Cytocompatibility of chitosan—based thermosensitive hydrogel to human periodontal ligament cell
2015-10-21WuHong1LiDan-dan1Li-Hui1
Wu Hong1Li Dan-dan1Li-Hui1
【Abstract】Objective: To investigate the effect of thermosensitive chitosan/β-glycerophosphate (CS/β-GP) hydrogel on proliferation of human periodontal ligament cells (HPDLCs). Methods: CS/β-GP were prepared into a thermosensitive hydrogel and its three-dimensional structure was observed under electron microscope. HPDLCs harvested and cultured in vitro were co-cultured with the thermosensitive CS/β-GP hydrogel. Growth of the cells in the hydrogel was observed with HE staining, and the effect of the extract on proliferation of HPDLCs was examined by CCK-8 assay. Results: Observations of SEM and HE staining showed that the thermosensitive CS/β-GP hydrogel was large in pore size and appropriate for cell growth. Different levels of CS/α,β-GP extracts could promote proliferation of HPDLCs. Conclusion: Thermosensitive CS/β-GP hydrogel can promote proliferation of HPDLCs and be a good carrier for periodontal tissue engineering because of its thermosensitivity.
【key words 】 CS/β-GP PDLCs CCK8
【CLC】R722.12 【Document code】B【Article number】1004-4949(2015)03-0516-02
High incidence of periodontal disease may result in destruction of periodontal supporting tissues. PDLC is of multiple differentiation potential, and plays a key role in periodontal tissue regeneration. Modern periodontal tissue engineering is expected to solve this problem, by which a certain amount of PDLCs are inoculated into an appropriate scaffold and form a cell-scaffold complex for co-culture. HPDLCs harvested and cultured in vitro are to be co-cultured with CS/β-GP hydrogel to investigate the biocompatibility of CS/β-GP with PDLCs.
Main materials and equipment FBS, α-MEM, CS , β-GP, Cell Counting Kit-8 , HE Staining Kit , magnetic stirrer , scanning electron microscope, CO2 Incubator , Thermo Scientific Microplate Reader.
Methods
Preparation and property detection of thermosensitive hydrogel Added CS to acetic acid, and dissolved β-GP into the double distilled water and stirred until complete dissolution. Finally added β-GP into the CS solution dropwise for preparation of thermosensitive CS/β-GP hydrogel.Placed the gelatinous thermosensitive CS/β-GP hydrogel in liquid nitrogen for rapid freezing, followed by lyophilization for 48 h, Finally detection of the surface structure of the hydrogel under electron microscope.
Isolation, culture of PDLCs
Scraped periapical tissues from the middle of the root of the orthodontically removed healthy premolar , digested with collagenase, centrifuged, discarded the supernatant, neutralized with α-MEM and collected the digested tissues. Added α-MEM containing FBS and penicillin/streptomycin to the obtained tissues, and transferred to a 5 ml culture bottle, followed by incubation at 37°C under an atmosphere of 5% CO2 and saturated humidity. monoclonal antibodies against vimentin and keratin were stained with IHC.
PDLCs compounded with thermosensitive CS/β-GP hydrogel
PDLCs were centrifuged and the supernatant was discarded, then mixed well with the CS/β-GP hydrogel solution at a cell concentration of 4×106 cells/ml. The cell/scaffold composite was added into a 24-well plate at 37°C for 10 min. Culture medium was added for a two-day routine culture, followed by HE staining.
Toxicity test of thermosensitive CS/β-GP hydrogel to PDLCs
Placed CS/α ,β-GP hydrogel to the α-MEM containing 10% FBS at a density of 3 cm2/ml and remained at 37°C for 72 h. The mixture was filtrated with a 0.22 μm filter membrane at 4°C for use. Grouping: Four groups were obtained according to the concentration of hydrogel extract in α-MEM. Group 1 acted as a normal control group, whereas Groups 2 to 4 were obtained according to different volume ratios of α-MEM containing 10% FBS to hydrogel extract (1:1, 1:3 and 1:7, respectively). PDLCs were inoculated into a 96-well plate following centrifugation and discard of the supernatant. After culture for 24 h, they were removed and the extract was added at a density of 0.1 ml/cell, with six wells in each group. Culture plates were removed at days 1, 3 and 5, respectively; plates were placed in an incubator following addition of 10 μl of CCK-8 solution in each well; culture was terminated after 1 h, and absorbance at 450nm was determined for each well by the Thermo Scientific Microplate Reader.
Statistical processing
Repeated measures ANOVA was used for comparison of optical densities (ODs) at different levels at different time points. Data were processed using SPSS18.0 software. Results were expressed as X±s). P < 0.05 was considered as statistically significant.
2. Results
Microstructure observation of thermosensitive CS/β-GP hydrogel: SEM reveals a loose surface of the hydrogel, presenting a porous cross-linked network (Fig. 1).
Morphological observation of PDLC and identification of its tissue origin.:
The time for primary cultured cells to free from the tissue block is between 3 and 15 days. Cells are long fusiform, or those oval ones are fibroblasts . At day 14, cells overspread approximately 80% of the bottom of the culture bottle, and passage can be obtained .
Observations of PDLCs compounded with thermosensitive CS/β-GP hydrogel :
The hydrogel is a loose and porous network structure. Quasi-circular cells are located in the network scaffolding structure and adhere to the scaffold; there are granular scaffolds adhering to some cell surfaces (Fig. 2)
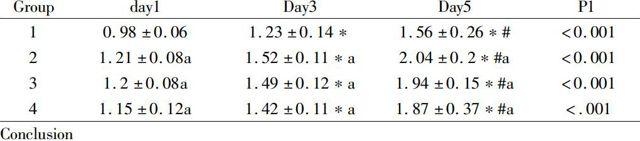
CCK-8 assay:HPDLCs were cultured in media containing different levels of hydrogel extracts for 1, 3and 5days, respectively, suggesting a significant role in promoting cell proliferation compared with Group 1, illustrates that the role of the hydrogel in HPDLCs is time-dependent and that different levels of hydrogel extracts can promote proliferation of HPDLCs. Compared with Group 1, differences were highly significant when at days 3 and 5 (P<0.001). A more marked proliferation-promoting effect was noted in Groups 2, 3 and 4 at days 3 and 5 compared with that at day 1 (P<0.001).
Group day1 Day3 Day5 P1 1 0.98±0.06 1.23±0.14* 1.56±0.26*# <0.0012 1.21±0.08a 1.52±0.11*a 2.04±0.2*#a <0.0013 1.2±0.08a 1.49±0.12*a 1.94±0.15*#a <0.0014 1.15±0.12a 1.42±0.11*a 1.87±0.37*#a <.001Conclusion
Th final goal of treatment of Periodontal disease is to achieve periodontal regeneration.Tissue engineering technique casts new light on effective periodontal regeneration and reconstruction, by which function-associated cells cultured in vitro are inoculated onto well-biocompatible extracellular matrix material and this cell-material complex, after a period of culture, will be implanted in the periodontal defects for the purpose of wound repair and tissue remodeling.Lots of literatures suggest that CS is characterized by good biocompatibility[1], nontoxicity[2] and biodegradability. CS and GP can prepare in situ forming, injectable thermosensitive hydrogels . Chenite et al. first obtained a neutral CS/GP complex, which remained liquid at room temperature for a long time, by mixing highly deacetylated CS with GP. The thermosensitive chitosan-based hydrogel prepared in this experiment is liquid at room temperature, with good fluidity. Sol-gel transition occurs if the CS/β-GP solution is placed at 37℃ for around 5 min, with pH is 7. Currently, considerable literature has showed that thermosensitive chitosan-based hydrogel is widely used in tissue engineering.Meanwhile, the CCK-8 assay was used for examining the effect of thermosensitive chitosan-based hydrogel on proliferation of PDLCs, suggesting that either high or low level of the extract could promote proliferation of PDLCs when determined at days 1, 3 and 5, respectively. In conclusion, the thermosensitive CS/β-GP hydrogel prepared in this study can be a good carrier for cells because of its nontoxicity to HPDLFs, with good biocompatibility.
Reference
[1]Molinaro G, Leroux JC, Damas J,etal. Biocompatibility of thermosensitive chitosan-based hydrogels: an in vivo experimental approach to injectable biomaterials. Biomaterials, 2002, 23: 2717-2722.
[2].Rao SB, Sharma CP. Use of chitosan as a biomaterial: tudies on its safety and hemostatic potential. Journal of biomedical materials research, 1997, 34: 21-28.
[3]. Muzzarelli RA. Biochemical significance of exogenous chitins and chitosansin animals and patients. Carbohydr Polym, 1993, 20: 7-16.
