulting in:
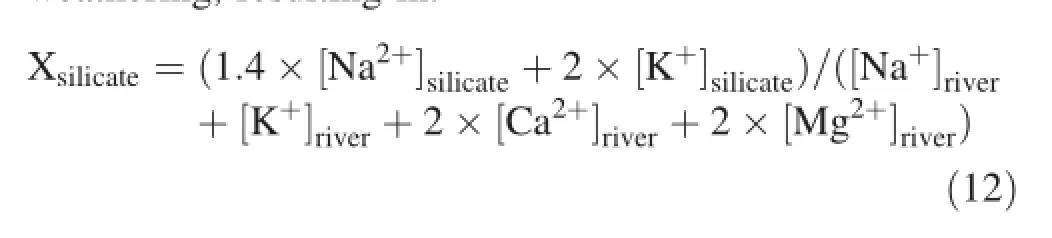
By calculation,in the dry period,Xsilicaterockof the Sanchahe River basin river water samples varied from 0.02 to 0.56,averaging0.18,and[X]carbonaterangedbetween0.44and 0.98,with an average value of 0.82;while in the wet seasons,Xsilicatevariation was 0.06—0.37,averaging 0.12,and[X]car-bonateranged from 0.63 to 0.94,with an average of 0.88.
Using Xsilicateand Xcarbonatevalues and hydrologic data of the watershed,the erosion rates of silicate and carbonate rock were estimated.The chemical weathering rate of silicate rock is represented as:

where Na+,K+,Ca2+,and Mg2+stem from silicate weathering and dissolving,and all SiO2originates from silicate weathering.
Carbonate weathering is widely distributed,and can occur rapidly.Several sources could be involved incarbonate weathering,such as H2CO3produced by CO2dissolving in the water;SO2input from the atmosphere;and H2SO4formed in sulfide mineral oxidation.In this study,we assumed silicate weathering did not contribute H2SO4as the study area is dominated by carbonate rock,and H2SO4tends to participate in carbonate weathering rather than in silicate weathering.The participation of H2CO3and H2SO4in carbonate weathering can be simplified as follows(Han and Liu 2004):
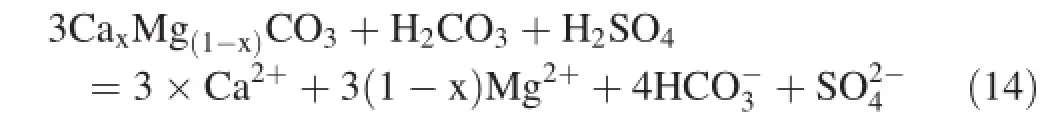
4.3.2 Chemical weathering and CO2consumption rate
As is shown in formula(14),1 mol of H2CO3and 1 mol of H2SO4are needed to dissolve 3 mol carbonate rock,and the equivalent ratio of SO42-and HCO3-is 0.9,which suggests that H2SO4plays a significant role in the watershed weathering.Li et al.(2008)applied carbon isotopes to demonstrate a similar weathering process in Beipanjiang,which is an upstream tributary of the Xijiang River.Liu et al.(2008b)used carbon and sulfur isotopes to verify H2SO4participation in watershed weathering processes of the Wujiang River,Nanpanjiang,and Beipanjiang.
The chemical weathering process of carbonate rock in the Sanchahe River basin could be affected by H2CO3and H2SO4.We assumed that Ca2+,Mg2+,and HCO3-are not impacted by human activities,and based on Eq.(14),the weathering rate of carbonate rock with the participation of both H2CO3and H2SO4can be expressed as below:

All HCO3-in the solutes(produced by silicate weathering)stem from atmospheric dissolved CO2.In the solutes generated from carbonate weathering with H2CO3as the only participant,half of the HCO3-was generated from atmospheric dissolved CO2;of the solutes affected by both H2CO3and H2SO4,one-fourth of the HCO3-was derived from atmospheric dissolved CO2.Thus,CO2consumed by silicate and carbonate weathering can be calculated by the following two equations:

CO2consumed by silicate rock weathering in the Sanchahe River basin was about 70.5×103and 255.0×103mol/(km2a)in the dry and wet periods,respectively.Approximately 325.5×103mol/(km2a)of CO2is consumed by silicate rock weathering,which is triple the average rate across the larger Wujiang drainage area(Han and Liu 2004)and far higher than the rate[128×103mol/(km2a)]in the Beipanjiang watershed(Xu and Liu 2010)(Table 3). Carbonate rock weathering consumed CO2(under bothimpact of carbonic acid and sulfuric acid)at rates of 128.2×103and 469.2×103mol/(km2a)in the dry and wet seasons,respectively.The annual CO2volume consumed by carbonate rock weathering was found to be about 597.4×103mol/(km2),which is similar to the rate in the Wujiang drainage area(Han and Liu 2004),while lower than that of Beipanjiang[966×103mol/(km2a)](Xu and Liu 2010)and higher than that of several rivers in noncarbonate rock areas,such as the Yangtze River,the Yellow River,and the Songhuajiang River(Table 3).The silicate rock weathering rates of the Sanchahe basin in the dry and wet seasons are 2.0 and 9.0 t/(km2a),respectively,while the carbonate rock weathering rates are 3.4 and 85.8 t/(km2a).This is consistent with rates of rivers flowing through carbonate rock(Table 3).

Table 3 Chemical weathering and CO2consumption rate of Sanchahe River Basin and other basins
5 Conclusions
Ca2+is the main cation of Sanchahe River solute,accounting for 64%and 73%of the total cations in the dry and wet periods,respectively;the second most prevalent cations are Mg2+and Na+.The main anions are HCO3-and SO42-,occupying 48%and 44%,respectively,of the total anions in the dry season,and 50%and 42%in the wet seasons;the next most common anions are NO3-and Cl-.
The weathering rates of carbonate,silicate,and total rock in the Sanchahe River basin during the hydrologic year were calculated to be about 109.2,11.0,and 120.2 t/(km2a),respectively,indicating an intense watershed erosion effect.Rates CO2consumed by carbonate,silicate,and total rock weathering were 597.4×103,325.5×103and 922.9×103mol/(km2a),respectively,similar to the regional rivers of the karst area in Southwest China.
According to the chemical calculation of the watershed weathering and CO2consumption rates,the Sanchahe River is primarily affected by carbonate weathering,followed by silicate weathering.Sulfuric acid participates in carbonate weathering,and the result suggests a high rate of watershed erosion and rock weathering.
AcknowledgmentsThis work was supported jointly by China Postdoctoral Science Foundation(No.2014M552388),the Guizhou Natural Science Foundation(Qiankehe-Z[2012]4012,Qiankehe-SY[2013]3133,Qiankehe-J[2013]2130,Qiankehe-J[2013]2298).
Aas W,Shao M,Jin L,Larssen T,Zhao D,Xiang R,Zhang J,Xiao J,Duan L(2007)Air concentrations and wet deposition of major inorganic ions at five non-urban sites in China,2001—2003. Atmos Environ 41:1706—1716
Blum JD,Gazis CA,Jacobson AD,Chamberlain CP (1998)Carbonate versus silicate weathering in the Raikhot watershed withintheHighHimalayanCrystallineSeries.Geology 26:411—414
Chetelat B,Liu CQ,Zhao Z,Wang Q,Li S,Li J,Wang B(2008)Geochemistry of the dissolved load of the Changjiang Basin rivers:anthropogenic impacts and chemical weathering.Geochim Cosmochim Acta 72:4254—4277
Das A,Krishnaswami S,Sarin MM,Pande K(2005)Chemical weathering in the Krishna Basin and Western Ghats of the Deccan Traps,India:rates of basalt weathering and their controls.Geochim Cosmochim Acta 69:2067—2084
Gaillardet J,Dupre B,Allegre CJ,Ne´grel P(1997)Chemical and physical denudation in the Amazon River Basin.Chem Geol 142:141—173
Gaillardet J,Dupre´B,Louvat P,Allegre C(1999)Global silicate weathering and CO2consumption rates deduced from the chemistry of large rivers.Chem Geol 159:3—30
Galy A,France-Lanord C(1999)Weathering processes in the Ganges-Brahmaputra basin and the riverine alkalinity budget. Chem Geol 159:31—60
Gibbs RJ(1970)Mechanisms controlling world water chemistry. Science 170:1088—1090
Han G,Liu CQ(2004)Water geochemistry controlled by carbonate dissolution:a study of the river waters draining karst-dominated terrain,Guizhou Province,China.Chem Geol 204:1—21
Han G,Wu QX,Tang Y(2011)Acid rain and alkalization in southwestern China:chemical and strontium isotope evidence in rainwater from Guiyang.J Atmos chem 68:139—155
Hong Y,Zhang H,Zhu Y(1993)Sulfur isotopic characteristics of coal in China and sulfur isotopic fractionation during coalburning process.Chin J Geochem 12:51—59(in Chinese)
综述,建筑精装修的施工质量管理能有效保障企业根本利益,还能为企业发展提供崭新平台、提高市场竞争力。因此,我们必须加强建筑精装修工程施工质量控制,仔细分析制定质量控制策略,进而促进企业自身发展。
Hren MT,Chamberlain CP,Hilley GE,Blisniuk PM,Bookhagen B(2007)Major ion chemistry of the Yarlung Tsangpo-Brahmaputra river:chemical weathering,erosion,and CO2consumption in the southern Tibetan plateau and eastern syntaxis of the Himalaya.Geochim Cosmochim Acta 71:2907—2935
Hu MH,Stallard RF,Edmond JM (1982)Major ion chemistry of some large Chinese rivers.Nature 298:550—553
Jacobson AD,Blum JD,Walter LM(2002)Reconciling the elemental and Sr isotope composition of Himalayan weathering fluxes:insights from the carbonate geochemistry of stream waters. Geochim Cosmochim Acta 66:3417—3429
Jiang YK,Liu CQ,Tao FX (2007)Sulfur isotope composition characters of Wujiang river water in Guizhou Province.Adv Water Sci 18:558-565(in Chinese)
Jiao SL,Liu L,Sun T,Tian QY,Ding R,Xiang S,Ye M(2013)Hydrological characteristics and the atmospheric carbon sink in the chemical weathering processes of the Sanchahe watershed. Geogr Res 32:1025—1032(in Chinese)
Kump LR,Brantley SL,Arthur MA(2000)Chemical weathering,atmospheric CO2,and climate.Annu Rev Earth Planet Sci 28:611—667
Larssen T,Seip HM,Semb A,Mulder J,Muniz IP,Vogt RD,Lydersen E,Angell V,Dagang T,Eilertsen O(1999)Acid deposition and its effects in China:an overview.Environ Sci Policy 2:9—24
Larssen T,Lydersen E,Tang D,He Y,Gao J,Liu H,Duan L,Seip HM,Vogt RD,Mulder J,Shao M,Wang Y,Shang H,Zhang X,Solberg S,Aas W,Okland T,Eilertsen O,Angell V,Li Q,Zhao D,Xiang R,Xiao J,Luo J(2006)Acid rain in China.Environ Sci Technol 40:418—425
Li SL,Calmels D,Han G,Gaillardet J,Liu CQ(2008)Sulfuric acid as an agent of carbonate weathering constrained by δ13CDIC:examplesfromsouthwestChina.EarthPlanetSciLett 270:189—199
Liu CQ,Zhao ZQ,Tao F,Li SL(2008a)Chemical weathering of Qinghai-Tibet Plateau:geochemical study of Jinsha Jiang,Lancang Jiang,and Nu Jiang river water,China.Geochim Cosmochim Acta 72:A556—A556
Liu CQ,Jiang Y,Tao F,Lang YC,Li SL(2008b)Chemical weathering of carbonate rocks by sulfuric acid and the carbon cycling in Southwest China.Geochimica 37:404—414
Liu Z,Dreybrodt W,Wang H(2010)A new direction in effective accounting for the atmospheric CO2budget:considering the combined action of carbonate dissolution,the global water cycle and photosynthetic uptake of DIC by aquatic organisms.Earth Sci Rev 99:162—172
Liu Z,Dreybrodt W,Liu H(2011)Atmospheric CO2sink:silicate weathering or carbonate weathering?Appl Geochem 26:S292—S294
Liu B,Liu CQ,Zhang G,Zhao ZQ,Li SL,Hu J,Ding H,Lang YC,Li XD(2013)Chemical weathering under mid-to cool temperate and monsoon-controlled climate:a study on water geochemistry of the Songhuajiang River system,northeast China.Appl Geochem 31:265—278
Liu L,Liang H,Jiao SL,Dai AN et al(2015)The study of the soil erosion sensitivity based on karst watershed for GIS—taking the three cha he basion in Guizhou province as an example. J Guizhou Normal Univ(Nat Sci)33(2):12—17
Meybeck M(1981)Pathways of major elements from land to ocean through rivers.In:Martin JM,Burton JD,Eisma D(eds)River inputs to ocean systems.United Nations Press,New York,pp 18—30
Meybeck M (1987)Global chemical weathering of surficial rocks estimated from river dissolved loads.Am J Sci 287:401—428
Moosdorf N,Hartmann J,Lauerwald R,Hagedorn B,Kempe S(2011)Atmospheric CO2consumption by chemical weathering in North America.Geochim Cosmochim Acta 75:7829—7854
Negrel P,Alle`gre CJ,Dupre´B,Lewin E(1993)Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in river water:the Congo Basin case. Earth Planet Sci Lett 120:59—76
Raymond PA,Cole JJ(2003)Increase in the export of alkalinity from North America's largest river.Science 301:88—91
Raymond PA,Oh NH (2009)Long term changes of chemical weathering products in rivers heavily impacted from acid mine drainage:insights on the impact of coal mining on regional and global carbon and sulfur budgets.Earth Planet Sci Lett 284:50—56
Viers J,Dupre B,Braun JJ,Freydier R,Greenberg S,Ngoupayou J,Nkamdjou L(2001)Evidence for Non-Conservative Behaviour of Chlorine in Humid Tropical Environments.Aquat Geochem 7:127—154
von Blanckenburg F(2005)The control mechanisms of erosion and weathering at basin scale from cosmogenic nuclides in river sediment.Earth Planet Sci Lett 237:462—479
White AF,Bullen TD,Vivit DV,Schulz MS,Clow DW(1999)The role of disseminated calcite in the chemical weathering of granitoid rocks.Geochim Cosmochim Acta 63:1939—1953
Williams M,Hopkinson C,Rastetter E,Vallino J,Claessens L(2005)Relationships of land use and stream solute concentrations in the Ipswich River basin,northeastern Massachusetts.Water Air Soil Pollut 161:55—74
Wu L,Huh Y,Qin J,Du G,van Der Lee S(2005)Chemical weathering in the Upper Huang He(Yellow River)draining the eastern Qinghai-Tibet Plateau.Geochim Cosmochim Acta 69:5279—5294
Wu Q,Han G,Tao F,Tang Y(2012)Chemical composition of rainwater in a karstic agricultural area,Southwest China:the impact of urbanization.Atmos Res 111:71—78
Xiao HW,Xiao HY,Long AM,Wang YL,Liu CQ(2013)Chemical composition and source apportionment of rainwater at Guiyang,SW China.J Atmos Chem 70:269—281
Xu Z,Liu CQ(2010)Water geochemistry of the Xijiang basin rivers,South China:chemical weathering and CO2consumption.Appl Geochem 25:1603—1614
10.1007/s11631-015-0074-2
21 June 2015/Revised:21 July 2015/Accepted:14 September 2015/Published online:23 September 2015 ©Science Press,Institute of Geochemistry,CAS and Springer-Verlag Berlin Heidelberg 2015
✉ Yanling An
re.ylan@gzu.edu.cn
✉ Qixin Wu
wuqixin@mails.gyig.ac.cn
1Key Laboratory of Karst Environment and Geohazard Prevention,Ministry of Education,Guizhou University,Guiyang 550003,China
2State Key Laboratory of Environmental Geochemistry,Institute of Geochemistry,Chinese Academy of Sciences,Guiyang 550002,China
3Guiyang Engineering Corporation,China Power,Guiyang 550081,China
Carbonate and silicate weathering·CO2consumption
猜你喜欢
杂志排行
Acta Geochimica的其它文章
- Characterization of biochars produced from seven biomasses grown in three different climate zones
- Identification of the four rearranged hopane series in geological bodies and their geochemical significances
- Study of an Early Silurian-Early Permian paleo-weathering profile in Zunyi,north Guizhou Province,China
- Geochemical and geochronological studies of the Aketas granite from Fuyun County,Xinjiang:the implications of the petrogenesis and tectonic setting
- Research on the ecological risk of heavy metals in the soil around a Pb-Zn mine in the Huize County,China
- Fluorite REE characteristics of the Diyanqinamu Mo deposit,Inner Mongolia,China
