A Fe-Ni-Cr system filler metal for brazing of stainless steel*
2015-09-05ZhaoHaishengXiongHuapingPanHuiandHuaiJunfeng赵海生熊华平淮军锋
Zhao Haisheng,Xiong Huaping,Pan Hui and Huai Junfeng赵海生,熊华平,潘 晖,淮军锋**
A Fe-Ni-Cr system filler metal for brazing of stainless steel*
Zhao Haisheng,Xiong Huaping,Pan Hui and Huai Junfeng
赵海生,熊华平,潘 晖,淮军锋**
New Fe-Ni-Cr system brazing alloyswere designed,in which elements Si and B aswell as Cu-Tibinary alloy were added as the temperature depressants.The brazing alloyswere fabricated into filler foilsby a rapidly-solidifying technique.It was found that,to acquire a suitable liquidus temperature of the filler alloy,the addition of Cu-Ti binary alloy decreased the needed amount of Si and B,and it had an effect on improvement in mechanical propertiesof the brazed joints.Based on the results ofmelting and wettability experiments,one fillermetalwas used to join stainless steel at1 140℃for 15 min.The microstructure of the joint was analyzed by means of a scanning electron microscope(SEM)equipped with X-ray energydispersive spectroscopy(EDS).Itwas found that the typical jointwasmainly composed of solid solution with a small quantity of Cr-rich borides strips,Ti-rich borideblocksand Cu-rich silicide particles.The brazed joints show an average tensile strength of 270.8 MPa and an average impact toughnessof35.6 J/cm2.
brazing,stainless steel,Fe-Ni-Cr fillermetal,microstructure,mechanical properties
0 Introduction
Austenitic stainless steelwith alloying elements of Cr and Nishows good corrosion resistance in any ofoxidizing,neutral or light reductivemedium.Due to its good plasticity and toughness aswell as cold-workability,the austenitic stainless steel has been widely used in the fields of automotive industry,food industry,medical apparatus and instruments,petrochemical industry and aerospace[1].The brazing technique of stainless steel covered awide range of fillermetals such as silver-based filler metal[2],manganese filler metal[3],nickel-based filler metal[4-5],goldbased fillermetal and copper filler metal[6].In a vacuum environment the majority of stainless steel brazing is carried out using nickel-based filler metals,but the gap clearances of the brazing jointsshould be strictly controlled to achieve good properties.Due to the fluctuating costs of raw materials such as nickel,new Ni-based filler metals with lower nickel contentor Fe-Cr-based fillermetalswere investigated recently[7-8].And in automotive industry,pure copper fillerwas previously used for the brazing of oil cooler[9],but the copper brazed jointswere easy to be corroded in an oilmedium,resulting in the failure of brazed oil coolers.Besides,for the stainless steel joints brazed with either Ni-based or copper filler,their mechanical strength and toughness remain to be improved in fact.
To solve the above problem,new types of Fe-Ni-Cr system fillermetalwere designed and fabricated in this paper.To ensure corrosion-and oxidation-resistance of these filler alloys,sufficient Ni and Cr were added in them. And elements of Si and B were added to lower theirmelting temperature.But the amount of Si and B should be strictly limited,because it is easy for them to combine with Ni to form brittle compound[6,10].According to the binary phase diagram,element Cu and element Ti can reactwith each other forming various lowmelting point intermetallics.So Cu and Tiwere added in these fillermetals to lower themelting temperature further and accordingly it can decrease the needed amount of Si and B.
1 Experiments
The experimental base metal is 1Cr18Ni9Ti stainless steel,and its nominal composition is shown in Table 1.New designed filler alloys were prepared by vacuum arcmeltingmethod,and subsequently the achieved ingots were broken up to pieces.Then every two pieces chosen randomly from these alloyswere heated to 1 140℃for15minutes in the ZKH-1 vacuum brazing furnace in order to study their melting behaviors and wettability on the base metal.In this paper,the selected alloy is named F46 with a nominal composition shown in Table 2.According to a differential thermal analysis(DTA),the solidus temperature and liquidus temperature of this filler alloy are 1 101℃and 1 124℃,respectively.The brazing foil of F46 filler metal was fabricated in a vacuum and argon-protecting furnacewith a rapidly-solidifying technique.Then,vacuum brazing was conducted with these foils under 1 140℃/15 min.
The mechanical properties of the brazed joints were tested and contrasted to the copper brazed joints under the same brazing and testing conditions.Themicrostructure of the joints brazed with F46 filler metal was analyzed by means of scanning electronmicroscope(SEM)and X-ray energy-dispersive spectroscopy(EDS),moreover the fracture location of the strength testing specimen was also observed.
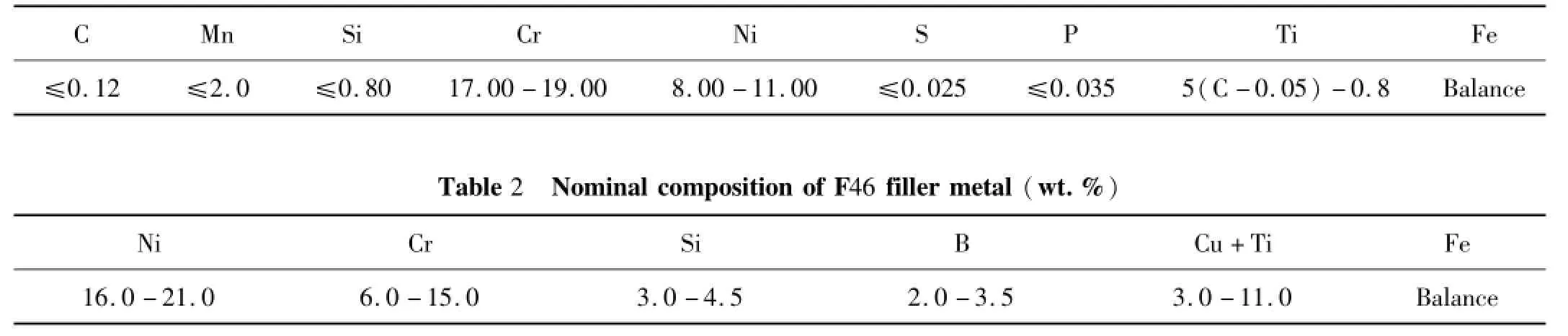
Table 1 Nom inal com position of 1C r18Ni9Ti stainless steel(w t.%)
2 Resu lts and discussion
2.1Microstructural exam ination
Fig.1 shows the microstructure of the typical joint brazed at 1 140℃for 15min with F46 filler metal.A sound joint has been achieved and it is composed of base metal,diffusion-reaction zone(Ⅰ),solid solution zone(Ⅱ)and central zone(Ⅲ).During the brazing procedure,the elements of B and Si diffused into the basemetal and reacted with Fe,Cr and Ni forming grayish phase(labeled“1”in Fig.1b).Near the interface in the basemetal,there exist some small black blocks which should be titanium carbides as labeled 2 shows.There are two solid solution zones between the diffusion-reaction zone“Ⅰ”and central zone“Ⅲ”.At the beginning of cooling stage after the brazing process,solid solution formed along the interfaces and grew into the seam center,accordingly low melting point liquids were pushed to the seam center and solidified with previous solution at a relatively low temperature[11].So the central zone is composed of solid solution and low melting point compounds of dark strips(microzone“3”),grayish blocks (microzone“4”),and a few of small black blocks(labeled“5”)as well as bright particles(labeled“6”). Thereby the central zone might be the weak zone determining themechanical properties of the joint.
EDS analysiswas carried out to detect the phase composition of the joint.According to the results shown in Table 3,the microzone“7”exhibited approximately the nominal chemical composition of the base metal,and microzone“8”in“Ⅱ”zone and microzone“9”in“Ⅲ”zone have similar compositions and are both Fe-based solid solutions with dissolved elements of Ni,Si,Cu and Cr,but the amount of Si in microzone“9”is a bit higher than that in microzone“8”due to its diffusion into base metal and gathering in the brazing seam center[12].Combined with Fe-Siand Ni-Si phase diagrams,the grayish phase in“Ⅰ”zone should be secondary(Fe,Cr,Ni,Si)SSin which Si is diffused from the fillermetal into the Fe-based solid solution.Because of the big size of silicon atom,it is hard to diffuse further into the basemetal,so zone“Ⅰ”is very thin,only about 10μm.The black blocks as labeled“2”shows in the basemetal near the interface contain lots of Tiand should be TiC compounds.In the“Ⅲ”zone,the dark strips contain lostof Fe,B and Cr,thereby theymay be(Fe,Cr)B according to the Fe-B and Cr-Bphase diagram.And the grayish blocks containingmore Si may be Si-rich Fe-based solid solution.The black blocks and bright particles in the center of“Ⅲ”zone are rich in Ti,B and Cu,Sielements respectively,then theymay be complex compounds such as(Ti,Fe,Cr)B2and(Cu,Fe,Ni)xSiy.

Fig.1 TheMicrostructure of the 1Cr18Ni9Ti brazed join t using F46 filler m etal w ith differentmagnifications

Table 3 EDS analysis of the typical phases and regions(at.%)
Element area distribution of the joint shown in Fig.1a is presented in Fig.2.Element Cr intends to reactwith element B to form CrB compounds as dark strips in“Ⅲ”zone.Most of Cu uniform ly distributed in the whole brazing seam,but only a few gathered in bright particles in“Ⅲ”zone.Then,almost all of element Ti concentrated in the compounds of black blocks only in“Ⅲ”zone.So a small quantity of Cu and Ti,added in the fillermetal,can form some compounds to lower the melting temperature of the fillermetal,and these small blocks and particlesmay strengthen the brazed joints to some extent.Because the size of Siatom is big and noteasy to diffuse,mostof them remained in the brazing seam and especially precipitated in“Ⅲ”zone.
2.2Mechanical properties
Table 4 shows the notched bar impact toughness and tensile strength of the brazed joints using F46 filler metal compared with those using copper filler under the same joining conditions.After 1 140℃/15 min brazing procedure,the average impact toughness of the joints using F46 fillermetal is 35.6 J/cm2,more than 3 times higher than that using copper fillermetal(10.8 J/cm2).In particular,an individual impact toughness value even reached 43.7 J/cm2with F46 filler metal.The average tensile strength of the F46 filler metal brazed joints is 270.8 MPa,about10%higher than that of copper brazed joints.
The fracture location of specimen brazed with F46 fillermetal is presented in Fig.3.Obviously,the fracture oc-curred at the weak zone“Ⅲ”which determines the mechanical properties of the joint.The edges of the fracture section are relatively rough and present ragged fracture pattern,indicating the relatively high strength and toughness.

Fig.2 Elem ent area distribution maps of the brazed joint using F46 filler metal
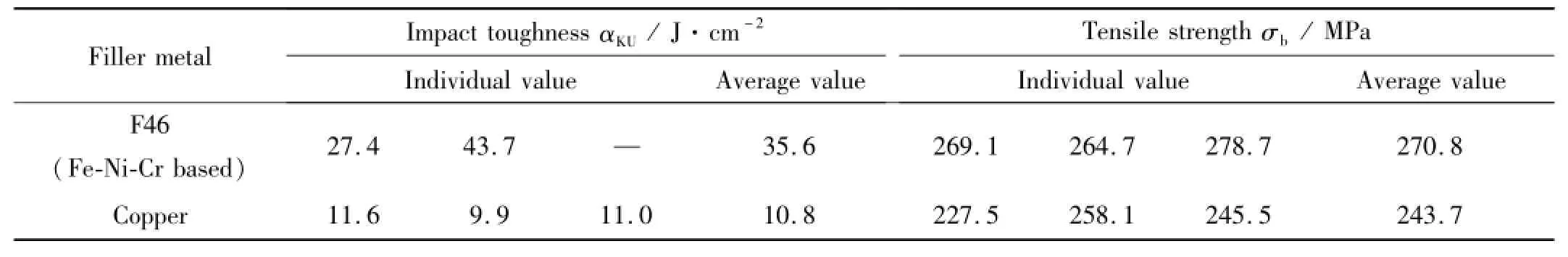
Table 4 Mechanical properties of the brazed join ts using F46 and copper filler metals
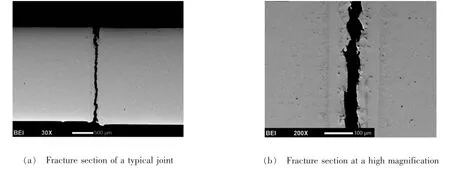
Fig.3 Fracture position of specimen
It is very important that the newly developed Fe-Ni-Cr system fillermetalmay be used to braze the oil coolers instead of pure copper fillermetal to solve corrosion failure problem and it should bemuch cheaper than the traditional Ni-based fillermetal.
3 Conclusions
Furnace vacuum brazing of1Cr18Ni9Tistainless steel was carried out under 1 140℃/15 min using new developed Fe-Ni-Cr system filler metals and sound joints were achieved in this paper.The mechanical properties of the brazed joints using new filler metal were evaluated compared with those using pure copper fillermetal,aswell as the microstructure examination in this study.Primary conclusions are summarized in the following:
(1)Typical joint ismade up with basemetal,diffusion-reaction zone,solid solution zone and central zone.
(2)The diffusion-reaction zone bases on primary Febased solid solution with secondary solution containing some Sielement,and the centralzone is composed of solid solution and compounds of Cr-rich boride strips,Ti-rich boride blocks and Cu-rich silicide particles.
(3)The brazed joints show an average impact toughness of35.6 J/cm2,more than 3 times of thatbrazed with copper filler,and an average tensile strength of 270.8 MPa,10%higher than that of copper brazed joints,under the same joining conditions.
(4)Fracture of specimen occurred at the central zone(zone“Ⅲ”)which determines themechanical properties of the joint.And the fracture edges are relatively rough and present ragged pattern indicating high strength and toughness.
References
[1] Zhang Q Y,Zhuang H S.Brazing and soldering manual. Beijing:China Machine Press,2008:199.(in Chinese)
[2] Pan H,Sun JS,Liu X F.Microstructure of brazemetal of stainless steel brazed with BAg25CuZn alloy.Journal of Aeronautical Materials,2000,20(3):94-97.(in Chinese)
[3] Ou C L,Liaw DW,Du Y C.Brazing of 422 stainless steel using the AWS classification BNi-2 braze alloy.Journal of Materials Science,2006,41(9):6353-6361.
[4] Yan G,Li N,Yan JZ,etal.Effects of fillermetalonmicrostructures of stainless steel brazed joint.Casting·Forging· Welding,2011,40(11):179-181.(in Chinese)
[5] Jing JN,Yu Z S,ChangW L,et al.Microstructure and microhardness of 316L stainless steel joint seam vacuum brazed by BNi7 Ni-Based fillermetal.Materials for Mechanical Engineering,2013,37(1):10-13.(in Chinese)
[6] Fan Y H,Yan J.Torch-Brazing of T2 copperwith 1Cr17Ni2 stainless steel.Development and Application of Materials,2009,24(3):42-45.(in Chinese)
[7] Ulrika P.New Iron-chromium based brazing filler metal for demanding stainless steel applications.Proceeding of the 4th International Brazing and Soldering Conference,Orlando,2009:125-129.
[8] Matsu K,Sawada T,Fukumoto S,et al.Mechanical properties of Fe-Cr based brazing fillermetals.Proceeding of 10th International Conference on Brazing,High Temperature Brazing and Diffusion Bonding Conference,Aachen,2010:48-51.
[9] Xu R H,Zhang W F,Lou H Y,et al.Corrosion resistance of pure copper brazed joint of oil cooler.Welding&Joining,2011,2:35-37.(in Chinese)
[10] Tokunaga T,Nishio K,Hasebe M.Thermodynamic study of phase equilibria in the Ni-Si-B system,basic and applied research:sectionⅠ.Journal of Phase Equilibria,2001,22 (3):294-298.
[11] Yeh M S,Chuang T H.Effects of applied pressure on the brazing of superplastic INCONEL 718 superalloy.Metallurgical and Materials Transactions A,1997,28(6):1367-1376.
[12] Grushko B,Weiss B Z.The BNi-5-Inconel 718“binary”system.Materials Science and Engineering,1982,74(1):19-27.
*Thiswork is sponsored by the National Natural Science Foundation of China(Grant No.51410105004).
**Zhao Haisheng,Xiong Huaping,Pan Hui and Huai Junfeng,Welding and Plastic Form ing Division,Beijing Institute of Aeronautical Materials,AVIC,Beijing,100095. Xiong Huaping,Corresponding author,E-mail:xionghuaping69@sina.cn
猜你喜欢
杂志排行
China Welding的其它文章
- Stress-strain characteristics of linear friction welding of TC11 and TC17 alloys*
- Effects of C/B4C ratio on Microstructure and property of Fe-based alloy coatings reinforced w ith in situ synthesized TiB2-TiC*
- Effects of Bi and rare earth metal on theMicrostructure and properties of Zn-based high-tem perature solder*
- Numerical analysis of keyhole establishment time in keyhole p lasma arc welding*
- Effect of pulse frequency on hardness characteristics of Al-Cu alloy HPVP-GTAW joints*
- Automatic defect detection in ultrasonic TOFD D-scan data using image processing m ethods*
