质子交换膜燃料电池非铂电催化剂研究进展
2015-08-20聂瑶丁炜魏子栋
聂瑶,丁炜,魏子栋
(重庆大学化学化工学院,重庆 400044)
引 言
质子交换膜燃料电池(PEMFCs)具有能量转换效率高、无污染的特点,非常适合作为绿色新能源汽车的动力能源[1]。燃料电池电汽车可以解决汽车工业发展带来的环境与能源问题,为汽车工业未来发展带来新的契机。然而,燃料电池的高成本问题是动力燃料电池大规模产业化与商业化道路上的巨大挑战。目前,燃料电池所使用的催化剂是Pt 基催化剂。根据2010年DOE年度报告,若以现有技术进行燃料电池汽车商业化,每年车用燃料电池对Pt资源的需求就高达1160 t,远远超过全球Pt 的年产量(约200 t)。从降低成本以及有限铂资源角度考虑,开发高活性非贵金属催化剂势在必行[1-2]。空气电极是燃料电池的正极,相对于负极氢电极,空气电极上的氧还原反应更为困难。在电流密度高达2 A·cm-2条件下,氢电极的过电位也不过50 mV。而空气(O2)电极,在这样大的电流密度下,即使以Pt 为催化剂,其过电位也达700~800 mV。因此,燃料电池的电压损失主要来自于空气电极。对氢电极,0.1 mg·cm-2或更少的Pt,即能满足燃料电池工作需要,燃料电池膜电极(MEA)上铂的需用量主要消耗在空气电极[3-6]。近年来,许多研究着眼于提高Pt 基阴极氧还原(ORR)催化剂的稳定性、利用率、改进电极结构以减少Pt 负载量,降低燃料电池成本[7]。但根本的出路应当是开发可以完全替代铂的、低成本的、资源丰富的非铂ORR 催化剂。本文结合本课题组的研究工作,综述了燃料电池非铂氧还原催化剂的最新研究进展。
1 钯基催化剂
金属Pd 具有储量丰富、价格便宜等优点,被视为铂的最理想替代金属[8-10]。然而,Pd 基催化剂的催化活性远不及铂类催化剂,无法满足商业化使用的要求。调节Pd 基催化剂的表面电子结构可使其获得与Pt 基催化剂相当的催化活性。通过与过渡金属如Fe、Ni、Au 等形成Pd 合金是一种有效调节Pd 电子结构的方法[11-12]。合金种类以及合金程度显著影响Pd 的电子结构,产生两种作用相异的效应,即晶格收缩效应和表面配位效应。其中,晶格收缩效应降低Pd 的d 带中心、减弱氧的吸附,被认为是活性提高的主要原因[11]。近年来,研究人员制备了多种活性组分的高分散钯基合金催化剂,在催化ORR 中显示了可与铂基催化剂相媲美的效果。Adzic 等[13]制备的Pd3Fe/C 催化剂,该催化剂的氧还原半波电位比商业化Pt/C 催化剂正约20 mV。Ding 等[14]以纳米多孔铜作为模板和还原剂合成了纳米管状PdCu 合金,与商业化Pt/C 和Pd/C 催化剂相比,PdCu 合金催化剂在酸性溶液中表现出更优异的ORR 性能和抗甲醇性能。FernaÂndez 等[15]研究了Pd-Co-Au/C 以及Pd-Ti/C 作为阴极ORR 催化剂在 PEMFC 中的表现。在相同负载量下,Pd-Co-Au/C 以及Pd-Ti/C 的初始性能表现可与商业化Pt/C 催化剂相媲美;200 mA·cm-2电流密度下持续12 h 后,Pd-Co-Au/C 性能明显衰减,而Pd-Ti/C性能基本没有变化。Xu 等[16]通过脱除PdTiAl 合金中的Al 制备了具有相互交联网状结构的纳米多孔PdTi 合金。该催化剂不仅表现出比Pt/C 更优异的氧还原和抗甲醇性能,而且在5000 次循环伏安(CV)老化实验中表现出较Pt/C 更优异的稳定性。DFT 理论研究表明,Ti 与Pd 合金化使Pd 的d 带中心下降,从而削弱了Pd-O 键能。
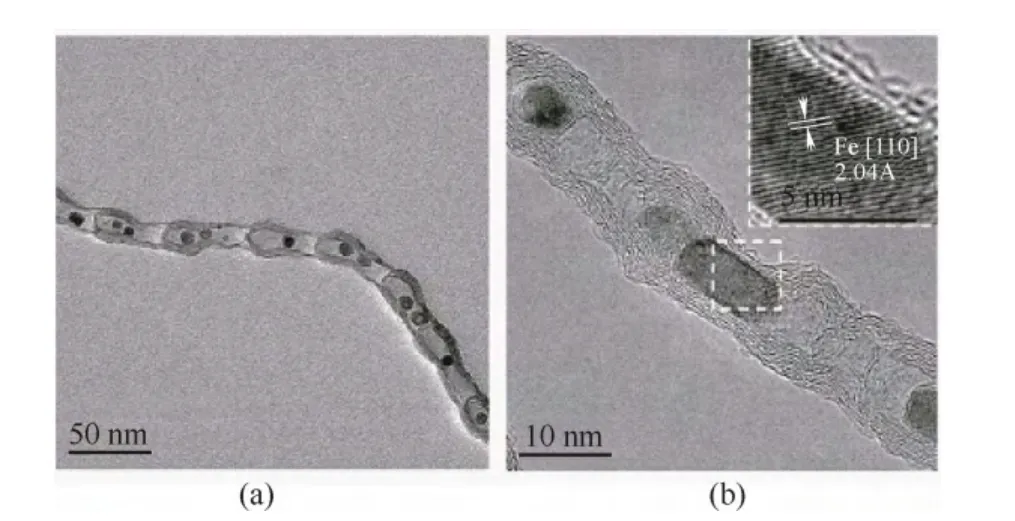
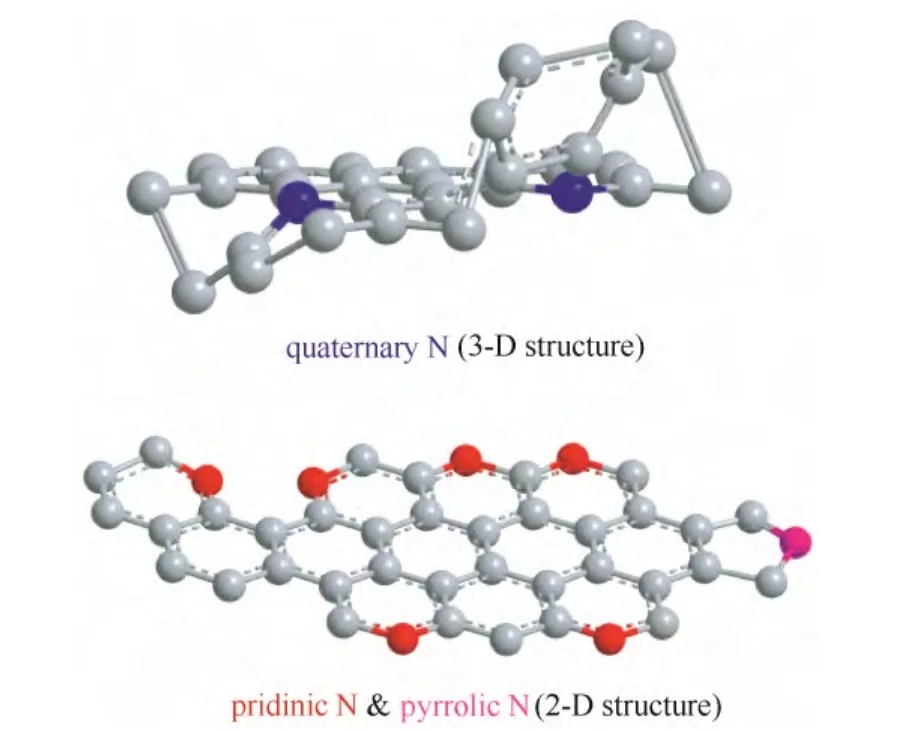
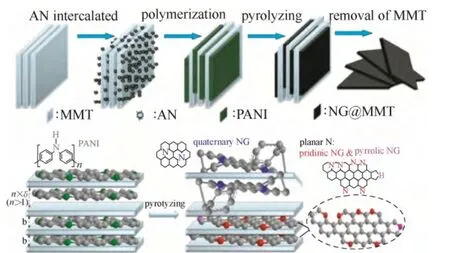
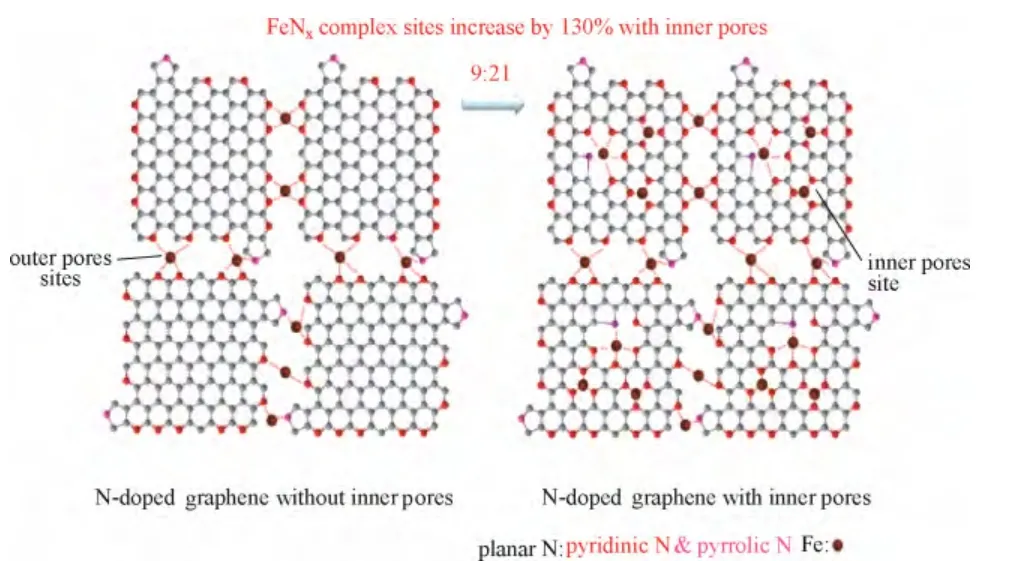
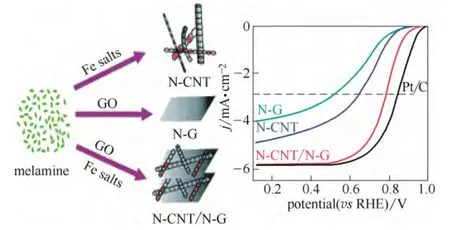
另外,Pd 的电子结构会随暴露的晶面改变而改变。因此,调控Pd 的纳米几何形态以暴露不同的晶面也是一种有效调节Pd 金属电子结构的方法[8,17-19]。Kondo 等[20]研究表明催化氧还原反应在以下Pd 单晶面上的活性递减,即Pd(110) 图1 Pd/C 四面体以及Pd/C 八面体在0.1 mol·L-1 HClO4溶液中的ORR 极化曲线Fig.1 ORR activities of Pd/C cubes and Pd/C octahedra in 0.1 mol·L-1 HClO4 利用载体和金属纳米颗粒之间的电子耦合效应也是优化金属纳米颗粒电子结构的一种手段。金属纳米颗粒在载体上可以暴露出多种复合位点,包括不同的晶面、边缘、棱角以及缺陷。这些复合位点会与载体产生较强的相互作用,从而对金属纳米颗粒的电子结构产生较大影响。Schalow 等[22]研究发现,在金属颗粒Pd 开始氧化时,在Pd 与载体Fe3O4的接触界面上形成了一层Pd 氧化物,并在载体的作用下稳定存在。该界面氧化物可以导致Pd电子状态或者是费米能级上升或下降,改变Pd 的电子结构。本课题组[23-24]通过采用具有单片层结构的剥离蒙脱土片(ex-MMT)负载纳米Pd 金属颗粒,调节Pd 催化剂的电子结构,增强稳定性和提高催化活性。蒙脱土的引入减少了因为碳载体的腐蚀而造成催化金属从载体脱落和流失的可能性,从而提高了催化剂的稳定性。此外,蒙脱土具有优异的质子传导能力,可加速质子在燃料电池催化层内部的传递,提高催化活性。电化学测试表明,Pd/ex-MMT具有与Pt/C 相似的催化活性(图2)。理论计算和实验数据表明催化剂活性、稳定性的提高是由于在Pd 金属颗粒与载体之间的界面上形成了一层界面氧化物PdOx或Pd-O-ex-MMT 价键。这种特殊的结构改变了Pd/ex-MMT 催化剂电子结构,使Pd 的d带宽化,d 带中心负移,使其具有更趋近于Pt 的电子结构,表现出与Pt 相当的ORR 催化活性以及酸性环境中良好的稳定性,如图2(a)所示。 图2 Pd 和Pd/ex-MMT 催化剂中的d 带结构与d 带中心以及Pd、Pd/ex-MMT 和Pt/C 在0.1 mol·L-1 HClO4 溶液中的ORR 活性Fig.2 D-band and relative center of Pt, Pd and Pd/ex-MMT; ORR activities of Pd/C, Pt/C and Pd/ex-MMT in 0.1 mol·L-1 HClO4 在众多非贵金属催化剂中,过渡金属-氮-碳化合物(M/N/C)因其具有可观的ORR 催化活性(在酸性溶液中)、低成本、寿命长、抗甲醇和环境友好等特点,被认为是最具潜力替代铂基催化剂的非贵金属燃料电池催化剂之一。自从1964年Jasinski[25]首次报道过渡金属卟啉和酞菁能有效催化ORR 后,M/N/C 便吸引了研究者的广泛关注。金属大环类催化剂具有较高的起始活性但稳定性较差[26]。高温处理后可提高催化剂的稳定性,但催化剂易烧结,导致比表面积减小,降低了催化剂的活性。该类催化剂主要以反应速率较慢的2 电子过程催化氧还原。Yeager[27]首次报道了以非-N4 大环化合物为前躯体高温热解制备M/N/C 催化剂用于 ORR。之后各种不同形式的金属、氮、碳前躯体被开发和应用于制备M/N/C 催化剂。目前该类催化剂使用的氮源主要包括无机氮源(氨气,sodium azide)、有机小分子(acetonitrile, pyrrole, 1-methylimidazole 等)和含氮有机聚合物(melamine resin,聚苯胺,聚吡咯,聚多巴胺等)[28-42]。与小分子前驱物相比,含氮有机聚合物有序化更高,可以在高温热解过程中指导形成更有序稳定的碳基活性层。聚吡咯是最早被应用的聚合物,之后研究发现聚苯胺-衍生的M/N/C 催化剂活性更好且更稳定[43]。最近,Wu 等[37]报道用聚苯胺结合铁和钴的热处理制备一类M/N/C(图3)。该类催化剂中催化活性最高的催化剂为PANI-Fe-C,其ORR 半波电位与Pt/C 相差60 mV;稳定性最优的催化剂为PANI-FeCo-C,其在0.4 V下稳定运行了700 h。Dodelet 等[33]于2011年报道了一种金属框架类作为前驱体制备的M/N/C,该前驱物具有优异的金属-有机配位结构,在经过两次热处理(一次在氮气气氛下1 h,再次为NH3气氛中15 min)后,该催化剂表现出了优异的催化性能,在0.8 V 下其体积活性高达230 A·cm-3(iR-free)[33],已经非常接近DOE 2020年所设定的目标(300 A·cm-3)。 图3 PANI-FeCo-C 催化剂的制备Fig.3 Schematic diagram of synthesis of PANI-M-C catalysts 此类催化剂的催化机理和活性中心尚不明确,一直是研究的重点。目前,有两条研究主线:① 催化剂表面的氮活性物种直接提供ORR 活性;② 含氮基团与金属配位成为活性中心。虽然此类材料的催化机理仍存在争论,但不能否认的是,过渡金属的类型和含量,碳源、氮源的类型与含量,以及热处理条件和持续时间对催化剂的性能有很大影响。许多研究工作致力于探究制备工艺条件与最终ORR 性能的关系[44-47]。就不同金属种类来说,Fe和Co 基M/N/C 催化剂活性一般比其他金属基(如Zn、Ni、Mn、Cu、Cr)M/N/C 催化剂活性高[48]。而且,不同金属的加入对活性位点形成所起的作用也不同。如对于有乙二胺或聚苯胺衍生的Co/N/C 催化剂,其表现的电化学性能(如起始电位、Tafel斜率)与无金属掺杂的氮掺杂碳基催化剂类似,这意味着Co 物种的存在可能只是单纯辅助氮原子更好地掺入碳晶格中,并不直接参与形成活性中心[41]。与Co 不同的是,Fe 物种可以与周围的氮配位(Fe-Nx),直接参与形成活性中心[30,44]。Kramm等[49]和Kattle 等[50]提出了几种不同的Fe-Nx物种,其中,FeN4/C 和 N-FeN2+2/C 位点ORR 活性最高。实验研究表明,同时加入Fe、Co 物种可以显著增强催化剂ORR 活性[51]。Xia 等[52]利用DFT 证明对于聚苯胺衍生的M/N/C 体系,其催化活性衰减次序依次为:CoFe-PANI > Fe-PANI > Co-PANI。这是由于掺入的不同金属之间产生了协调作用,加快了电子向吸附氧物种的转移。Co 的加入可能还降低了催化剂中最高占据分子轨道(HOMO)-最低占据分子轨道(LUMO) 带,使得催化剂更加稳定。 除了催化剂机理不明确,传统热解方法制备的M/N/C 还存在孔结构少,比表面积低,暴露的活性位点有限等缺点。在M/N/C 中引入足够的活性位点,最常规的方法便是通过硬模板或柔模板增加催化剂的比表面积,如Liang 等[53]以硅胶球、介孔硅和蒙脱土为模板,VB12或PANI 为前驱体,制备了介孔的Fe/Co-N-C 材料,显著提高了催化剂的比表面积。 在M/N/C 催化剂高温制备过程中,金属颗粒通常会包覆在石墨化碳壳中,而被包覆的金属对催化活性的贡献已被探究[54]。包信和等[31,55-56]的一系列研究表明,当金属纳米颗粒限域在碳纳米管中时(如图4 所示,是他们制备的金属铁纳米粒子包裹在豆荚状氮掺杂纳米管催化剂),金属颗粒不与酸性介质、氧和硫等污染物直接接触,也不妨碍活化氧分子电催化氧还原反应,它们之间特殊的电荷转移降低了碳纳米管表面的局部功函从而形成ORR 电催化活性中心。本课题组[47]开发了一种Co-N-C 壳层包覆钴纳米颗粒催化剂(Co@Co-N-C),其中高分散的Co@N-C 和表面Co-N 物种产生的电子效应协同增强了氧还原活性,如图5 所示。最近,Li 等[57]制备了一种空心球形的石墨碳层包覆Fe3C 纳米催化剂。包覆在内部的Fe3C 纳米颗粒虽然没有与外界电解液直接接触,但它们却使得周围的石墨化碳层活化而更有利于ORR 的发生和进行,这与包信和等的研究结果类似。此外,该催化剂表面的氮和金属含量极少可忽略,却在酸性和碱性溶液中表现出很好的ORR 活性,为此类包覆型催化剂活性位点的探究提供了新的模型。 图4 Pod-Fe 催化剂的透射电镜图Fig.4 TEM images of Pod-Fe 图5 Co@Co-N-C 催化机理Fig.5 Schematic diagram of ORR on Co@Co-N-C 过渡金属氧化物,尤其是锰基和钴基氧化物在碱性溶液中表现出很好的催化氧还原活性[58-60]。Dai等[61-62]通过水热法制备了Co3O4、CoO 纳米颗粒并担载于氮掺杂碳类载体上(CNT,石墨烯),协同增强氧还原活性。通过X 射线近边吸收精细结构分析可知,该催化剂形成了金属-碳-氧和金属-碳-氮共价键,电子由氮传至金属氧化物,从而赋予了金属氧化物好的导电性和电化学活性。将不同价态的过渡金属氧化物复合形成尖晶石结构的催化剂是过渡金属氧化物催化剂研究的重点。Dai 等[63]发现,用Mn3+取代部分 Co3+得到的具有尖晶石结构的MnCo2O4可以显著增强氧还原活性。Sun 等[64]通过热解乙酰丙酮盐前驱体,油胺油酸作稳定剂,制备了单分散、粒径小于10 nm 的= Fe, Cu, Co, Mn)纳米颗粒。这些纳米颗粒即便担载在传统碳载体上,也表现出与Pt/C 相当的氧还原催化活性。近期,本课题组[65]开发了一种新型钴基催化剂,碱式碳酸钴(CCH),并发现催化剂相比于贵金属催化剂Pt/C 具有更优的氧还原催化性能。研究还发现,随着水热时间的延长,所制备的催化剂发生了明显的相变和形变,并具有不同的催化活性,如图 6 所示。其中正交相的碱式碳酸钴[Co(CO3)0.5(OH)·0.11H2O]同由单斜[Co2(OH)2CO3]和正 v c 交相组成的混合相的碱式碳酸钴相比具有更高的氧还原活性。 图6 碱式碳酸钴催化机理以及反应时间对碱式碳酸钴 在0.1 mol·L-1 KOH 中ORR 活性的影响Fig.6 Schematic diagram of ORR and OER on CCH in presence of carbon powders and influence of reaction time on ORR activity for CCH in 0.1 mol·L-1 KOH 其他金属氧化物,如TiO2、NbO2和 Ta2O5也具有ORR 催化活性[66-68]。近年来,钙钛矿型氧化物因其同时具有电子和离子导电性,越来越多地用作高温燃料电池中的氧还原催化剂。钙钛矿型氧化物ABO3中稀土元素占据A 位,过渡金属占据 B 位。其中,通过阳离子取代很容易调控Ba0.5Sr0.5Co0.8Fe0.2O3-δ(BSCF5582)-基钙钛矿型氧化物组成,BSCF5582 被认为是此类材料中最具潜力的氧还原催化剂[69]。Suntivich 等[69]提出钙钛矿型氧化物在燃料电池中的氧还原活性与eg(σ*-轨道占据)和A-B-O 型中的B 位密切相关,且eg-填充接近1的钙钛矿型氧化物可以表现出最好的氧还原活性。最近,Risch 等[70]采用脉冲激光沉积法制备了BSCF|LSMO|NSTO 催化剂,表现出很好的氧还原和析氧(OER)活性。 过渡金属硫属化合物M-X(其中,M=Co, Ru, Re, 或 Rh,X=S, Se, Te)高温处理后能形成纳米微晶[71],在酸性介质中具有高的ORR 催化活性[72-73]。金属硫化物(如Co9S8)被认为是硫属化合物中活性ORR最高的一类[74]。DFT 研究表明,在Co9S8中,氧气的吸附是在硫元素上,且氧气在(202)晶面上还原的过电势与Pt 相当[74]。此外,Co1-xS、Co4S3、CoSe2[73,75-77]等在碱性溶液中均可以表现出近4 电子过程,然而在酸性溶液中,这类催化剂通常表现为2 电子过程。Wu 等[78]开发的 Co9S8-N-C 催化剂,在0.1 mol·L-1NaOH 溶液中,其ORR 活性明显优于Pt/C 催化剂。Wang 等[79]以还原氧化石墨烯负载Co1-xS 纳米颗粒,协同增强ORR 活性。 过渡金属氮化物和氧氮化合物由于其较好的导电性和耐腐蚀性也被广泛应用于ORR。表面氮化物的形成可以调控催化剂的电子结构,使得d-带收缩,电子密度增大更接近费米能级。这样加快了电子向氧吸附物种的转移,从而使得活性金属更容易还原氧[80]。之前,4~6 主族的单金属氮化物/氧氮化合物被广泛研究[81-84],如ZrOxNy和TaOxNy,它们在硫酸溶液中有很好的电化学稳定性; MoN 和Mo2N 表现出可观的ORR 活性且反应接近4 电子过程。之后研究者们开发了双金属氧氮化合物并发现它们发挥了协同增强的优势。如碳担载双金属 Co-W-O-N 催化剂在0.5 mol·L-1H2SO4中ORR 起始电位为 0.749 V, 显著优于单金属 W 或 Co 氧氮化合物催化剂[85]。最近,Cao 等[86]采用溶液浸渍法合成了CoxMo1-xOyNz催化剂,其在酸中表现出可观的氧还原活性,其在碱性溶液中活性与Pt/C 相差0.1 V。 非金属催化剂的研究主要是各种杂原子掺杂的纳米碳材料,主要包括硼掺杂、氮掺杂、磷掺杂、硫掺杂以及多原子的双掺杂或三掺杂[87-101]。研究表明,碳材料掺杂后,无论是否与过渡金属复合,都显示出明显的氧还原催化活性。目前关于不同原子掺杂碳材料的催化剂机理仍不明确。Dai 等[88]认为,对于氮原子掺杂碳材料,由于氮原子电负性较碳原子大(氮电负性为3.04;碳电负性为2.55),它的引入使得邻近碳原子带正电荷,这有利于氧气的吸附从而保障氧还原反应的进行。然而这种解释并不适用于电负性较碳原子小的磷原子和硼原子(磷电负性为2.19;硼电负性为2.04)。Hu 等[87]认为,无论掺杂原子的电负性与碳原子相比是大还是小,只要破坏了sp2杂化的碳原子的电中性,生成了利于氧吸附的带电位点就可以提升催化剂活性。对于电负性与碳接近的硫原子(硫电负性为2.58),Zhang等[102]认为其催化活性增强的原因是自旋密度变化改变了表面电子结构。 图7 氮在石墨结构中掺入位置以及相应的结合能数据Fig.7 Schematic representation of common N bonding configurations. 各类杂原子掺杂碳类材料中,氮掺杂碳(NC)研究最多。氮原子的分子结构对最终催化剂的性能具有至关重要的影响。掺氮碳材料中,氮有5 种键合结构,如图7 所示,分别为石墨氮、吡啶氮、吡喏氮、氨基氮以及氧化氮。哪一种掺氮碳材料氧还原电催化活性最好,目前尚有争议。吡啶氮掺杂的石墨烯,其ORR 的过程系2 电子还原过程,据此认为吡啶氮不是有效的ORR 催化中心[103]。与此相反,还有发现,酸性条件下催化剂氧还原活性随吡啶氮含量增加而升高[104];在碱性介质,其电催化活性随吡喏氮含量增加而升高[105]。故氮掺杂碳材料的活性中心,须考虑如下要点:首先氮键合结构不同时,其催化剂的导电性是否处于同一水平;再者催化剂中sp2杂化C 含量、石墨化程度是否一致。通常,石墨氮形成的温度较高,更有利于碳材料石墨化,也影响着材料的导电性和sp2杂化C 结构。因此,“高石墨氮含量-高ORR 活性”可能与碳基材料的导电性有关。除了氮的分子结构类型,掺入氮的总含量、碳边缘位的含量、比表面积等也是影响最终NC 催化剂性能的重要因素。 纳米碳材料氮掺杂的方法大致可分为3类[106-108]:①原位掺杂,即在纳米碳材料期间掺入氮,如化学气相沉积法(CVD),这种方法得到的产品掺杂率很高,但不适用于实际大规模批量生产;②后掺杂,即合成纳米碳材料后,再用含氮原子的前驱体对其进行后处理,这种方法得到的产品氮掺杂率不高;③直接热解含氮原子丰富的有机物,这种方法简单易操作,得到的产品掺杂率高,然而由于过高的含氮量,破坏了碳材料原共轭大π 键结构,使得产品电导率低。Bao 等[109]报道了大批量高质量氮掺石墨烯的方法,如图8 所示。其采用溶剂热反应将四氯化碳和氮化锂直接反应生成氮掺杂的石墨烯(NG),实现了克量级制备氮掺杂石墨烯。 图8 溶剂热法氮掺杂石墨烯制备及产品的电镜照片Fig.8 Schematic representation of solvothermal synthesis of NG and TEM of NG 设计、制备含氮量高、导电性好且比表面积大的氮掺杂碳材料是提高氮掺杂类碳材料性能亟需解决的问题。通常采用软模板或硬模板法可以显著增加催化剂的比表面积。如通过多孔二氧化硅模板辅助法[110]、热解具有优异金属配位效应的金属有机框架化合物(MOFs)或多孔有机聚合物(POP)制备得到的NC 材料[111-113],氮含量高,且比表面积大,然而在酸性溶液中,它们的氧还原活性与Pt 相比仍相差很远。这是因为,在酸性介质体系中,平面结构的吡啶氮和吡喏氮氧还原电催化更为重要[114-116]。吡啶型和吡咯型的二维平面结构使NG 保持了石墨烯原有的平面共轭大π 键结构,具有良好的导电性,因而具有优异的ORR 催化活性;而石墨型氮为三维空间不平整结构,破坏了石墨烯原有的共轭大π键,导电性差,ORR 催化活性低,如图9 所示。如何在高度石墨化的条件下选择性的合成具有平面构型的吡啶氮和吡喏氮(平面氮)并尽可能减少甚至 抑制石墨氮的形成则是获得高活性ORR 催化剂的关键。 图9 石墨氮和平面N 示意图Fig.9 Schematic representation of quaternary N and planar N 针对上述问题,本课题组[117]在分子结构的基础上,认识到“NG 分子结构-NG 电导率-ORR 催化活性”的关联,利用层状材料(LM)的层间限域效应,通过调制LM 层间距,在LM 层间插入苯胺单体,层间聚合,然后热解的方法,获得平面氮掺杂达90%以上的NG 材料,如图10 所示。其催化ORR 的半波电位仅比Pt/C 催化剂落后60 mV,是传统方法下获得的NG 材料ORR 催化活性的54 倍,以该材料为正极催化剂的质子交换膜燃料电池的输出功率达320 mW·cm-2,如图11 所示。LM 层间近乎封闭的扁平反应空间不仅克服了传统开放体系下合成的NG 以石墨型为主,导电性差,活性低的弊病,而且也克服了开放体系下因掺N 效率低而导致合成NG 成本高的问题。 除了增加活性位点数量,活性位点充分暴露在三相界面也是非常重要的。氧还原反应是一个多相反应,涉及氧气,质子、电子和水的传导,因而一个高效的ORR 催化剂须含有足够多的小孔以承载活性位点,同时这些小孔还需联通至能有效传输反应气体、生成水、电子导体以及质子导体的中孔或大孔网络结构中。然而对于传统直接热解前驱体的方法,难以控制所制备的催化剂的孔结构,导致活性位点难以暴露到可以被ORR 催化反应利用的区 域中[118-120]。 图10 NG@MMT 制备Fig.10 Schematic representation of NG@MMT synthesis 图11 NG@MMT 在0.1 mol·L-1 HClO4 中ORR 极化曲线以及以NG@MMT 为阴极催化剂制备的MEA 单电池测试 极化曲线Fig.11 ORR activity in 0.1 mol·L-1 HClO4 and MEA test of NG@MMT catalyst 图12 基于形态控制通过盐重结晶方法的示意图和 MEA 单电池测试极化曲线Fig.12 Schematic diagram of “Shape Fixing via Salt Recrystallization” method and result of MEA test 图13 PANI 三维网状、PANI 纳米管、PANI 纳米壳 以及其相应碳化后产品的扫描电镜图Fig.13 SEM images of 3D PANI network, PANI nanotubes, PANI nanoshell, and their corresponding carbonized products 上述本课题组设计的扁平纳米反应器制备平面氮掺杂的石墨烯,可有效地提高催化活性位的密度,增加反应界面。但由于缺少传质通道,在制备成膜电极(MEA)后其活性位暴露的概率大大降低,影响了电池性能。在此工作的基础上,本课题组进一步开发了一种基于形态控制转换纳米聚合物制备高效氧还原碳纳米材料催化剂的方法——“NaCl重结晶固型热解法”[121],可以有效地使大量的活性位暴露在ORR 催化反应的三相界面上,制备过程如图12 所示。通过对含氮聚合物无机盐水溶液混合物的蒸发重结晶,将含氮聚合物固化在无机盐NaCl晶体中,利用无机盐结晶的盐封效应,避免了传统直接碳化过程中活性位严重烧失、高石墨氮掺杂和结构坍塌等问题;避免了传统模板法模板去除与纳米催化剂分离的困难问题;巧妙地将低温下聚合物的形态最大限度地保留到高温碳化后的终极产品,如图13 所示。此外,由于盐封局域空间的限域效应,掺氮石墨烯中以具有二维平面结构吡啶型和吡咯型为主,最大限度地抑制了撑开型石墨氮掺杂型NG;同时,由于盐封效应,在碳化过程中NG 内部形成大量的气蚀孔,NG 片边沿和及内孔边沿的大量存在,有利于吡啶型和吡咯型氮参杂NG 的形成,使活性中心数量倍增。如图14、图15 所示,与没有微孔生成的对比样品相比,以NaCl 固型热解法制备得到的催化剂其平面氮含量增加了68%,FeNx位点增加了130%。大量的活性位点结合高效的传质量通道使活性位暴露在三相界面的概率增高从而极大地提高活性位点的利用率。以该材料为正极催化剂的质子交换膜燃料电池输出功率达 600 mW·cm-2,较之前以扁平纳米反应器制备平面氮掺杂的石墨烯有大幅提高,为世界领先水平。加速老化实验显示该催化剂非常稳定。该方法具有广泛的应用性和通用性,并可以有效地控制碳材料的孔结构、活性位点以及纳米形貌。 图14 边缘位及孔内平面氮活性位点示意图Fig.14 Schematic diagram of planar N active sites on edges and in pores 图15 边缘位及孔内FeNx 活性位点示意图Fig.15 Schematic diagram of FeNx active sites on edges and in pores 碳材料之间的复合也是一种有效制备非金属催化剂的方法。Chen 等[122]在氧化石墨烯表面,以Fe 催化三聚氰胺热解,实现Fe-N 同时掺杂石墨烯和碳纳米管的同步合成路线(N-CNT/N-G),如图16 所示,该复合催化剂中纳米管分散均匀,管径均一,其特殊的3D 结构有利于提高传质和电催化活性。最近,Wei 等[123]以FeMo-MgAl 层状双氢氧化物为模板,采用CVD 法制备了氮掺杂石墨烯/单壁碳纳米管复合物(NGSHs)。FeMo-MgAl 层状双氢氧化物中的Fe 纳米颗粒不仅可以作为氮掺杂单壁碳纳米管生长的催化剂,还可以作为氮掺杂石墨烯沉积的基底。以此制备得到的NGSHs 催化剂具有 高比表面积和高石墨化程度。研究发现,NGSHs复合物表现出比其单组分更好的氧还原活性,因而氮掺杂石墨烯与单壁碳纳米管的复合很有可能协同增强最终氧还原活性。 图16 N-CNT/N-G 制备路线及其在0.1 mol·L-1 KOH 中氧还原活性Fig.16 Schematic illustration of formation of N-CNT/N-G and ORR activity of N-CNT/N-G in 0.1 mol·L-1 KOH 值得指出的是,大多数使用的碳材料在制备过程中都有一些金属的参与,如通过 Hummers 法制备氧化石墨烯,CVD 法制备碳纳米管和以生物或自然材料为前躯体或模板制备碳材料。以Hummers 法制备氧化石墨烯为例,最终石墨烯产品中的金属杂质可达到整个材料的2%(质量分数)[124-129]。这些残留在sp2碳材料中的金属杂质包括Fe、Ni、Co、 Mo、Mn、V 和 Cr,它们可以很大程度上影响最终碳材料的电化学性能[130-134]。因而,在制备过程卷入的痕量金属(trace metal)对最终催化剂的氧还原活性的影响是不能忽略的。Masa 等[135]证明了无定形碳中的痕量金属残余对ORR 活性是有贡献的。研究表明,在整个制备周期不涉及任何金属参与的非金属催化剂,其ORR 活性低于制备时有少量金属参与的催化剂。而且,加入低至0.05% 含量的Fe 就会对最终ORR 活性和选择性有很大影响。最近,Pumera 等[136]研究了痕量金属杂质对杂原子掺杂石墨烯ORR 性能的影响。为了探究锰基金属杂质的影响,他们采用Hummers 氧化法(得到的产品标记为G-HU) 和Staudenmaier 氧化法(利用氯酸盐氧化剂制备肼还原的石墨烯,得到的产品标记为G-ST)制备两组不同的石墨烯材料,并且利用耦合等离子质谱法 (ICP-MS)分析制备原料及产品中的金属杂质含量。研究结果表明,富含锰基杂质(>8000 mg·kg-1)的G-HU 催化剂的氧还原起始电位比含有少量锰基杂质(约18 mg·kg-1)的G-ST 催化剂正50 mV,有力证明了锰基杂质的ORR 催化作用。此外,即便是锰基杂质含量低至18 mg·kg-1(0.0018%,质量分数),G-ST 催化剂表现的ORR 电位比裸露的玻碳电极(GC)正80 mV,进一步证明痕量金属杂质足以改变石墨烯材料的氧还原电催化性能。 (1)由于钯具有与铂相媲美的催化性质,且Pd储量远高于Pt,因而开发高效Pd 基催化剂是替代Pt,降低商业化成本的有效途径。然而,迄今为止,在酸性条件下,Pd 基催化剂的活性和稳定性很难与铂基催化剂相当。此外,由于需求/价格波动的关系,用Pd 基催化剂完全替代Pt 不能从根本上摆脱贵金属的资源限制。 (2)非贵金属催化剂和非金属催化剂完全摆脱了对贵金属的依赖。在众多的非贵金属催化剂中,包含或不包含过渡金属的氮掺杂碳基催化剂 (M/N/C 或 NC) 表现出可观的ORR 催化活性。尽管对于金属物种是否直接参与形成活性中心仍存在争议,但碳结构中氮原子的掺入对提高ORR 活性的作用是不可否认的。基于当前对非铂催化剂的理论认识和实验探究,非铂催化剂的催化活性已有大幅提高,但其稳定性仍与Pt 基催化剂有很大差距。探究非金属催化剂的活性与原子组成、电子构型、表面形貌的构效关系,结合理论计算在分子、电子水平确定非金属催化剂的活性位点,开发提高活性位密度的技术,构筑高效新型非铂催化剂结构,提高催化剂的稳定性,是未来非铂氧还原催化剂研究发展的主要方向。 [1]Yi Baolian(衣宝廉).Fuel Cells—Principle, Technologies and Applications (燃料电池——原理·技术·应用)[M].Beijing: Chemical Industry Press, 2003. [2]Bashyam R, Zelenay P.A class of non-precious metal composite catalysts for fuel cell [J].Nature, 2006, 443: 63-66. [3]Xiong W, Du F, Liu Y.3-D carbon nanotube structures used as high performance catalyst for oxygen reduction reaction [J].J.Am.Chem.Soc., 2010, 132: 15839-15841. [4]Snyder J, Fujita T, Chen M W.Oxygen reduction in nanoporous metal-ionic liquid composite electrocatalysts [J].Nat.Mater., 2010, 9: 904-907. [5]Lim B, Jiang M, Cho E C.Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction [J].Science, 2009, 324: 1302-1305. [6]Chen Z W, Waje M, Li W Z.Supportless Pt and PtPd nanotubes as electrocatalysts for oxygen-reduction reactions [J].Angew.Chem.Int.Ed., 2007, 46: 4060-4063. [7]Nie Y, Li L, Wei Z D.Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction [J].Chem.Soc.Rev., 2015,44: 2168-2201. [8]Jukk K, Alexeyeva N, Ritslaid P, Kozlova J, Sammelselg V, Tammeveski K.Electrochemical reduction of oxygen on heat-treated Pd nanoparticle/multi-walled carbon nanotube composites in alkaline solution [J].Electrocatalysis, 2013, 4(1): 42-48. [9]Sha Y, Yu T H, Merinov B V.Oxygen hydration mechanism for the oxygen reduction reaction at Pt and Pd fuel cell catalysts [J].J.Phys.Chem.Lett., 2011, 2(6): 572-576. [10]Antolini E.Palladium in fuel cell catalysis [J].Energy Environ.Sci., 2009, 2(9): 915-931. [11]Suo Y, Zhuang L, Lu J T.First-principles considerations in the design of Pd-alloy catalysts for oxygen reduction [J].Angew.Chem.Int.Ed., 2007, 46(16): 2862-2864. [12]Wei Y C, Liu C W, Wang K W.Improvement of oxygen reduction reaction and methanol tolerance characteristics for PdCo electrocatalysts by Au alloying and CO treatment [J].Chem.Commun., 2011, 47(43): 11927-11929. [13]Shao M H, Sasaki K, Adzic R R.Pd-Fe nanoparticles as electrocatalysts for oxygen reduction [J].J.Am.Chem.Soc., 2006, 128(11): 3526-3527. [14]Xu C, Zhang Y, Wang L, Xu L, Bian X, Ma H, Ding Y.Nanotubular mesoporous PdCu bimetallic electrocatalysts toward oxygen reduction reaction [J].Chem.Mater., 2009, 21(14): 3110-3116. [15]FernaÂndez Jose L, Raghuveer Vadari, Manthiram Arumugam, Bard Allen J.Pd-Ti and Pd-Co-Au electrocatalysts as a replacement for platinum for oxygen reduction in proton exchange membrane fuel cells [J].J.Am.Chem.Soc.,2005, 127(38) : 13100-13101. [16]Liu Y, Xu C.Nanoporous PdTi alloys as non-platinum oxygen-reduction reaction electrocatalysts with enhanced activity and durability [J].ChemSusChem, 2013, 1(6): 78-84. [17]Shao M, Yu T, Odell J H.Structural dependence of oxygen reduction reaction on palladium nanocrystals [J].Chem.Commun., 2011, 47(23): 6566-6568. [18]Shao M, Odell J, Humbert M.Electrocatalysis on shape-controlled palladium nanocrystals: oxygen reduction reaction and formic acid oxidation [J].J.Phys.Chem.C, 2013, 117(8): 4172-4180. [19]Zhang L, Hou F, Tan Y W.Shape-tailoring of CuPd nanocrystals for enhancement of electro-catalytic activity in oxygen reduction reaction [J].Chem.Commun., 2012, 48(57): 7152-7154. [20]Kondo S, Nakamura M, Maki N, Hoshi N.Active sites for the oxygen reduction reaction on the low and high index planes of palladium [J].J.Phys.Chem.C, 2009, 113(29) : 12625-12628. [21]Xiao L, Zhuang L, Liu Y, Lu J, Abruna H D.Activating Pd by morphology tailoring for oxygen reduction [J].J.Am.Chem.Soc., 2009, 131(2) : 602-608. [22]Schalow T, Brandt B, Starr D E, Laurin M.Size-dependent oxidation mechanism of supported Pd nanoparticles [J].Angew.Chem.Int.Ed., 2006, 45(22): 3693-3697. [23]Ding W, Xia M, Wei Z, Wan L.Enhanced stability and activity with Pd-O junction formation and electronic structure modification of palladium nanoparticles supported on exfoliated montmorillonite for the oxygen reduction reaction [J].Chem.Commun., 2014, 50: 6660-6663. [24]Xia M R, Ding W, Wei Z D.Anchoring effect of exfoliated-montmorillonite-supported Pd catalyst for the oxygen reduction reaction [J].J.Phys.Chem.C, 2013, 117 (20) : 10581-10588. [25]Jasinski R.A new fuel cell cathode catalyst [J].Nature, 1964, 201: 1212-1213. [26]Beck F.Redox mechanism of chelate-catalyzed oxygen cathode [J].J.Appl.Electrochem., 1977, 7: 239-245. [27]Yeager E.Electrocatalysts for O2reduction [J].Electrochim.Acta, 1984, 29(11): 1527-1537. [28]Liu H, Song C, Tang Y, Zhang J.High-surface-area CoTMPP/C synthesized by ultrasonic spray pyrolysis for PEM fuel cell electrocatalysts [J].Electrochim.Acta, 2007, 52(13) : 4532-4538. [29]Ren Qizhi(任奇志), Ma Xiaoxia(麻晓霞), Xie Xianyu(谢先宇),Yan Tao(阎陶), Ma Zifeng (马紫峰).Heat-treated metalloporphyrin compounds supported on different carbons as electrocatalyst for oxygen reduction [J].Journal of Chemical Industry and Engineering(China)(化工学报), 2006, 57(11): 2597-2603. [30]Lefèvre M, Proietti E, Jaouen F, Dodelet J P.Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells [J].Science, 2009, 324: 71-74. [31]Deng D, Yu L, Chen X, Wang G, Jin L, Pan X, Deng J, Sun G, Bao X.Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction [J].Angew.Chem.Int.Ed., 2013, 52(1): 371-375. [32]Wan Shuwei(万术伟), Zhang Jing(张靖), Deng Peng (邓棚).Research progress of non-platinum Fe/N/C and Co/N/C cathode electrocatalyst for fuel cell [J].Chinese Journal of Power Sources (电源技术), 2010, 34(10): 1087-1092. [33]Proietti E, Jaouen F, Lefèvre M, Larouche N, Tian J, Dodelet J, Herranz J P.Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells [J].Nat.Commun., 2011, 2: 416. [34]Xiao H, Shao Z G, Zhang G, Gao Y, Lu W, Yi B.Fe-N-carbon black for the oxygen reduction reaction in sulfuric acid [J].Carbon, 2013, 57: 443-451. [35]Wohlgemuth S A, Fellinger T P, Jäker P.Tunable nitrogen-doped carbon aerogels as sustainable electrocatalysts in the oxygen reduction reaction [J].Journal of Materials Chemistry A, 2013, 1(12): 4002-4009. [36]Su P, Xiao H, Zhao J, Yao Y, Shao Z, Li C, Yang Q.Nitrogen-doped carbon nanotubes derived from Zn-Fe-ZIF nanospheres and their application as efficient oxygen reduction electrocatalysts with in situ generated iron species [J].Chem.Sci., 2013, 4: 2941-2946. [37]Wu G, More K L, Johnston C M, Zelenay P.High-performance electrocatalysts for oxygen reduction derived from polyaniline, iron, and cobalt [J].Science, 2011, 332: 443-447. [38]Zhang P, Sun F, Xiang Z, Shen Z, Yun J, Cao D.ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction [J].Energy Environ.Sci., 2014, 7: 442-450. [39]Wu Z S, Chen L, Liu J, Parvez K, Liang H, Shu J, Sachdev H, Graf R, Feng X, Müllen K.High-performance electrocatalysts for oxygen reduction derived from cobalt porphyrin-based conjugated mesoporous polymers [J].Adv.Mater., 2013, 26(9) : 1450-1455. [40]Lee J S, Park G S, Kim S T.A highly efficient electrocatalyst for the oxygen reduction reaction: N-doped ketjenblack incorporated into Fe/Fe3C-functionalized melamine foam [J].Angewandte Chemie, 2013, 125(3): 1060-1064. [41]Wu G, Johnston C M, Mack N H, Artyushkova K, Ferrandon M, Nelson M, Lezama-Pacheco J S, Conradson S D, More K L, Myers D J, Zelenay P.Synthesis-structure-performance correlation for polyaniline-Me-C non-precious metal cathode catalysts for oxygen reduction in fuel cells [J].J.Mater.Chem., 2011, 21: 11392- 11405. [42]Ai K, Liu Y, Ruan C, Lu L, Lu G.Sp2C-dominant N-doped carbon sub-micrometer spheres with a tunable size: a versatile platform for highly efficient oxygen-reduction catalysts [J].Adv.Mater., 2013, 25(7): 998-1003. [43]Shao M H, Adzic R R.Pd-Fe nanoparticles as electrocatalysts for palladium alloy electrocatalysts for oxygen reduction [J].Langmuir, 2006, 22: 10409-10415. [44]Li Shang(李赏), Zhou Yanfang(周彦方), Qiu Peng(邱鹏), et al.Preparation of Co-based non-noble metal catalyst and its electrocatalytic activity for oxygen reduction.[J].Chinese Sci.Bull.(科学通报), 2009, 54(7): 881-887. [45]Wu G, Zelenay P.Nitrogen-doped graphene-rich catalysts derived from heteroatom polymers for oxygen reduction in nonaqueous lithium—O2battery cathodes [J].ACS Nano, 2012, 6(11): 9764-9776. [46]Zhang Yuhui(张玉晖), Yi Qingfeng(易清风).Effect of Fe/Co mass ratio on activity of non-noble metal catalyst for oxygen reduction reaction.[J].CIESC Journal (化工学报), 2014, 65(6): 2113-2119. [47]Wang Y, Nie Y, Wei Z D.Unification of catalytic oxygen reduction and hydrogen evolution reactions: highly dispersive Co nanoparticles encapsulated inside Co and nitrogen co-doped carbon [J].Chemical Communications, 2015, DOI: 10.1039/c5cc02400e. [48]Ohms D, Herzog S, Franke R, Neumann V, Wiesener K, Gamburcev S , Kaisheva A, Iliev I.Influence of metal ions on the electrocatalytic oxygen reduction of carbon materials prepared from pyrolyzed polyacrylonitrile [J].J.Power Sources, 1992, 38(3): 327-334. [49]Kramm U I, Dodelet J P.Structure of the catalytic sites in Fe/N/C-catalysts for O2-reduction in PEM fuel cell [J].Phys.Chem.Chem.Phys., 2012, 14: 11673-11688. [50]Kattel Shyam, Wang Guofeng.Reaction pathway for oxygen reduction on FeN4embedded graphene [J].J.Phys.Chem.Lett., 2014, 5(3): 452-456. [51]Nallathambi V, Lee J W, Kumaraguru S P, Wu G.Development of high performance carbon composite catalyst for oxygen reduction reaction in proton exchange membrane fuel cells [J].J.Power Sources, 2008, 183(1): 34-42 . [52]Chen X, Sun S, Xia D.DFT study of polyaniline and metal composites as nonprecious metal catalysts for oxygen reduction in fuel cells [J].J.Phys.Chem.C, 2012, 116(43): 22737-22742. [53]Liang H W, Feng X, Müllen K.Mesoporous metal—nitrogen-doped carbon electrocatalysts for highly efficient oxygen reduction reaction [J].J.Am.Chem.Soc., 2013, 135(43): 16002-16005. [54]Faubert G, Cote R, Dodelet J P, Lefèvre M, Bertrand P.Oxygen reduction catalysts for polymer electrolyte fuel cells from the pyrolysis of FeIIacetate adsorbed on 3,4,9,10-perylenetetracarboxylic dianhydride [J].Electrochim.Acta, 1999, 44(15): 2589-2603. [55]Zhang F, Pan X, Hu Y, Yu L, Chen X, Jiang P, Zhang H, Deng S, Zhang J, Bolin T B, Zhang S, Huang Y, Bao X.Tuning the redox activity of encapsulated metal clusters via the metallic and semiconducting character of carbon nanotube [J].Acad.Sci.USA , 2013, 110(37): 14861-14866. [56]Chen W, Fan Z, Pan X, Bao X.Effect of confinement in carbon nanotubes on the activity of Fischer-Tropsch iron catalyst [J].J.Am.Chem.Soc., 2008, 130(29): 9414-9419. [57]Hu Y, Xing W, Li Q.Hollow spheres of iron carbide nanoparticles encased in graphitic layers as oxygen reduction catalysts [J].Angew.Chem.Int.Ed., 2014, 53(14): 3675-3679. [58]Wu G, Li N, Zhou D R.Anodically electrodeposited Co+Ni mixed oxide electrode: preparation and electrocatalytic activity for oxygen evolution in alkaline media [J].J.Solid State Chem., 2004, 177(10): 3682-3692. [59]Liang Y, Dai H.Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction [J].Nature Materials, 2011, 10: 780-786. [60]Liang Y, Dai H.Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts [J].J.Am.Chem.Soc., 2012, 134(7): 3517-3523. [61]Liang Y Y, Wang H L, D P, Chang Wesley, Hong G S, Li Y G, G M, Xie L, Zhou J, Wang J, Regier Tom Z, Wei F, Dai H.Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes [J].J.Am.Chem.Soc., 2012, 134 (38): 15849-15857. [62]Liang Y, Li Y, Wang H, Zhou J, Wang J, Regier T, Dai H.Co3O4nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction [J].Nat.Mater., 2011, 10: 780-786. [63]Liang Y, Li Y, Wang H, Zhou J, Wang J, Regier T, Dai H.Covalent hybrid of spinel manganese-cobalt oxide and graphene as advanced oxygen reduction electrocatalysts [J].J.Am.Chem.Soc., 2012, 134(7): 3517-3523. [64]Zhu H, Zhang S, Huang Y, Wu L, Sun S.Monodisperse MxFe3-xO4(M = Fe, Cu, Co, Mn) nanoparticles and their electrocatalysis for oxygen reduction reaction [J].Nano Lett., 2013, 13(6): 2947-2951. [65]Wang Yao, Ding Wei, Chen Siguo, Nie Yao, Xiong Kun, Wei Zidong.Cobalt carbonate hydroxide/C: an efficient dual electrocatalyst for oxygen reduction/evolution reactions [J].Chem.Commun., 2014, 50: 15529-15532. [66]Wu G, Zelenay P.Titanium dioxide-supported non-precious metal oxygen reduction electrocatalyst [J].Chem.Commun., 2010, 46: 7489-7491. [67]Sasaki K, Adzic R R.Niobium oxide-supported platinum ultra-low amount electrocatalysts for oxygen reduction [J].Phys.Chem.Chem.Phys., 2008, 10: 159-167. [68]Imai H.Structural defects working as active oxygen-reduction sites in partially oxidized Ta-carbonitride core-shell particles probed by using surface-sensitive conversion-electron-yield X-ray absorption spectroscopy[J]Appl.Phys.Lett., 2010, 96(19): 191905. [69]Suntivich J, Gasteige H A, Yabuuchi N, Nakanishi H, Goodenough J B, Shao-Horn Y.A perovskite oxide optimized for oxygen evolution catalysis from molecular orbital principles [J].Nat.Chem., 2011, 334 (6061): 1383-1385. [70]Risch M, Horn Y S.La0.8Sr0.2MnO3ˉδdecorated with Ba0.5Sr0.5Co0.8Fe0.2O3ˉδ: a bifunctional surface for oxygen electrocatalysis with enhanced stability and activity [J].J.Am.Chem.Soc., 2014, 136 (14): 5229-5232. [71]Feng Y J, Alonso-Vante N.Nonprecious metal catalysts for the molecular oxygen-reduction reaction [J].Phys.Status.Solidi.B, 2008, 245(9): 1792-1806. [72]Behret H, Binder H, Sandstede G.Electrocatalytic oxygen reduction with thiospinels and other sulphides of transition metals [J].Electrochim.Acta, 1975, 20(2): 111-117. [73]Feng Y J, He T, Alonso-Vante N.In situ free-surfactant synthesis and ORR-electrochemistry of carbon-supported Co3S4and CoSe2nanoparticles [J].Chem.Mater., 2007, 20(1): 26-28. [74]Sidik R A, Anderson A B.Co9S8as a catalyst for electroreduction of O2: quantum chemistry predictions [J].The Journal of Physical Chemistry B, 2006, 110(2): 936-941. [75]Ganesan P, Prabu M, Sanetuntikul J, Shanmugam S.Cobalt sulfide nanoparticles grown on nitrogen and sulfur codoped graphene oxide: an efficient electrocatalyst for oxygen reduction and evolution reactions [J].ACS Catal., 2015, 5 (6): 3625-3637. [76]Feng Y J, He T, Alonso-Vante N.Carbon-supported CoSe2nanoparticles for oxygen reduction reaction in acid Medium [J].Fuel Cells, 2010, 10(1): 77-83. [77]Zhou Y X, Yao H B, Wang Y.Hierarchical hollow Co9S8microspheres: solvothermal synthesis, magnetic, electrochemical, and electrocatalytic properties [J].Chemistry-A European Journal, 2010, 16(39): 12000-12007. [78]Wu G, Chung H T, Nelson M.Graphene-riched Co9S8-NC non-precious metal catalyst for oxygen reduction in alkaline media [J].ECS Transactions, 2011, 41(1): 1709-1717. [79]Wang H, Liang Y, Li Y.Co1ˉxS-graphene hybrid: a high-performance metal chalcogenide electrocatalyst for oxygen reduction [J].Angewandte Chemie International Edition, 2011, 50(46): 10969- 10972. [80]Ham D J, Lee J S.Transition metal carbides and nitrides as electrode materials for low temperature fuel cells [J].Energies, 2009, 2(4): 873-899. [81]Zhong H, Zhang H, Liu G.A novel non-noble electrocatalyst for PEM fuel cell based on molybdenum nitride [J].Electrochemistry Communications, 2006, 8(5): 707-712. [82]Xia D, Liu S, Wang Z.Methanol-tolerant MoN electrocatalyst synthesized through heat treatment of molybdenum tetraphenylporphyrin for four-electron oxygen reduction reaction [J].Journal of Power Sources, 2008, 177(2): 296-302. [83]Kim J H, Ishihara A, Mitsushima S.Catalytic activity of titanium oxide for oxygen reduction reaction as a non-platinum catalyst for PEFC [J].Electrochimica Acta, 2007, 52(7): 2492-2497. [84]Ishihara A, Lee K, Doi S.Tantalum oxynitride for a novel cathode of PEFC [J].Electrochemical and Solid-State Letters, 2005, 8(4): A201-A203. [85]Ando T, Izhar S, Tominaga H.Ammonia-treated carbon-supported cobalt tungsten as fuel cell cathode catalyst [J].Electrochimica Acta, 2010, 55(8): 2614-2621. [86]Cao B, Veith G M, Diaz R E.Cobalt molybdenum oxynitrides: synthesis, structural characterization, and catalytic activity for the oxygen reduction reaction [J].Angewandte Chemie, 2013, 125(41): 10953-10957. [87]Yang L, Jiang S, Zhao Y, Hu Z.Boron-doped carbon nanotubes as metal-free electrocatalysts for the oxygen reduction reaction [J].Angew.Chem.Int, Ed., 2011, 50(31): 7132-7135. [88]Gong K, Du F, Xia Z, Dai L.Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction [J].Science, 2009, 323(5915): 760-764. [89]Qu L, Liu Y, Baek J B.Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells [J].ACS Nano, 2010, 4(3): 1321-1326. [90] Yu D, Zhang Q, Dai L.Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction [J].Journal of the American Chemical Society, 2010, 132(43): 15127-15129. [91] Sheng Z H, Shao L, Chen J J.Catalyst-free synthesis of nitrogen-doped graphene via thermal annealing graphite oxide with melamine and its excellent electrocatalysis [J].ACS Nano, 2011, 5(6): 4350-4358. [92] Liu R, Wu D, Feng X.Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction [J].Angewandte Chemie, 2010, 122(14): 2619-2623. [93] Xiong C, Wei Z, Hu B.Nitrogen-doped carbon nanotubes as catalysts for oxygen reduction reaction [J].Journal of Power Sources, 2012, 215: 216-220. [94] Yang Z, Yao Z, Li G.Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction [J].ACS Nano, 2011, 6(1): 205-211. [95] Yang D S, Bhattacharjya D, Inamdar S.Phosphorus-doped ordered mesoporous carbons with different lengths as efficient metal-free electrocatalysts for oxygen reduction reaction in alkaline media [J].Journal of the American Chemical Society, 2012, 134(39): 16127-16130. [96] Liu Z W, Peng F, Wang H J.Phosphorus-doped graphite layers with high electrocatalytic activity for the O2reduction in an alkaline medium [J].Angewandte Chemie, 2011, 123(14): 3315-3319. [97] Sun X, Zhang Y, Song P.Fluorine-doped carbon blacks: highly efficient metal-free electrocatalysts for oxygen reduction reaction [J].ACS Catalysis, 2013, 3(8): 1726-1729. [98] Choi C H, Park S H, Woo S I.Phosphorus-nitrogen dual doped carbon as an effective catalyst for oxygen reduction reaction in acidic media: effects of the amount of P-doping on the physical and electrochemical properties of carbon [J].Journal of Materials Chemistry, 2012, 22(24): 12107-12115. [99] Liang J, Jiao Y, Jaroniec M.Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance [J].Angewandte Chemie International Edition, 2012, 51(46): 11496-11500. [100]Zheng Y, Jiao Y, Ge L.Two-step boron and nitrogen doping in graphene for enhanced synergistic catalysis [J].Angewandte Chemie, 2013, 125(11): 3192-3198. [101]Wang S, Zhang L, Xia Z.BCN graphene as efficient metal-free electrocatalyst for the oxygen reduction reaction [J].Angewandte Chemie International Edition, 2012, 51(17): 4209-4212. [102]Zhang L, Xia Z.Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells [J].The Journal of Physical Chemistry C, 2011, 115(22): 11170-11176. [103]Luo Z, Lim S, Tian Z.Pyridinic N doped graphene: synthesis, electronic structure, and electrocatalytic property [J].Journal of Materials Chemistry, 2011, 21(22): 8038-8044. [104]Rao C V, Cabrera C R, Ishikawa Y.In search of the active site in nitrogen-doped carbon nanotube electrodes for the oxygen reduction reaction [J].The Journal of Physical Chemistry Letters, 2010, 1(18): 2622-2627. [105]Unni S M, Devulapally S, Karjule N.Graphene enriched with pyrrolic coordination of the doped nitrogen as an efficient metal-free electrocatalyst for oxygen reduction [J].Journal of Materials Chemistry, 2012, 22(44): 23506-23513. [106]Jin Z, Yao J, Kittrell C.Large-scale growth and characterizations of nitrogen-doped monolayer graphene sheets [J].ACS Nano, 2011, 5(5): 4112-4117. [107]Gao F, Zhao G L, Yang S.Nitrogen-doped fullerene as a potential catalyst for hydrogen fuel cells [J].Journal of the American Chemical Society, 2013, 135(9): 3315-3318. [108]Zhao Y, Watanabe K, Hashimoto K.Self-supporting oxygen reduction electrocatalysts made from a nitrogen-rich network polymer [J].J.Am.Chem.Soc., 2012, 134 (48): 19528-19531. [109]Deng D H, Pan X L,Yu L, et al.Toward N-doped graphene via solvothermal synthesis [J].Chem.Mater., 2011, 23(5): 1188-1193. [110]Liu R L, Wu D Q, Feng X L, Müllen K.Nitrogen-doped ordered mesoporous graphitic arrays with high electrocatalytic activity for oxygen reduction [J].Angewandte Chemie, 2011, 122(14): 2619- 2623. [111]Proietti E, Jaouen F, Lefèvre M.Iron-based cathode catalyst with enhanced power density in polymer electrolyte membrane fuel cells [J].Nat.Commun., 2011, 2: 416. [112]Yuan S, Shui J L, Grabstanowicz L.A highly active and support-free oxygen reduction catalyst prepared from ultrahigh-surface-area porous polyporphyrin [J].Angew.Chem., 2013, 125(32): 8507-8511. [113]Tian J, Morozan A, Sougrati M T.Optimized synthesis of Fe/N/C cathode catalysts for PEM fuel cells: a matter of iron-ligand coordination strength [J].Angew.Chem.Int.Ed., 2013, 52(27): 6867. [114]Kundu S, Nagaiah T C, Xia W.Electrocatalytic activity and stability of nitrogen-containing carbon nanotubes in the oxygen reduction reaction [J].The Journal of Physical Chemistry C, 2009, 113(32): 14302-14310. [115]Dorjgotov A, Ok J, Jeon K Y.Activity and active sites of nitrogen-doped carbon nanotubes for oxygen reduction reaction [J].J.Appl.Electrochem., 2013, 43: 387-397. [116]Sidik R A, Anderson A B, Subramanian N P.O2reduction on graphite and nitrogen-doped graphite: experiment and theory [J].The Journal of Physical Chemistry B, 2006, 110(4): 1787-1793. [117]Ding W, Wei Z, Chen S.Space-confinement-induced synthesis of pyridinic and pyrrolic-nitrogen-doped graphene for the catalysis of oxygen reduction [J].Angewandte Chemie, 2013, 125(45): 11971-11975. [118]Ignaszak A, Ye S, Gyenge E.A study of the catalytic interface for O2electroreduction on Pt: the interaction between carbon support meso/microstructure and ionomer (Nafion) distribution [J].J.Phys.Chem.C, 2008, 113(1): 298-307. [119]Antolini E.Carbon supports for low-temperature fuel cell catalysts [J].Appl.Catal.B: Environ., 2009, 88(1): 1-24. [120]Jaouen F, Proietti E, Lefèvre M.Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells [J].Energy Environ.Sci., 2011, 4(1): 114-130. [121]Ding W, Wei Z D.Shape fixing via salt recrystallization: a morphology-controlled approach to convert nanostructured polymer to carbon nanomaterial as a highly active catalyst for oxygen reduction reaction [J].J.Am.Chem.Soc., 2015, 137 (16): 5414-5420. [122]Zhang S M , Zhang H Y, Chen S L, et al.Fe-N doped carbonnanotube/graphene composite: facile synthesis and superior electrocatalytic activity [J].Journal of Materials Chemistry A, 2013, 1: 3302-3308. [123]Tian G L, Zhao M Q, Yu D, Wei F.Graphene hybrids: nitrogen-doped graphene/carbon nanotube hybrids: in situ formation on bifunctional catalysts and their superior electrocatalytic activity for oxygen evolution/reduction reaction [J].Small, 2014, 10(11): 2113-2113. [124]Liu S, Loper C R Kish.A source of crystalline graphite [J].Carbon, 1991, 29(8): 1119-1124. [125]Mayer H K.Elemental analysis of graphite//The American Carbon Society’s 24th Biennial Conference on Carbon–CARBON[C].1999: 99. [126]Koshino Y, Narukawa A.Determination of trace metal impurities in graphite powders by acid pressure decomposition and inductively coupled plasma atomic emission spectrometry [J].Analyst, 1993, 118(7): 827-830. [127]Zaghib K, Song X, Guerfi A.Purification process of natural graphite as anode for Li-ion batteries: chemical versus thermal [J].Journal of Power Sources, 2003, 119: 8-15. [128]McKee D W.Effect of metallic impurities on the gasification of graphite in water vapor and hydrogen [J].Carbon, 1974, 12(4): 453-464. [129]Heintz E A, Parker W E.Catalytic effect of major impurities on graphite oxidation [J].Carbon, 1966, 4(4): 473-482. [130]Dai X, Wildgoose G G, Compton R G.Apparent ‘electrocatalytic’ activity of multiwalled carbon nanotubes in the detection of the anaesthetic halothane: occluded copper nanoparticles [J].Analyst, 2006, 131(8): 901-906. [131]Batchelor-McAuley C, Wildgoose G G, Compton R G.Copper oxide nanoparticle impurities are responsible for the electroanalytical detection of glucose seen using multiwalled carbon nanotubes [J].Sensors and Actuators B: Chemical, 2008, 132(1): 356-360. [132]Jurkschat K, Ji X, Crossley A.Super-washing does not leave single walled carbon nanotubes iron-free [J].Analyst, 2006, 132(1): 21-23. [133]Dai X, Wildgoose G G, Salter C.Electroanalysis using macro-, micro-, and nanochemical architectures on electrode surfaces.Bulk surface modification of glassy carbon microspheres with gold nanoparticles and their electrical wiring using carbon nanotubes [J].Analytical Chemistry, 2006, 78(17): 6102-6108. [134]Wong C H A, Chua C K, Khezri B.Graphene oxide nanoribbons from the oxidative opening of carbon nanotubes retain electrochemically active metallic impurities [J].Angewandte Chemie, 2013, 125(33): 8847-8850. [135]Masa J, Zhao A, Xia W.Trace metal residues promote the activity of supposedly metal-free nitrogen-modified carbon catalysts for the oxygen reduction reaction [J].Electrochemistry Communications, 2013, 34: 113-116. [136]Wang L, Ambrosi A, Pumera M.“Metal-free” catalytic oxygen reduction reaction on heteroatom-doped graphene is caused by trace metal impurities [J].Angewandte Chemie International Edition, 2013, 52(51): 13818-13821.

2 非贵金属催化剂
2.1 金属-氮-碳催化剂


2.2 过渡金属氧化物、硫属化合物、金属氧氮化合物和金属碳氮化合物

3 非金属催化剂






4 结 论
