Genomewide association study of Aegilops tauschii traits under seedling-stage cadmium stress
2015-08-15PengQinLangWangKunLiuShuangshuangMaoZhanyiLiShangGaoHaoranShiYaxiLiu
Peng Qin,Lang Wang,Kun Liu,Shuangshuang Mao,Zhanyi Li,Shang Gao,Haoran Shi,Yaxi Liu,*
aCollege of Agronomy and Biotechnology,Yunnan Agricultural University,Kunming 650201,ChinabTriticeae Research Institute,Sichuan Agricultural University,Chengdu 611130,China
Genomewide association study of Aegilops tauschii traits under seedling-stage cadmium stress
Peng Qina,b,1,Lang Wangb,1,Kun Liub,Shuangshuang Maob,Zhanyi Lib,Shang Gaob,Haoran Shib,Yaxi Liub,*
aCollege of Agronomy and Biotechnology,Yunnan Agricultural University,Kunming 650201,ChinabTriticeae Research Institute,Sichuan Agricultural University,Chengdu 611130,China
A R T I C L E I N F O
Article history:
Received in revised form 22 April 2015
Accepted 1 June 2015
Available online 6 June 2015
Aegilops tauschii
GWAS
Cadmium tolerance Biomass
A B S T R A C T
Aegilops tauschii Aisawildrelativeofcommonwheat(Triticumaestivum)andactsasanimportant resourceofelitegenesincludinggenesforresistancetobioticandabioticstresses.Toimprovethe cadmium(Cd)toleranceofwheatvarietiesusingA.tauschiiresources,weinvestigatedthegenetic variation of biomass-based Cd tolerance in 235 A.tauschii accessions treated with 0(control)and 100 μmol L−1CdCl2(as Cd stress).Simultaneously,we performed a genomewide association study(GWAS)using a single-nucleotide polymorphism chip containing 7185 markers.Six markerswerefoundtobesignificantlyassociatedwithCdtolerancebyagenerallinearmodeland a mixed linear model.These markers were close to several candidate/flanking genes associated with Cd tolerance according to results in public databases,including pdil5-1,Acc-1,DME-5A,
TaAP2-D,TaAP2-B,Vrn-B1,and FtsH-like protein gene.The A.tauschii accessions were classified as high,moderate,and low Cd-tolerant according to a secondary index,the synthetic index(SI),in proportions of 9%,57%,and 34%,respectively.By the average SI,accessions from Afghanistan,Turkey,Azerbaijan,and Iran showed relatively high Cd tolerance.
©2015 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Cadmium (Cd)is one of the most plant-toxic heavy metals and is a pollutant for humans and animals.It is released into the environment mainly through industrial processes and the application of Cd-containing phosphate fertilizers[1]. Excessive Cd accumulation in soils leads to severe problems such as visible phytotoxic symptoms(for example,chlorosis and growth reduction of shoots and roots)[2].Cd can induce oxidative stress in plant cells and inhibit or stimulate the activities of several antioxidant enzymes before toxicity symptoms are visible[3].In China,with the development of modern industry and agriculture,Cd has become a serious problem for safe crop production,and at least 13,330 ha of farmland has been contaminated with varying Cd levels[4].
Upon Cd stress,plants display many types of responses,including morphological and physiological responses.For example,in wheat,marked changes in plant length,dryweight,chlorophyll,total soluble phenolics,and free proline are observed[5].In maize,at relatively low external Cd levels(<100 μmol L−1),shoot height and biomass increase are stimulated,and growth is inhibited at Cd levels greater than 200 μmol L−1[6].In addition,in maize,chlorophyll b is more sensitive to Cd stress than chlorophyll a,and maize can adapt to the negative effects by changing proline content[6]. In some rice cultivars,a cyanide-resistant respiration mechanism important in Cd detoxification is also promoted under Cd stress[7].In general,the ability to tolerate and accumulate Cd varies among plant species and varieties of the same plant species[8,9].Crop species and cultivars differ in their genetic potential to take up trace elements,and this variation provides new opportunities to minimize harmful elements in the food chain by selecting and breeding crops with lowuptakepotential[10].Thus,exploitingthegenetic potential of plants is an option for remediating heavy metalcontaminated soils,and the development of Cd-tolerant accessions within a given plant species has accordingly become a top priority.
Wheat(Triticum aestivum L.)is one of the most important food crops worldwide.Cd is easily absorbed and accumulated in wheat compared with other heavy metals,so that the goal of no or low Cd content in wheat has become imperative. However,the low genetic diversity in the Triticum genus has hindered the development of improved Cd-tolerant wheat varieties[11]and it is difficult to screen for Cd-tolerant accessions of wheat.Thus,researchers have paid greater attention to the wild relatives of wheat,which survive under various stress conditions and retain many favorable characters including resistances to drought,salinity and alkalinity,and disease[12].Aegilops tauschii is one of the ancestral species of wheat and the donor species of the D genome of wheat.The species carries a wide range of resistances and tolerances to biotic/abiotic factors[13,14]that can contribute to wheat improvement.However,to date,little work has been performed on the selection of Cd-tolerant A.tauschii accessions,let alone the elucidation of the genetic mechanisms behind these phenotypes.
Genomewide association studies(GWAS)provide a powerful approach for identifying genes underlying complex diseases at an unprecedented rate[15]and is widely used in plant research.During the past few years,an increasing number of association studies based on the analysis of candidate genes such as flowering-time genes in barley[16],Dwarf8 and the phytoene synthase locus in maize[17,18],rhg-1 in soybean[19],the Psy1-A1 locus in wheat[20],and many candidate genes in Arabidopsis[21,22]have been published.However,to date,few GWAS of Cd tolerance traits have been performed in A.tauschii.
In the present study we performed a GWAS in a worldwide collection of 235 diverse A.tauschii accessions using 7185 informative single-nucleotide polymorphism(SNP)markers. Among these accessions we aimed to identify accessions with excellent Cd tolerance using biomass-associated traits.We also investigated marker—trait associations(MTAs)for Cd tolerance based on a whole-genome association-mapping approach employing SNP markers.This work is one of the first to identify genomic loci for Cd tolerance in A.tauschii. Thus,it is a tentative exploration in A.tauschii and can be a useful reference for the D-genome of wheat in further identification of Cd tolerance-conferring loci.
2.Materials and methods
2.1.Plant materials
A set of 235 A.tauschii accessions(2n=14,DD)was provided by the Triticeae Research Institute of Sichuan Agricultural University(SAU).Detailed information on the tested accessions is summarized in Supplementary Table S1.
2.2.Growth conditions
All A.tauschii plants were grown in a phytotron in Wenjiang,Sichuan Province,China,from March to June 2013.Plant materials were evaluated using hydroponic experiments under 0(control)and 100 μmol L−1CdCl2(Cd stress)conditions in a randomized block design,each treatment with four replicates.The normal nutrient solution was modified based on Hoagland's nutrient solution[23].The modified Hoagland's nutrient solution consisted of Ca(NO3)2·4H2O(4 mmol L−1),KNO3(6 mmol L−1),MgSO4·7H2O(2 mmol L−1),H3BO3(46 μmol L−1),Na·Fe·EDTA (100 μmol L−1),MnCl2·4H2O (9.146 μmol L−1),ZnSO4·7H2O(0.76 μmol L−1),CuSO4·5H2O (0.32 μmol L−1),and(NH4)6Mo7O24·4H2O(0.0161 μmol L−1).
Uniform seedlings,grown from seeds germinated for 8 days,were transplanted into holes in a foamboard.A sponge strip was tied around each seedling to prevent falling and the foamboard with the seedlings was placed in a box filled with nutrient solution.All the roots of the seedlings were fully exposed to the nutrient solution for proper nutrient absorption.To ensure the regular growth of all seedlings after transplanting,theywereplantedinanormalsolution supplemented with phosphorus for 4 days.Subsequently,a normal solution with or without CdCl2was used to water A. tauschii accessions under control and Cd stress conditions,respectively.Thesolutionswerereplacedevery4 days. The growth environment of all seedlings was 25/22(±1)°C day/night temperature,65%/85%day/night relative humidity,and a 16-h photoperiod with 500 μmol m−2s−1photon flux density at the plant canopy level.
2.3.Data collection and phenotypic evaluation
Total fresh weight(FW)and dry weight(DW)were measured 15 days after transplantation.Each seedling from the four replicates was carefully washed in clean water and the FW was obtained with an electronic balance.Plants were then placed in paper bags,heated at 105°C for 30 min to kill the cells,and then dried at 75°C until a constant mass was obtained,recorded as DW.
To eliminate inherent biological and genetic differences of the A.tauschii accessions,the Cd stress tolerance index(CTI)was used as an indicator of the diversity of the accessions following the formula:CTI=(TC−TS)/TS[24],where TSand TCare the values of the traits measured under Cd stress and control conditions,respectively.The synthetic index(SI)wasused to evaluate Cd-tolerant accessions and determined by considering the average CTI across FW and DW measures. Heritability was estimated as H=VG/(VG+VE),where VGand VErepresent estimates of genetic and environmental variance,respectively[25].Descriptive analysis and analysis of variance were conducted for the tested traits using IBM SPSS Statistics for Windows,version 20.0(IBM Corp.,Armonk,NY,USA).
2.4.10K Infinium iSelect SNP array and SNP genotyping
The construction of the A.tauschii 10K SNP array has been previously reported in detail[26].A total of 7185 polymorphic SNP markers in the array were uniquely mapped to the A. tauschii genetic map and to the physical map of the A.tauschii genome built based on bacterial artificial chromosome clones. SNPs were assayed according to the manufacturer's protocol (Illumina,San Diego,CA,USA)at the Genome Center,University of California,Davis,CA,USA.The detailed information for SNP genotyping of the 235 A.tauschii accessions has been described in our previous study[27].
2.5.Marker-trait associations
In our study,only 6905 SNP markers with a minor allele frequency(MAF)greater than 0.05 were used for marker—trait association.To identify marker—trait associations(MTAs),TASSEL v2.1[28]was used,employing a general linear model (GLM)based on principal components(PCs)derived from the TASSEL v2.1 principal component analysis(PCA).To control efficiently for type I and II errors,a mixed linear model(MLM),incorporating both the PCs and kinship matrix(K matrix),was also fitted.Bonferroni-corrected thresholds at α=1 were used as cutoffs.When the number of markers was 6905 SNPs,at α=1,the Bonferroni-corrected threshold for the P-values was 144.823×10−6,with a corresponding−lg(P)-value of 3.84. Significant markers are shown in a Manhattan plot drawn with SAS 9.2(SAS Institute,Cary,NC,USA).Important P-value distributions(observed P-values against cumulative P-values on a−lg scale)are shown in a quantile—quantile plot also drawn with SAS.
Putative candidate genes were proposed for each significant MTA based on the best match to the National Center for Biotechnology Information(NCBI,http://www.ncbi.nlm.nih. gov/)GenBank nonredundant database using the corresponding extended SNP marker sequence,which was derived from the 5000 bp around each SNP marker and was acquired using BLASTattheInternationalWheatGenomeSequencing Consortium(IWGSC,http://www.wheatgenome.org/)site.

Table 1-Analysis of variance for total fresh and dry weights in 235 accessions of Aegilops tauschii under Cd stress and a control condition.
3.Results
3.1.Genetic variation as revealed by analysis of variance
The analysis of variance revealed significant variation among accessions for FW and DW (Table 1).The level of variation isreflectedinthedistributionsofthetwotraits(Fig.1).Genotypic variationforthetwotraitswassignificantatP<0.01.Inaddition,significant differences in treatment×genotype interaction were observed for all traits(Table 1).The results show that the two traits were significantly influenced by Cd supply among the 235 A.tauschii accessions,which were subjected to further genetic analysis.
Phenotypic variation among accessions for each trait was confirmed by the trait mean,standard deviation,and coefficient of variation(Table 2).In comparison with the Cd stress condition,the mean values of the two traits decreased substantially under the control condition.The coefficients of variation under the control condition were 52.52%and 50.03%for FW and DW,respectively.In contrast,under the Cd stress condition,the two traits showed lower coefficients of variation,50.14%and 48.57%for FW and DW,respectively.
3.2.Heritability
Medium-to-high heritability estimates were obtained for the two traits(Table 2).Of the two,FW had the higher heritability under the Cd stress condition(0.78)but a heritability of 0.74 under the control condition.For DW,heritability estimates were 0.74 and 0.69 for the control and Cd stress conditions,respectively.Thus,the heritability estimates were not very different from each other.This result shows that DW is highly heritable under the control condition compared with the result obtained under the Cd stress condition.In contrast,FW is highly heritable under Cd stress compared with the result obtained under the control condition.These results also suggest that DW and FW are good indicators for screening Cd tolerance in A.tauschii.
3.3.Cd stress tolerance index(CTI)and synthetic index(SI)
The CTI for FW and DW in all 235 A.tauschii accessions and itsSIacrossthetwotraitswerecalculated.Pearson's correlations between FW,DW,CIT,and SI were also computed (Table 3).FW and DW under the control condition were significantly and positively correlated with SI(r=0.358**and r=0.348**,respectively,P<0.01).The CTI for FW was positively correlated with the CTI for DW(r=0.875,P<0.01).TheSI values ranged from −0.828 to 0.656,indicating that A. tauschii accessions differ in Cd tolerance(Fig.2,Supplementary Table S1).Based on the SI values,the tested A.tauschii accessions could be classified into three groups:20 accessions (9%)showed high Cd tolerance with an SI value of>0,135 (57%)showed moderate Cd tolerance with an SI value between−0.5 and 0,and 80(34%)showed low Cd tolerance with an SI value of<−0.5.Overall,among the 235 A. tauschii accessions,AS623321 showed the highest SI with a value of 0.656 and AS623008 showed the lowest SI with a value of−0.828.These two were selected as extremely tolerant and sensitive accessions,respectively.Among highly Cd-tolerant accessions,the top five A.tauschii accessions were AS623321,AS623402,AS623194,AS623186,and AS623173 with SI values of 0.656,0.629,0.629,0.614,and 0.399,respectively.

Fig.1-Distribution of total fresh weight and total dry weight phenotyped under 0(control)and 100 μmol L−1CdCl2(Cd stress)conditions in 235 Aegilops tauschii accessions.
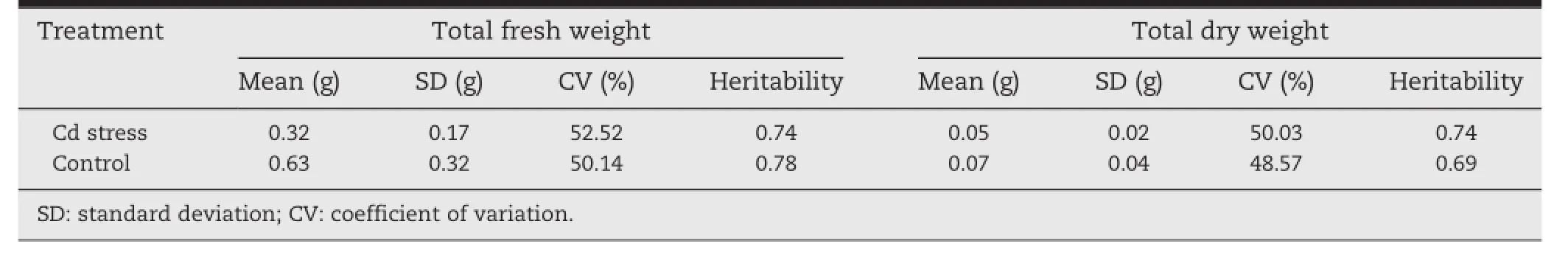
Table 2-Variation and heritability of total fresh and dry weights in 235 accessions of Aegilops tauschii under Cd stress and a control condition.
3.4.Cd tolerance is associated with the geographic origin of A.tauschii
The average SI values were used to compare Cd tolerance levels among the different origins of A.tauschii.Table 4 lists the average SI values for A.tauschii accessions from each origin.Among the origins that provided more than 10 A. tauschii accessions,Afghanistan,Turkey,Azerbaijan,and Iran had high SI values(−0.321,−0.323,−0.326,and −0.371,respectively).The percentages of the selected tolerant accessions were 10.0%,12.9%,8.0%,and 6.7%of the total accessions from Afghanistan,Turkey,Azerbaijan,and Iran,respectively. The higher SI values of these four countries suggest that A. tauschii accessions from these countries have higher than average Cd tolerance.

Table 3-Genetic correlations among FW,DW,CTI,and SI.
3.5.Association analysis
We tested two models for detecting associations between SNP markers and traits:GLM with PC to correct for population structure,and MLM with PC and K matrix to correct for population structure.In the PCA analysis performed with TASSEL v2.1 and 6905 SNP markers,the first 5 PCs explained 82.84%of the variance(data not shown),reflecting the complex pedigrees of A.tauschii.Thus,the first 5 PCs were used for computing both GLM and MLM.At a significance level of 3.84,seven and six significant markers were detected by GLM and MLM,respectively(Table 5;Fig.3A,B,D,E,G,H). Under the control condition,significant markers for DW and FW were detected by both GLM and MLM,whereas under the Cd stress condition,significant markers were detected by GLM and MLM for FW,but none for DW.The R2values provide an estimate of the amount of phenotypic variation explained by the markers(Table 5).Six significant markers were detected by both GLM and MLM.The distribution of the observed−lg(P)values should ideally follow a uniform distribution with little deviation from the expected−lg(P)values.In our study,GLM and MLM showed a good fit for the−lg(P)values(Fig.3C,F,I).
Basedonapublicdatabase,weidentifiedputative candidate/flankinggenes (Table6).Forthesignificant markers GA8KES401BYTBK_134(on chromosome 1D)and BE398279ATwsnp1(on chromosome 3D),we failed to identify any candidate genes and speculated that they are two new loci associated with control of Cd tolerance.For the remainingthreesignificantmarkers,sevencandidate/ flanking genes were identified,including an FtsH-like protein gene,pdil5-1,Acc-1,TaAP2-D,TaAP2-B,Vrn-B1,and DME-5A. The annotation of these genes is shown in Table 7.It is noteworthy that marker GBUVHFX01COE01_159 was associated with DW and FW under the control condition.

Fig.2-Frequency distribution of the synthetic index(SI)for Cd tolerance in 235 Aegilops tauschii accessions.
4.Discussion
4.1.Growth response to Cd toxicity
Cd produces disturbances in both the uptake and the distribution of elements in many plant species[35,36].In addition,Cd toxicity varies depending on growth conditions,experimental design,Cd availability,duration of Cd treatment,and plant age [24].Effects of Cd toxicity in plants are of two types:stimulatory and inhibitory.The main explanation for stimulatory effects is that metalionsmay serve asactivators of enzymes incytokinin metabolism,which accelerates the growth of plants[37—39];also,low doses of stress may cause changes in plant hormones and cytokinins,which regulate plant growth and development [40].There are two main explanations for the inhibitory effects of Cd.First,these effects may be caused by direct interaction with proteins owing to their affinities for thioyl,histidyl,and carboxyl groups,causing the metals to target structural,catalytic,and transport sites of the cell[41].Second,they may be due to the displacement of essential cations from specificbinding sites,impairing function.Several studies have shown that Cdcan enter cells using the same uptake systememployed by cations such as Fe,Cu,Ca,and Zn,and excess Cd could compete with these elements for transporters,promoting a reduction in both uptake and accumulation of these cations [42].For instance,Cd2+replaces Ca2+in the photosystem II reaction center,inhibiting PSII photoactivation[43].In the present study,A.tauschii growth was inhibited in general under Cd stress in comparison with control conditions,and the mean values of FW and DW were reduced by nearly half under the Cd stress condition.The results suggest that A.tauschii growth was restricted under a 100 μmol L−1Cd concentration.
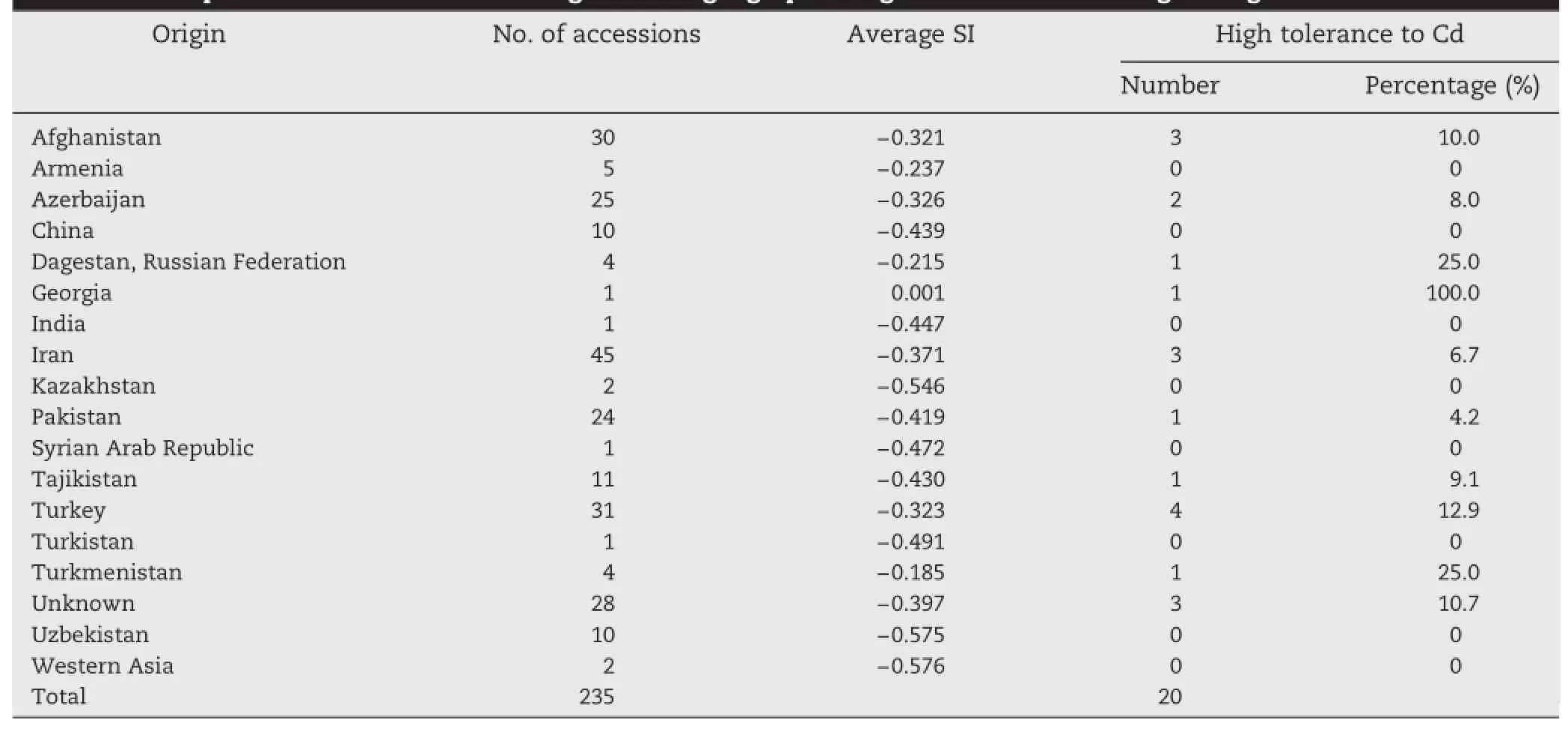
Table 4-Comparison of Cd tolerance among different geographic origins of A.tauschii using average SI.
4.2.Biomass as a crucial trait for evaluating Cd tolerance
Numerous growth parameters can be used to estimate the ability of plants to endure and survive at a contaminated site. For example,root growth and visible symptoms have been used to assess tolerance to heavy metals in ryegrass[44]. Biomass and root and shoot length have also been used to evaluate tolerance to heavy metal toxicity[45],as well as toxic symptoms and shoot biomass to screen for plants that can effectivelyremedyheavymetal-contaminatedsoils[46]. Relative growth rate and leaf structural properties have been used to measure Cd tolerance in 10 grass species[47],and root growth and density have been used to estimate the different Cd tolerances of Pinus pinaster and Pinus pinea[48].Among the different parameters used,plant biomass has been used to evaluate Cd tolerance in many plant species[49—51].In the present study,the biomass traits FW and DW decreased significantly upon Cd treatment,possibly because Cd stress generally reduces chlorophyll content,resulting in the inhibition of photosynthesis in A.tauschii.The inhibition of photosynthesis is the result of the damage to the PSII reaction center in the leaf[52,53].

Table 5-Genomewide association study of Cd tolerance in 235 Aegilops tauschii accessions.
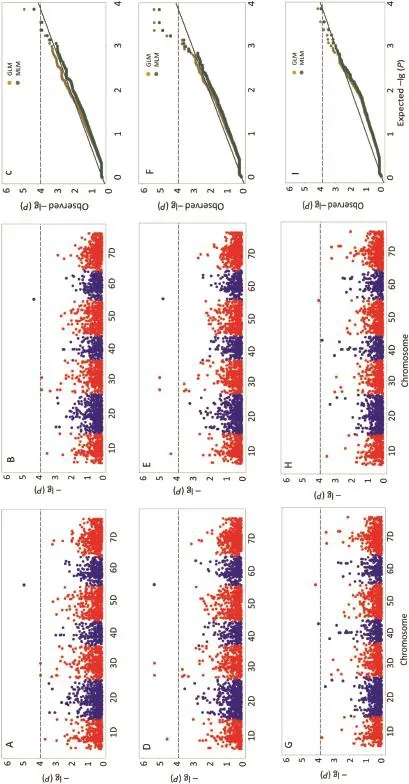
Fig. 3 - GWAS scan for trait DW under Cd stress condition (A, B, C), FW under Cd stress condition (D, E, F), FW under control condition (G, H, I). (A, B) GLM and MLM results for association of DWunder Cd stress condition. (C) Q-Q plots of GLM and MLMfor DWunder Cd stress condition. (D, E) GLM and MLM results for association of FW under Cd stress condition. (F) Q-Q plots of GLM and MLM for FW under Cd stress condition. (G, H) GLM and MLM results for association of FW under control condition. (I) Q-Q plots of GLM and MLM for FW under control condition.
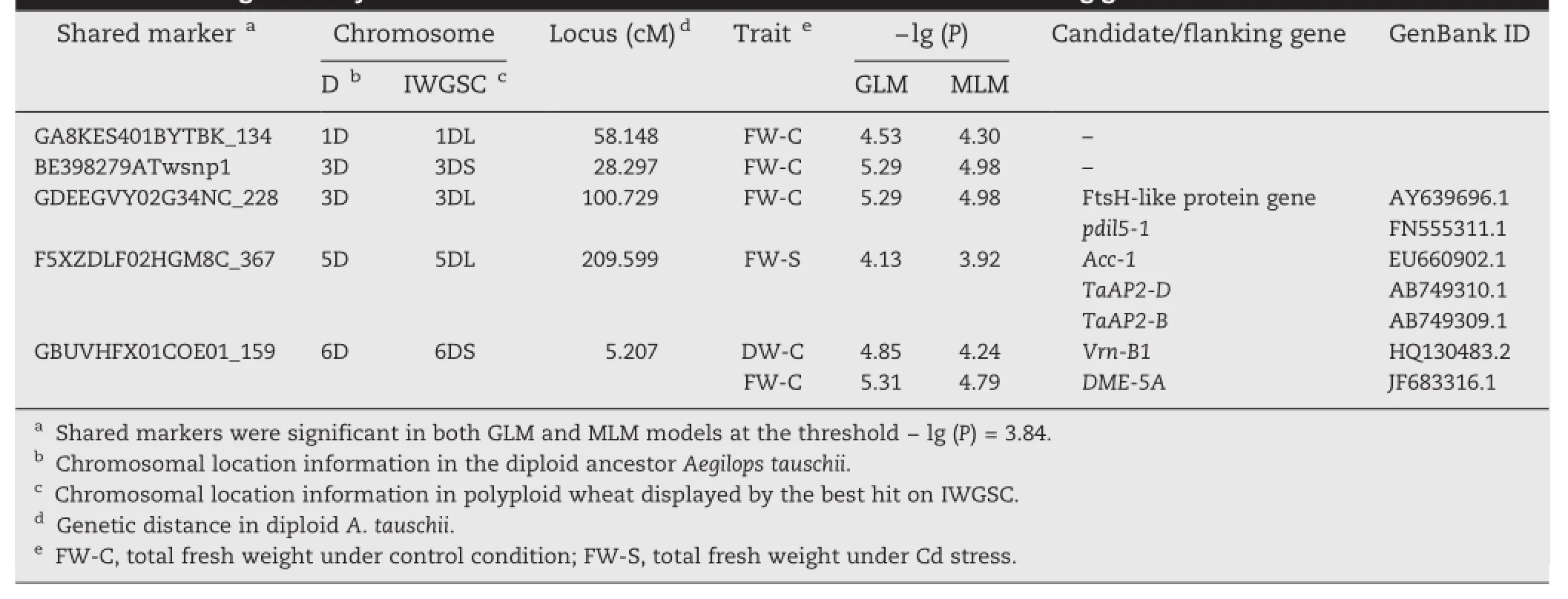
Table 6-SNPs significantly associated with Cd-tolerance traits and candidate/flanking genes.
4.3.Comparison of identified Cd tolerance-conferring quantitative trait loci(QTL)with those from previous reports
In the present study,we report the outcome of a GWAS approach for identifying genomic regions associated with the control of Cd tolerance-conferring traits in A.tauschii using 7185 SNP markers genotyped in a core collection of 235 natural A.tauschii accessions.Linkage mapping can also be used to detect quantitative trait loci(QTL)using different segregating populations and testing them under different environmental conditions.To date,a few QTL associated with Cd tolerance-conferring traits have been identified in various plants using this classical approach.Compared to those analyses,the present approach offers more useful information for identifying loci present in A.tauschii that control Cd tolerance-conferring traits and accordingly present in the D-genome of wheat.
Genetically,Cd accumulation in plants may be regulated by multiple genes with combined effects on uptake,translocation,and sequestration [54].In Arabidopsis halleri,QTL mapping was used to identify chromosomal regions involved in Cd tolerance and,in addition to the identification of three QTL,a gene named HMA4 encoding a predicted heavy metal ATPase was cloned and functionally characterized[55]. Several QTL associated with Cd accumulation in rice(Oryza sativa L.)have been reported[56,57].In durum wheat,Cd accumulation is governed by Cdu1,which is located on chromosome arm 5BL[58,59].Prior to the current study,few loci associated with control of Cd tolerance-conferring traits had been identified in A.tauschii.Because the genome of A.tauschii could be used as a reference for common wheat,the loci we have identified can be further used in searching for Cd tolerance-conferring genes in wheat.The candidate genes associated with Cd tolerance identified in this study encode diverse enzymes(e.g.pdil5-1 gene,Acc-1,and DME-5A),transcription factors(e.g.TaAP2-D and TaAP2-B),a vernalization-associated gene(Vrn-B1),and a gene(FtsH-like protein gene)with unclear function.In addition,we detected a SNP,GBUVHFX01COE01_159,located on 6D significantly associated with two different traits(FW and DW under the control condition).Although this association may be the result of pleiotropy,it was supported by Pearson's correlation analysis (r=0.936**for FW-C and DW-C,P<0.01).These identified candidate genes indicated once again that the regulation of Cd tolerance is complex and requires deeper study.
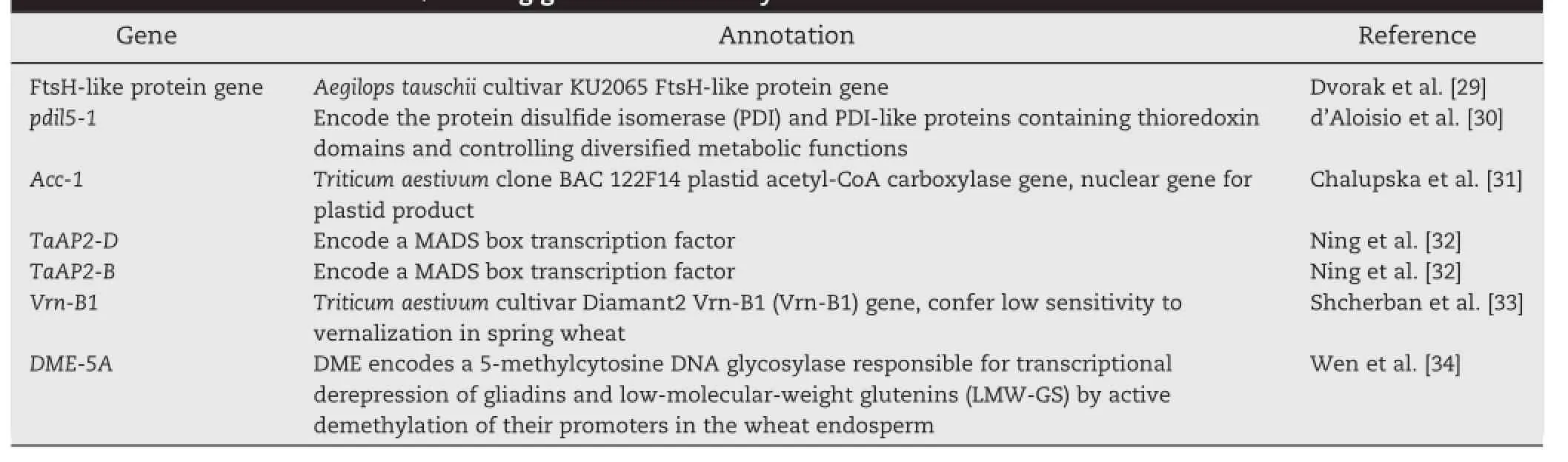
Table 7-Definition of candidate/flanking genes identified by BLAST.
4.4.Use of A.tauschii in wheat variety improvement
To date,wild species have been considered more as sources of resistance to biotic and abiotic stresses than as sources ofdiversity,permitting the deep modification of the architecture and physiology of cultivated species[60,61].A.tauschii is one of the ancestral species of wheat and the donor species of the D genome of wheat.Aegilops species are found in Mediterranean climates,being indigenous from the Canary Islands to the western part of Asia,in Afghanistan and western China [62].Their ability to spread widely is probably associated with their great adaptability,which also explains why they carry many agronomically valuable traits including tolerance to drought,salt,low phosphorus,and extreme environments,all of which could be used in plant breeding[63—65].Given that the D genome was involved in the evolution of bread wheat[66,67],the genetic diversity present in Aegilops species bearing the D genome is of considerable interest[68].These potential sources of genetic variation and alleles are useful for wheat breeding purposes,and can serve as a secondary gene pool for this species[69,70].The rich genetic diversity for resistance to various biotic and abiotic stresses has already contributed to wheat improvement[14,71,72].In the present study,we identified 20 A.tauschii accessions with high tolerance to Cd.Most of them were originally from the Turkey—Iran—Afghanistan zone,a finding in keeping with the path of A.tauschii range expansion.The five accessions with highest Cd tolerance were AS623321,AS623402,AS623194,AS623186,and AS623173,and may be used as germplasm resources to widen the genetic diversity of cultivated wheat. Their use could shorten the procedure of wheat breeding for no or low Cd content.
5.Conclusions
WeperformedagenomewideassociationstudyofCd tolerance-conferring traits in a population of 235 A.tauschii plants using 7185 single-nucleotide polymorphism markers. Six significant markers were detected using both general and mixed linear models.At significant loci and in flanking regions,we identified candidate genes that might control Cd tolerance-conferring traits,including pdil5-1,Acc-1,DME-5A,TaAP2-D,TaAP2-B,Vrn-B1,and FtsH-like protein gene.The identified SNPs and genes offer information for cloning genes associated with Cd tolerance in A.tauschii and wheat. Concurrently,we screened the 235 A.tauschii accessions for Cd-tolerant accessions using biomass-associated traits.The results indicate that A.tauschii accessions from Afghanistan,Turkey,Azerbaijan,and Iran have higher Cd tolerance than those from other origins.Using the average SI value,20 A. tauschii accessions with high tolerance to applied Cd were identified.The five most tolerant accessions,AS623321,AS623402,AS623194,AS623186,and AS623173,could be useful for wheat improvement.Collectively,our results may provide a foundation for breeding Cd tolerant wheat cultivars.
Acknowledgments
We thank Assad Siham(ICARDA,Syria),Jon W.Raupp(Kansas State University,USA),Shuhei Nasuda(Komugi,Japan)and Harold Bockelman(USDA,USA)for plant materials.This study was supported by the International Science&Technology CooperationProgramofChina(2015DFA30600)andthe National Natural Science Foundation of China(31301317).
Supplementary data
Supplementary table to this article can be found online at http://dx.doi.org/10.1016/j.cj.2015.04.005.
R E F E R E N C E S
[1]M.Prasad,Cadmium toxicity and tolerance in vascular plants,Environ.Exp.Bot.35(1995)525—545.
[2]U.Shukla,J.Singh,P.Joshi,P.Kakkar,Effect of bioaccumulation of cadmium on biomass productivity,essential trace elements,chlorophyll biosynthesis,and macromolecules of wheat seedlings,Biol.Trace Elem.Res.92(2003)257—273.
[3]L.Sandalio,H.Dalurzo,M.Gomez,M.Romero-Puertas,L. Del Rio,Cadmium-induced changes in the growth and oxidative metabolism of pea plants,J.Exp.Bot.52(2001)2115—2126.
[4]W.Liu,Q.Zhou,Y.Sun,R.Liu,Identification of Chinese cabbage genotypes with low cadmium accumulation for food safety,Environ.Pollut.157(2009)1961—1967.
[5]I.Öncel,Y.Keleş,A.Üstün,Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings,Environ.Pollut.107(2000)315—320.
[6]C.Wang,L.Tao,J.Ren,The response of maize seedlings to cadmium stress under hydroponic conditions,Russ.J.Plant Physiol.60(2013)295—299.
[7]M.J.Hassan,G.Shao,G.Zhang,Influence of cadmium toxicity on growth and antioxidant enzyme activity in rice cultivars with different grain cadmium accumulation,J.Plant Nutr.28 (2005)1259—1270.
[8]D.Chan,B.Hale,Differential accumulation of Cd in durum wheat cultivars:uptake and retranslocation as sources of variation,J.Exp.Bot.55(2004)2571—2579.
[9]P.Verma,K.George,H.Singh,R.Singh,Modeling cadmium accumulation in radish,carrot,spinach and cabbage,Appl. Math.Model.31(2007)1652—1661.
[10]C.Grant,J.Clarke,S.Duguid,R.Chaney,Selection and breeding of plant cultivars to minimize cadmium accumulation,Sci.Total Environ.390(2008)301—310.
[11]X.Jia,C.J.Zhou,S.M.Dong,Progress of research on the effects of Cd~(2+)stress on wheat and the response of wheat to Cd~(2+),J.Triticeae Crops 31(2011)786—792.
[12]J.Zhang,Z.Liu,J.Xiao,W.Wang,Study and utilization of wild relatives of wheat in xinjiang uygur autonomous regin,Chin. Agric.Sci.Bull.27(2011)29—32.
[13]J.Valkoun,Dostal,D.Kučerová,Triticum×Aegilops hybrids through embryo culture,in:Y.P.S.Bajaj(Ed.),Biotechnology in Agriculture and Forestry,Springer,Berlin 1990,pp.152—166.
[14]T.Cox,W.Raupp,D.Wilson,S.Leath,W.Bockus,L.Browder,Resistance to foliar diseases in a collection of Triticum tauschii germ plasm,Plant Dis.(USA)76(1992)1061—1064.
[15]D.Altshuler,M.J.Daly,E.S.Lander,Genetic mapping in human disease,Science 322(2008)881—888.
[16]S.Stracke,G.Haseneyer,J.B.Veyrieras,H.H.Geiger,S. Sauer,A.Graner,H.P.Piepho,Association mapping reveals gene action and interactions in the determination of flowering time in barley,Theor.Appl.Genet.118(2009)259—273.
[17]J.M.Thornsberry,M.M.Goodman,J.Doebley,S.Kresovich,D.Nielsen,E.S.Buckler,Dwarf8 polymorphisms associate with variation in flowering time,Nat.Genet.28(2001)286—289.
[18]K.A.Palaisa,M.Morgante,M.Williams,A.Rafalski,Contrasting effects of selection on sequence diversity and linkage disequilibrium at two phytoene synthase loci,Plant Cell 15(2003)1795—1806.
[19]Y.H.Li,C.Zhang,Z.S.Gao,M.J.M.Smulders,Z.Ma,Z.X.Liu,H.Y.Nan,R.-Z.Chang,L.J.Qiu,Development of SNP markers and haplotype analysis of the candidate gene for rhg1,which confers resistance to soybean cyst nematode in soybean,Mol. Breed.24(2009)63—76.
[20]A.Singh,S.Reimer,C.Pozniak,F.Clarke,J.Clarke,R.Knox,A. Singh,Allelic variation at Psy1-A1 and association with yellow pigment in durum wheat grain,Theor.Appl.Genet. 118(2009)1539—1548.
[21]I.M.Ehrenreich,Y.Hanzawa,L.Chou,J.L.Roe,P.X.Kover,M.D. Purugganan,Candidate gene association mapping of Arabidopsis flowering time,Genetics 183(2009)325—335.
[22]K.Zhao,M.J.Aranzana,S.Kim,C.Lister,C.Shindo,C.Tang,C. Toomajian,H.Zheng,C.Dean,P.Marjoram,An Arabidopsis example of association mapping in structured samples,PLoS Genet.3(2007)e4.
[23]D.R.Hoagland,D.I.Arnon,The water-culture method for growing plants without soil,Circular,Calif.Agric.Exp.Station. 347(1950).
[24]A.Metwally,V.I.Safronova,A.A.Belimov,K.J.Dietz,Genotypic variation of the response to cadmium toxicity in Pisum sativum L,J.Exp.Bot.56(2005)167—178.
[25]S.Smith,R.Kuehl,I.Ray,R.Hui,D.Soleri,Evaluation of simple methods for estimating broad-sense heritability in stands of randomly planted genotypes,Crop Sci.38(1998)1125—1129.
[26]M.C.Luo,Y.Q.Gu,F.M.You,K.R.Deal,Y.Ma,Y.Hu,N.Huo,Y. Wang,J.Wang,S.Chen,A 4-gigabase physical map unlocks the structure and evolution of the complex genome of Aegilops tauschii,the wheat D-genome progenitor,Proc. Natl.Acad.Sci.U.S.A.110(2013)7940—7945.
[27]J.Wang,M.C.Luo,Z.Chen,F.M.You,Y.Wei,Y.Zheng,J. Dvorak,Aegilops tauschii single nucleotide polymorphisms shed light on the origins of wheat D-genome genetic diversity and pinpoint the geographic origin of hexaploid wheat,New Phytol.198(2013)925—937.
[28]P.J.Bradbury,Z.Zhang,D.E.Kroon,T.M.Casstevens,Y. Ramdoss,E.S.Buckler,TASSEL:software for association mapping of complex traits in diverse samples,Bioinformatics 23(2007)2633—2635.
[29]J.Dvorak,E.D.Akhunov,Tempos of gene locus deletions and duplications and their relationship to recombination rate during diploid and polyploid evolution in the Aegilops-Triticum Alliance,Genetics 171(2005)323—332.
[30]E.d'Aloisio,A.Paolacci,A.Dhanapal,O.Tanzarella,E. Porceddu,M.Ciaffi,The protein disulfide isomerase gene family in bread wheat(T.aestivum L.),BMC Plant Biol.10 (2010)e101.
[31]D.Chalupska,H.Y.Lee,J.D.Faris,A.Evrard,B.Chalhoub,R. Haselkorn,P.Gornicki,Acc homoeoloci and the evolution of wheat genomes,Proc.Natl.Acad.Sci.U.S.A.105(2008)9691—9696.
[32]S.Ning,N.Wang,S.Sakuma,M.Pourkheirandish,J.Wu,T. Matsumoto,T.Koba,T.Komatsuda,Structure,transcription and post-transcriptional regulation of the bread wheat orthologs of the barley cleistogamy gene Cly1,Theor.Appl. Genet.126(2013)1273—1283.
[33]A.B.Shcherban,T.T.Efremova,E.A.Salina,Identification of a new Vrn-B1 allele using two near-isogenic wheat lines with difference in heading time,Mol.Breed.29(2012)675—685.
[34]W.Shanshan,W.Nuan,P.Jinsong,G.Langen,R.A.T. Brew-Appiah,J.H.Mejias,C.Osorio,Y.Mingming,R.Gemini,C.P.Moehs,R.S.Zemetra,K.Karl-Heinz,L.Bao,W.Xingzhi,D.von Wettstein,S.Rustgi,Structural genes of wheat and barley 5-methylcytosine DNA glycosylases and their potential applications for human health,Proc.Natl.Acad. Sci.U.S.A.109(2012)20543—20548.
[35]E.E.Rogers,D.J.Eide,M.L.Guerinot,Altered selectivity in an Arabidopsis metal transporter,Proc.Natl.Acad.Sci.U.S.A.97 (2000)12356—12360.
[36]V.E.Tsyganov,A.A.Belimov,A.Y.Borisov,V.I.Safronova,M. Georgi,K.J.Dietz,I.A.Tikhonovich,A chemically induced new pea(Pisum sativum)mutant SGECdt with increased tolerance to,and accumulation of,cadmium,Ann.Bot.99(2007)227—237.
[37]M.Kamínek,Progress in cytokinin research,Trends Biotechnol.10(1992)159—164.
[38]P.Nyitrai,K.Bóka,L.Gáspár,É.Sárvári,Á.Keresztes,Rejuvenation of ageing bean leaves under the effect of low-dose stressors,Plant Biol.6(2004)708—714.
[39]W.Liu,Q.Zhou,J.An,Y.Sun,R.Liu,Variations in cadmium accumulation among Chinese cabbage cultivars and screening for Cd-safe cultivars,J.Hazard.Mater.173(2010)737—743.
[40]H.Yu,J.Wang,W.Fang,J.Yuan,Z.Yang,Cadmium accumulation in different rice cultivars and screening for pollution-safe cultivars of rice,Sci.Total Environ.370(2006)302—309.
[41]C.Foy,R.T.Chaney,M.White,The physiology of metal toxicity in plants,Annu.Rev.Plant Physiol.29(1978)511—566.
[42]S.Clemens,Toxic metal accumulation,responses to exposure and mechanisms of tolerance in plants,Biochimie 88(2006)1707—1719.
[43]P.Faller,K.Kienzler,A.Krieger-Liszkay,Mechanism of Cd2+toxicity:Cd2+inhibits photoactivation of Photosystem II by competitive binding to the essential Ca2+site,Biochim. Biophys.Acta Bioenerg.1706(2005)158—164.
[44]M.Wong,A.Bradshaw,A comparison of the toxicity of heavy metals,using root elongation of rye grass,Lolium perenne,New Phytol.91(1982)255—261.
[45]A.Baker,P.L.Walker,Physiological responses of plants to heavy metals and the quantification of tolerance and toxicity,Chem.Speciat.Bioavailab.1(1989)7—17.
[46]S.H.Wei,Q.X.Zhou,X.Wang,W.Cao,L.P.Ren,Y.F.Song,Potential of weed species applied to remediation of soils contaminated with heavy metals,J.Environ.Sci.16(2004)868—873.
[47]S.Sabreen,S.I.Sugiyama,Trade-off between cadmium tolerance and relative growth rate in 10 grass species,Environ.Exp.Bot.63(2008)327—332.
[48]I.Arduini,D.L.Godbold,A.Onnis,Cadmium and copper change root growth and morphology of Pinus pinea and Pinus pinaster seedlings,Physiol.Plant.92(1994)675—680.
[49]G.Shi,Q.Cai,Cadmium tolerance and accumulation in eight potential energy crops,Biotechnol.Adv.27(2009)555—561.
[50]Y.Sun,Q.Zhou,C.Diao,Effects of cadmium and arsenic on growth and metal accumulation of Cd-hyperaccumulator Solanum nigrum L,Bioresour.Technol.99(2008)1103—1110.
[51]M.J.Wang,W.X.Wang,Cadmium in three marine phytoplankton:accumulation,subcellular fate and thiol induction,Aquat.Toxicol.95(2009)99—107.
[52]D.Tanyolac,Y.Ekmekçi,Ş.Ünalan,Changes in photochemical and antioxidant enzyme activities in maize (Zea mays L.)leaves exposed to excess copper,Chemosphere 67(2007)89—98.
[53]M.Li,L.J.Zhang,L.Tao,W.Li,Ecophysiological responses of Jussiaea rapens to cadmium exposure,Aquat.Bot.88(2008)347—352.
[54]P.Tanhuanpää,R.Kalendar,A.Schulman,E.Kiviharju,A major gene for grain cadmium accumulation in oat,Genome 50(2007)588—594.
[55]M.Courbot,G.Willems,P.Motte,S.Arvidsson,N.Roosens,P. Saumitou-Laprade,N.Verbruggen,A major quantitative trait locus for cadmium tolerance in Arabidopsis halleri colocalizes with HMA4,a gene encoding a heavy metal ATPase,Plant Physiol.144(2007)1052—1065.
[56]T.Kashiwagi,K.Shindoh,N.Hirotsu,K.Ishimaru,Evidence for separate translocation pathways in determining cadmium accumulation in grain and aerial plant parts in rice,BMC Plant Biol.9(2009)e8.
[57]D.Ueno,I.Kono,K.Yokosho,T.Ando,M.Yano,J.Ma,A major quantitative trait locus controlling cadmium translocation in rice(Oryza sativa),New Phytol.182(2009)644—653.
[58]J.Clarke,D.Leisle,G.Kopytko,Inheritance of cadmium concentration in five durum wheat crosses,Crop Sci.37 (1997)1722—1726.
[59]G.Scoles,R.Knox,C.Pozniak,F.Clarke,J.Clarke,S. Houshmand,A.Singh,Chromosomal location of the cadmium uptake gene(Cdu1)in durum wheat,Genome 52 (2009)741—747.
[60]P.Monneveux,M.Zaharieva,D.Rekika,The utilisation of Triticum and Aegilops species for the improvement of durum wheat,in:C.Royo(Ed.),Durum Wheat Improvement in the Mediterranean Region:New Challenges,Ciheam,Zaragoza 2000,pp.71—81.
[61]K.Mguis,A.Albouchi,M.Abassi,A.Khadhri,M.Ykoubi-Tej,A.Mahjoub,N.B.Brahim,Z.Ouerghi,Responses of leaf growth and gas exchanges to salt stress during reproductive stage in wild wheat relative Aegilops geniculata Roth.and wheat(Triticum durum Desf.),Acta Physiol.Plant.35(2013)1453—1461.
[62]M.W.van Slageren,Wild wheats:a monograph of Aegilops L. and Amblyopyrum(Jaub.&Spach)Eig(Poaceae),Wageningen Agricultural University Papers,1994.7—95.
[63]I.Molnár,L.Gáspár,É.Sárvári,S.Dulai,B.Hoffmann,M. Molnár-Láng,G.Galiba,Physiological and morphological responses to water stress in Aegilops biuncialis and Triticum aestivum genotypes with differing tolerance to drought,Funct.Plant Biol.31(2004)1149—1159.
[64]T.D.Colmer,T.J.Flowers,R.Munns,Use of wild relatives to improve salt tolerance in wheat,J.Exp.Bot.57(2006)1059—1078.
[65]L.Wang,K.Liu,S.Mao,Z.Li,Y.Lu,J.Wang,Y.Liu,Y.Wei,Y. Zheng,Large-scale screening for Aegilops tauschii tolerant genotypes to phosphorus deficiency at seedling stage,Euphytica(2014)http://dx.doi.org/10.1007/s10681-014-1327-6.
[66]J.Dvorak,M.Luo,Z.Yang,H.Zhang,The structure of the Aegilops tauschii genepool and the evolution of hexaploid wheat,Theor.Appl.Genet.97(1998)657—670.
[67]G.Petersen,O.Seberg,M.Yde,K.Berthelsen,Phylogenetic relationships of Triticum and Aegilops and evidence for the origin of the A,B,and D genomes of common wheat(Triticum aestivum),Mol.Phylogenet.Evol.39(2006)70—82.
[68]F.Bordbar,M.R.Rahiminejad,H.Saeidi,F.R.Blattner,Phylogeny and genetic diversity of D-genome species of Aegilops and Triticum(Triticeae,Poaceae)from Iran based on microsatellites,ITS,and trnL-F,Plant Syst.Evol.291(2011)117—131.
[69]M.Feldman,E.R.Sears,The wild gene resources of wheat,Sci. Am.244(1981)102—112.
[70]B.Kilian,K.Mammen,E.Millet,R.Sharma,A.Graner,F. Salamini,K.Hammer,H.Ozkan,Aegilops,in:C.Kole(Ed.),Wild Crop Relatives:Genomic and Breeding Resources,Cereals,Springer,Berlin 2011,pp.1—76.
[71]T.Hussien,R.Bowden,B.Gill,T.Cox,Chromosome location of leaf rust resistance gene Lr43 from Aegilops tauschii in common wheat,Crop Sci.37(1997)1764—1766.
[72]A.Mujeeb-Kazi,L.Gilchrist,G.Fuentes-Davila,R.Delgado,Production and utilization of D genome synthetic hexaploids in wheat improvement,Proceedings of the Third International Triticeae Symposium,ICARDA,Science Publishers Enfield,NH,1998.
*Corresponding author.Tel.:+86 28 86290951;fax:+86 28 82650350.
E-mail address:liuyaxi@sicau.edu.cn(Y.Liu).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.1These authors contributed equally to this work.
http://dx.doi.org/10.1016/j.cj.2015.04.005
2214-5141/©2015 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
27 January 2015
杂志排行
The Crop Journal的其它文章
- Tillage and straw mulching impacts on grain yield and water use efficiency of spring maize in Northern Huang-Huai-Hai Valley
- Genetic diversity and association mapping for salinity tolerance in Bangladeshi rice landraces
- Physiological responses of Brassica napus to fulvic acid under water stress:Chlorophyll a fluorescence and antioxidant enzyme activity
- Field evaluation of durum wheat landraces for prevailing abiotic and biotic stresses in highland rainfed regions of Iran
- Genetic diversity and DNA fingerprinting in jute (Corchorus spp.)based on SSR markers
- Crop rotation-dependent yield responses to fertilization in winter oilseed rape(Brassica napus L.)
