Physical Properties of[AMIM]Cl with Different Organic Solvents to Account for Underlying Interactions
2015-08-11SABAHinaZHANGYumei张玉梅WANGHuaping王华平
SABA Hina,ZHANG Yu-mei(张玉梅),WANG Hua-ping(王华平)
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University,Shanghai 201620,China
Physical Properties of[AMIM]Cl with Different Organic Solvents to Account for Underlying Interactions
SABA Hina,ZHANG Yu-mei(张玉梅)*,WANG Hua-ping(王华平)
State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University,Shanghai 201620,China
In order to understand the interactions of the mixtures of ionic liquids with different organic solvents,density,conductivity and refractive index have been investigated for binary mixtures of 1-allyl-3-methyl-imidazolium chloride([AMIM]Cl)with acetonitrile,ethanol,and dimethyl sulfoxide(DMSO)at 298.15 K.Density and refractive index were found to increase with increasing[AMIM]Cl concentration.Conductivity was found to increase initially by the addition of[AMIM]Cl.However,as the amount of[AMIM]Cl increases,a gradual decrease in conductivity of the mixtures has been observed.The resultant properties were caused by subsequent ion-ion and/or ion-solvent interactions depending on the extent of polarity,dielectric constants and miscibility of organic solvents being used.
ionic liquid mixtures; density; refractive index; conductivity;interactions
Introduction
Ionic liquids could be defined as polymeric hydrogen-bonded macromolecules,and when ionic liquids were mixed with other solvents(water or organic solvents),they could be considered as nanostructured aggregates comprising both polar and non-polar regimes rather than homogeneous solvents[1].Various methods have been used to illustrate interactions within these mixtures such asinfrared (IR) spectroscopy[2],nuclearmagnetic resonance(NMR)[3],molecular dynamics[4],and high-pressure infrared spectroscopic[5].Water and organic solvents mixtures with different ionic liquids,constitute by far the most commonly used media for many technological processes[6].In particular,they play an important role in the recovery of acetone,butanol,and ethanol produced in fermentation processes[7]. The physiochemical properties of several mixtures of ionic liquids within aqueous media[8-10]and with organic solvents[11-18]have already been widely scrutinized.[AMIM]Cl has shown potential in organic synthesis and polymer processing.Swatloski et al.were the first ones to demonstrate the utilization of 1-butyl-3-methyl-imidazolium([BMIM]Cl)chloride as a solvent for cellulose processing[19]. Afterwards, 1-allyl-3-methylimidazolium chloride([AMIM]Cl)was discovered as an alternative solvent for cellulose processing[20].However,to deal with high viscosities of these ionic liquids and to develop different technologies,it would be quite interesting to study the physical properties of these ionic liquids in the presence of different organic solvents. Presently, the amount of physicochemical data available for ionic liquids is insufficient to create a database of knowledge relating to their applicability in separation processes[18].
In current studies,various physical parameters have been investigated for 1-allyl-3-methyl-imidazolium chloride[AMIM]Cl+organic solvent mixtures.Previously,aqueous mixtures of[AMIM]Cl have been investigated by Wu et al.[8]However,due to extensive applications of ionic liquids in organic synthesis, polymer processing and electrochemical studies in the presence of organic solvents,we find it intriguing to study physical properties of certain organic solvents such as dimethyt sulfoxide(DMSO),ethanol and acetonitrile with[AMIM]Cl.These organic solvents have been selected due to their quite distinct solvent properties such as dielectric constants and extent ofmiscibility with[AMIM]Cl.
1 Experimental
[AMIM]Cl was purchased from Chengjie Chemical Co.,Ltd.,Shanghai.DMSO and ethanol have been bought from Shanghai Chemical Reagents Company.Doubly distilled water was used in all experiments.The details of the chemicals being used with their percentage purity and water fraction have been illustrated in Table 1.The purity of[AMIM]Cl was verified in terms of NMR analysis.The water mass fraction of[AMIM]Cl was determined by the Karl Fisher titration(ZSD-2 KF Cany Precision Instruments Co.,Ltd.).A 2WAJ Abbe refractometer had been used for the measurements of the refractive index.Calibration of the instrument has been done by means of deionized water.The temperature was maintained at(298.15±0.01)K with an externaltemperature controller(HX-1008 low constant temperature circulating water baths,Shanghai BILON Instrament Manufacturing Co.,Ltd.).The uncertainty inrefractive index measurements was found to be(±1.0×10-4).

Table 1 Purity grades with experimental and theoretical values for properties of the pure components and[AMIM]Cl
The conductivity measurements were made with a DDSJ-308A conductometer(Shanghai Optical Instrument Factory); cell constant was 1.0 S/cm and uncertainty was found to be 0.5%.The temperature was maintained at(298.15±0.01)K with a DC-2006 low-temperature thermostat (Shanghai Hengping Instrument Factory).Before measuring the densities,DA-130N Density Specific Gravity Meter(Kyoto Electronics Manufacturing Co.,Ltd.,Japan)was calibrated with pure water at(298.15±0.01)K.The uncertainty in density was estimated to be(5×10-4g/cm3).Repetition of all the data was carried out three times.
2 Results and Discussion
Certain physical properties such as density,refractive index,viscosity and conductivity have been measured formixtures of[AMIM]Cl with acetonitrile,DMSO and ethanol.Various models have been used for describing the underlying interactions between ionic liquids and organic solvents.
In order to elucidate the extent of interactions within solutesolvent molecules,the excess molar volumes(VE) were calculated for all the systems by Eq.(1)[24]:
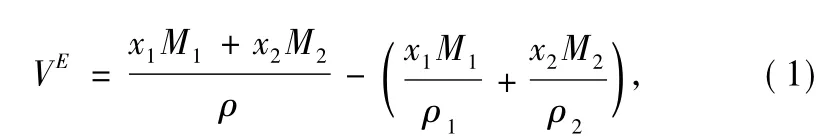
where ρ depicts density of mixtures;x and M represent mole fractions and molar masses.Subscripts 1 and 2 correspond to pure ionic liquid and organic solvents,respectively.All the values of excess molar volume have been fitted by using Redlich-Kister polynomial equation:

where Aiis the adjustable parameter.The data of excess molar volume and coefficients of the Redlich-Kister equation are given in Table 2 and 3,respectively.The presence of negative values of excess molar volumes clearly indicates the existence of considerable intermolecular interaction within these mixtures.
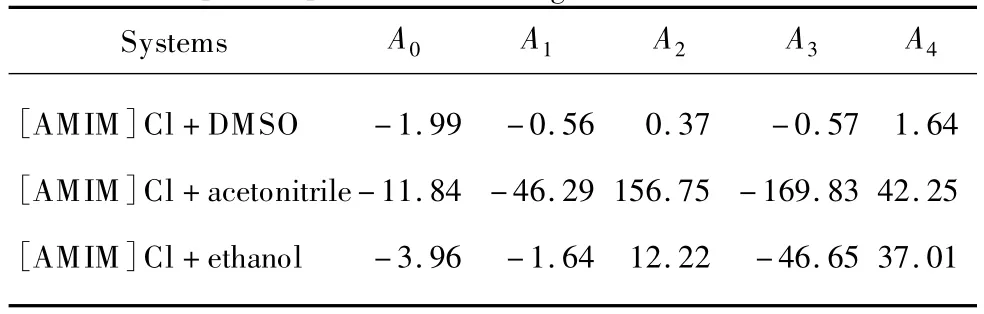
Table 2 Coefficients of the Redlich-Kister equation for VEof[AMIM]Cl in different organic solvents at 298.15 K
2.1 Effect of organic solvent on the density of[AMIM]Cl
In current studies,acetonitrile,DMSO and ethanol have been selected for exploring the effect of organic solvents on density of the[AMIM]Cl.All organic solvents being used represent quite diminished classes of solvents(being protic and aprotic)and hence exhibit varying extent of interactions within the binary system.The relationship of densities and mole fractions(x)of[AMIM]Cl with acetonitrile,DMSO and ethanol is shown in Fig.1.Related data is shown in Table 3.It is clearly indicated that density of the mixtures increase with increasing concentration.
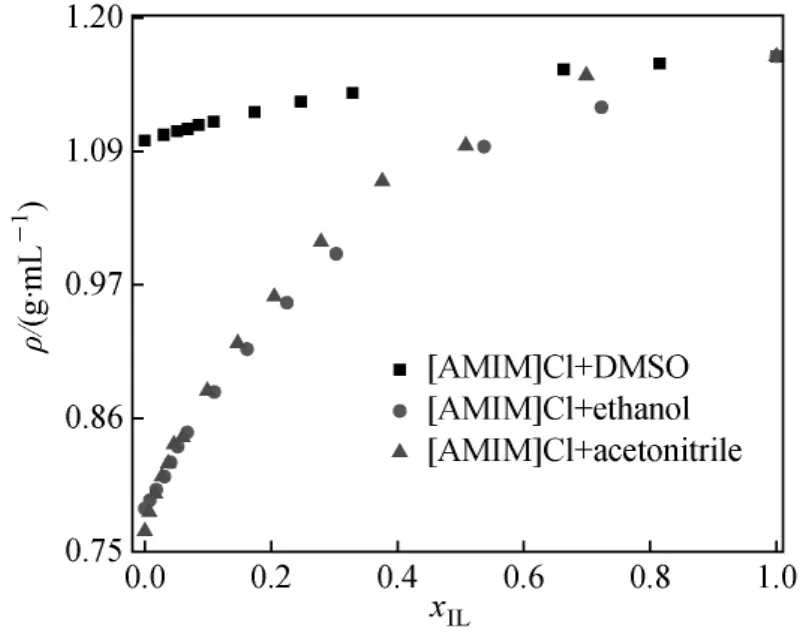
Fig.1 Relationship between density and mole fraction of[AMIM]Cl(xIL) in different organic solvents at 298.15 K
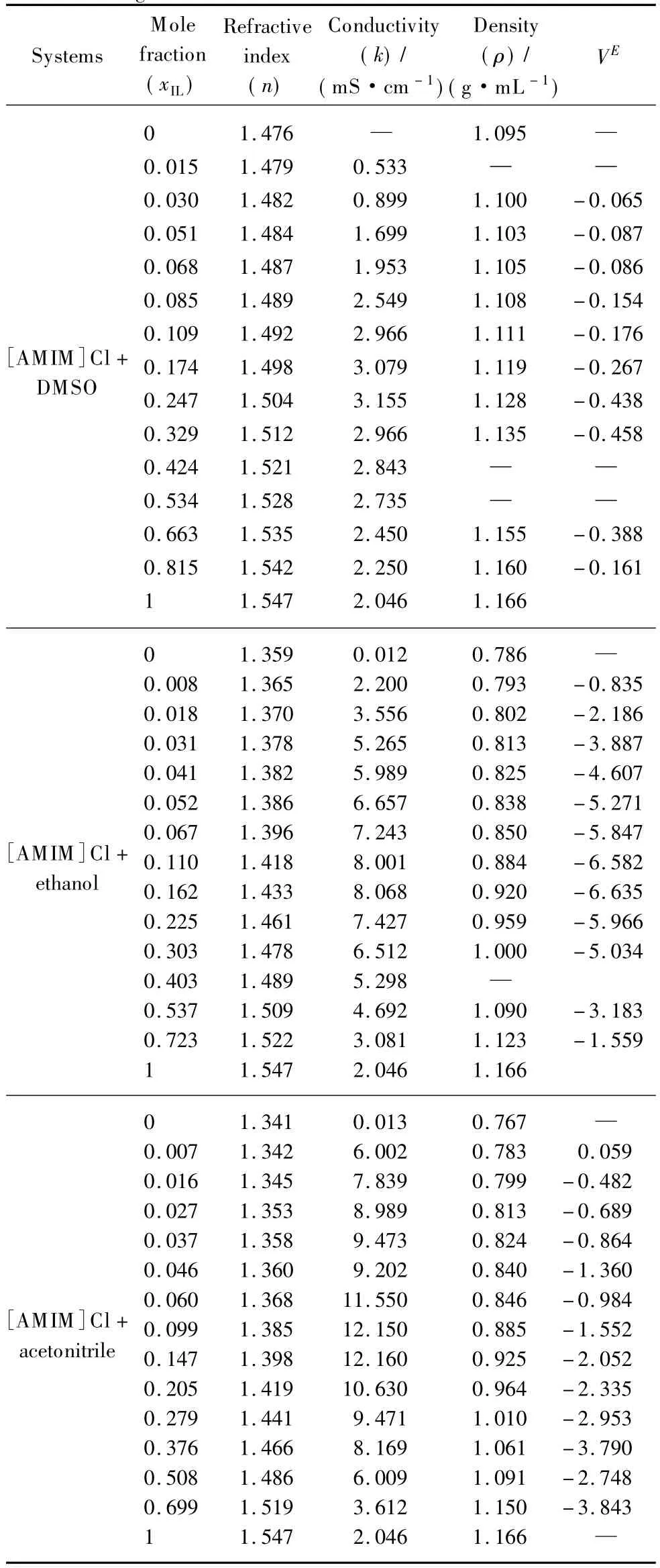
Table 3 Physical data for mixtures of[AMIM]Cl with different organic solvents at 298.15 K
These results are in quite agreement with the results of our group published previouslyfor aqueous mixtures ofionic liquid[8]as well as with several studies acquired for[AMIM]Cl +organic solvents mixtures[25-27].The density of the mixtures was found to be dependent on the molar masses of the ions and ionic liquids with heavy atoms are generally denser.It is found that the[AMIM]Cl mixtures with acetonitrile and ethanol showed the same trend in density measurements, while[AMIM]Cl+DMSO mixtures show the highest densities compared with other systems being reported as shown in Fig.1[11-13].
The[AMIM]Cl+DMSO mixtures showed higher densities compared with[AMIM]Cl mixtures with acetonitrile.And ethanol can be related to high molecular weight and hence higher density of DMSO itself.So the addition of DMSO will lead to denser mixtures as compared with ethanol.It can be suggested that the varying extent of polarity as well as the different structure of solvents being used has enabled them to impart varying extent of interactions within the mixtures(Table 4).In order to elucidate further interactions,excess molar volumes(VE)were calculated by means of Eq.(1).The data has been fitted by Redlich-Kister equation and is shown in Fig.2 for[AMIM]Cl+acetonitrile mixture.Similar behavior has been observed for other mixtures.For all[AMIM]Cl+organic solvents systems,the VEis negative over all the concentration range being used.The negative values clearly indicate the existence of interactions and certain associations among the molecules of[AMIM]Cl and organic solvents.VEis found to be the resultant of contributions from some opposing effects.These effects could be chemical,physical or structural.

Table 4 Hildebrand,Hansen solubility parameters and dielectric constants for the organic solvents at 298.15 K

Fig.2 Relationship between excess molar volumes and mole fraction of acetonitrile(xs)with[AMIM]Cl at 298.15 K
The negative values of VEare due to the chemical intermolecular interactions which lead to volume decrease,and include chargetransfertypeforces and complex forming interactions.The excess molar volume is quite in agreement with other studies carried out previously and clearly indicates the extent of considerable associations.However,it could be suggested that in case of[AMIM]Cl+DMSO,these interactions are the maximum and are more dominated by the nature of solvent being used rather than its composition as being suggested in previous studies[30].This behavior could be justified by the dominance of structure of DMSO exhibiting high polarity(Table 4)and two bulky methyl groups.Therefore,the structure of DMSO is the main driving factor in determining the fate of resultant properties.Whereas in the cases of acetone and ethanol,the hindrance ofthe side groups is almost negligible and even with different extent of polarities,both mixtures of both solvents follow the same pattern and the composition of the solvent is found to play crucial role in overall interactions rather than their nature.The plausible scheme of the extent of interactions can be seen in Scheme 1.
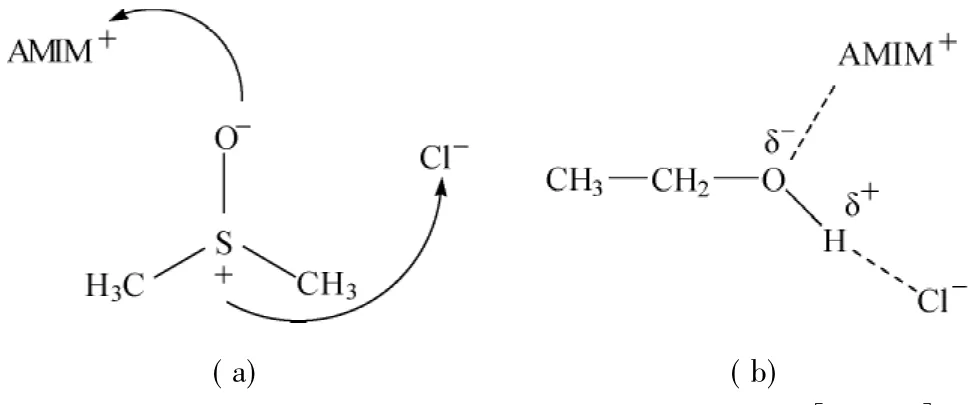
Scheme 1 Plausible scheme for the interactions between[AMIM]Cl with(a)DMSO and(b)ethanol
2.2 Effect of organic solvent on the refractive index of[AMIM]Cl
Refractive index is mostly used to have an insight about the polarizability/dipolarity of the system.The relationship of refractive index with mole fraction shows a gradual trend with increasing mole fraction(Fig.3)for all the[AMIM]Cl+ organic solvents mixtures as reported in Refs.[31-32].Relevant data for the all mixtures have been illustrated in Table 3.For all the systems being studied,refractive index has shown quite critical trend characteristic of each organic solvent.As the dielectric constant is believed to be the measure of miscibility[18],the behavior of these solvents could be related to their extent of polarity and interaction within mixtures as follows:DMSO>ethanol>acetonitrile.Generally,polar and dipolar solventsofhigh dielectric constantsshow better miscibility with ionic liquids indicating polar solvents have stronger molecular interactions with ionic liquids.As[AMIM]Cl+DMSO mixture has highest dielectric constant and solubility values compared with[AMIM]Cl+ethanol mixtures,its mixture shows the highest refractive index(Table 2).It could be suggested that DMSO has been able to disrupt the ionic liquid network within solution by means of strong hydrogen bonding thus leading to more pronounced intermolecular interactions and aggregate formation.

Fig.3 Relationship between refractive index(n)and mole fraction of[AMIM]Cl(xIL) in different organic solvents at 298.15 K
2.3 Effect of organic solvent on conductivity[AMIM]Cl+ organic solvents binary mixtures
Conductivity measurements are quite crucial in liquid systems as it depends on the extent ofassociations anddissociationswithin solventmolecules in mixtures. The conductivity of the mixture was found to be increased with increasing concentration of[AMIM]Cl.Except[AMIM]Cl+ DMSO mixtures(Fig.4),both the systems follow the general trend,i.e.,the conductivity increases rapidly in the organic solvent-rich region and then gradually decreases with increasing concentration of[AMIM]Cl.

Fig.4 Relationship between conductivity(K)and mole fraction of[AMIM] Cl(xIL) in different organic solvents at 298.15 K
It is quite evident that ion mobility increases in organic-rich region which enhancesitsconductivity.The increase of conductivity in high organic solvent concentration region can be attributed to the rapid and free movement of ions more easily as compared with[AMIM]Cl-rich region which can obstruct the motion of ions due to aggregates formation/association.
The mobility of ions could be related to overall resultant conductivity as follows[33]:

where nirepresents the number of charged moieties of species i,qidepicts the charge and μishows the ion mobility.So it can be elucidated that increasing the concentration of[AMIM]Cl will lead to strong interactions and thus hinder the ion mobility.
However,in caseof[AMIM]Cl+DMSO mixture,increasing concentration of the ionic liquid will make conductivity constant after reaching a maxima rather than being decreasing or vice versa.It could be deduced that[AMIM]Cl and DMSO were found not to form any type of considerable interactions or associations and hence conductivity not decreases efficiently leading to constant behavior.This behavior of DMSO is related to its structure as two methyl groups as well as lone pair of sulfur inhibits oxygen to interact from all direction and also provides aprotic character to the solvent.On the contrary,ethanol and acetonitrile provide the maximum availability of its oxygen atom.
3 Conclusions
All mixtures have shown traditional trend during density and refractive indexmeasurements,i.e.,density and refractive index increase with increasing concentration of[AMIM]Cl.The conductivity data show quite varying results for solvents being used.[AMIM]Cl+DMSO mixture depicts the nonconventional results as being expected and is suggested to be related to the absence of strong solute-solvent interactions.It is evaluated that the molar mass and hydrogen bonding ability of[AMIM]Cl,solubility parameters and dielectric constants of solvents being used play an important role in determining the extent of solute-solvent interactions within these mixtures.
[1]DupontJ.On the Solid, Liquid and Solution Structural Organization of Imidazolium Ionic Liquids[J].Journal of Brazilian Chemical Society,2004,15(3):341-350.
[2] Wu B,Liu Y,Zhang Y M,et al.Probing Intermolecular Interactions in Ionic Liquid-Water Mixtures by Near-Infrared Spectroscopy[J].Chemistry-A European Journal,2009,15 (28):6889-6893.
[3]Zhai C P,Wang J J,Zhao Y,et al.An NMR Relaxation Study for the Interactions of Some 1-Alkyl-3-Methylimidazolium Ionic Liquids with Acetone[J].Zeitschrift für Physikalische Chemie,2009,223(8):839-847.
[4]Brehm M,Weber H,Pensado A S,et al.Proton Transfer and Polarity Changes in Ionic Liquid-Water Mixtures:a Perspective on Hydrogen Bondsfrom Abinitio MolecularDynamicsatthe Example of 1-Ethyl-3-methylimidazolium Acetate-Water Mixtures-Part 1[J].Physical Chemistry Chemical Physics,2012,14(1): 5030-5044.
[5]Umebayashi Y,Jiang J C,Shan Y L,et al.Structural Change of Ionic Association in Ionic Liquid/Water Mixtures: a High-Pressure Infrared Spectroscopic Study[J].Journal of Chemical Physics,2009,130(12):124503-124503-6.
[6]Najdanovic-Visak V,Esperanca J M S S,Rebelo L P N,et al.Phase Behavior of Room Temperature Ionic Liquid Solutions:an Unusually Large Co-solvent Effect in(Water+Ethanol)[J].Physical Chemistry Chemical Physics,2002,4(10):1701-1703.
[7]Fadeev A G,Megher M M.Opportunities for Ionic Liquids in Recovery of Biofuels[J].Chemical Communications,2001(3): 295-296.
[8]Wu D,Wu B,Zhang Y M,et al.Density,Viscosity,Refractive Index and Conductivity of 1-Allyl-3-Methylimidazolium Chloride +Water Mixture[J].Journal of Chemical Engineering Data,2010,55(2):621-624.
[9]Liu W W,Cheng L Y,Zhang Y M,et al.The Physical Properties of Aqueous Solution of Room-Temperature Ionic Liquids Based on Imidazolium:Database and Evaluation[J].Journal of Molecular Liquid,2008,140(1/2/3):68-72.
[10]Gardas R L,Dagade H D,Coutinho J A P,et al.Thermodynamic Studies of Ionic Interactions in Aqueous Solutions of Imidazolium-Based Ionic Liquids[Emim][Br]and[Bmim][Cl][J].Journal of Physical Chemistry B,2008,112(11):3380-3389.
[11]Seddon K R,Stark A,Torres M J.Influence of Chloride,Water,and Organic Solvents on the Physical Properties of Ionic Liquids[J].Pure Applied Chemistry,2000,72(12):2275-2287.
[12]Ciocirlan O,Croitoru O,Iulian O.Densities and Viscosities for Binary Mixtures of 1-Butyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquid with Molecular Solvents[J].Journal of Chemical Engineering Data,2011,5(4):1526-1534.
[13]Sarkar S,Pramanik R,Ghatak C,et al.Probing the Interaction of 1-Ethyl-3-Methylimidazolium Ethyl Sulfate([Emim][EtSO4]) with Alcohols and Water by Solvent and Rotational Relaxation[J].Journal of Physical Chemistry B,2010,114(8):2779-2789.
[14]Singh S,Aznar M,Deenadayalu N.Densities,Speeds of Sound,and Refractive Indices for Binary Mixtures of1-Butyl-3-Methylimidazolium Methyl Sulphate Ionic Liquid with Alcohols at T=(298.15,303.15,308.15,and 313.15)K[J].Journal of Chemical Thermodynamics,2013,57(1):238-247.
[15]Matkowska D,Hofman T.Volumetric Properties of the Ionic Liquids:[C6mim][MeSO4],[C6mim][EtSO4],[C4mim][EtSO4] and their Mixtures with Methanol or Ethanol[J].Journal of Molecular Liquid,2013,177(1):301-305.
[16]Rathnam M V,Sayed R T,Bhanushali K R,et al.Density and Viscosity of Binary Mixtures of n-Butyl Acetate with Ketones at (298.15,303.15,308.15,and 313.15)K[J].Journal of Chemical Engineering Data,2012,57(6):1721-1727.
[17]Xu Y J,Yao J,Wang C M,et al.Density,Viscosity,and Refractive Index Propertiesforthe Binary Mixturesofn-Butylammonium Acetate Ionic Liquid+Alkanols at Several Temperatures[J].Journal of Chemical Engineering Data,2012,57(2):298-308.
[18]Shekaari H,Bezaatpour A,Elhami-Kalvanagh R.Thermodynamic Properties of Salophen Schiff Base+Ionic Liquid([CnmIm][Br])+Dimethylformamide Ternary Mixtures at 298.15 K[J].Journal of Chemical Engineering Data,2012,57(2):345-351.
[19]Swatloski R P,Spear S K,Holbrey J D,et al.Dissolution of Cellulose with Ionic Liquids[J].Journal of American Chemical Society,2002,124(18):4974-4795.
[20]Zhang H,Wu J,Zhang J,et al.1-Allyl-3-Methylimidazolium Chloride Room Temperature Ionic Liquid:a New and Powerful Nonderivatizing Solvent for Cellulose[J].Macromolecule,2005,38(20):8272-8277.
[21] Côté J F,Brouillette D,Desnoyers J E,et al.Dielectric Constants of Acetonitrile,γ-Butyrolactone,Propylene Carbonate,and 1,2-Dimethoxyethane as a Function ofPressure and Temperature[J].Journal of Solution Chemistry,1996,25(12): 1163-1173.
[22]Barton A F M.Handbook of Solubility Parameters and Other Cohesion Parameters[M].2nd ed.Florida,U.S.A:CRC Press,1983:55-65.
[23]Hansen C M.Hansen Solubility Parameters:a Users Handbook[M].2nd ed.Florida,U.S.A:CRC Press,2007:104-105.
[24]Ren R,Zuo Y,Zhou Q,et al.Density,Excess Molar Volume and Conductivity of Binary Mixtures of the Ionic Liquid 1,2-Dimethyl-3-Hexylimidazolium bis(trifluoromethylsulfonyl)imide and Dimethyl Carbonate[J].Journal of Chemical Engineering Data,2011,56(1):27-30.
[25]Pereiro A B,Tojo E,Rodríguez A,et al.Properties of Ionic Liquid HMIMPF6with Carbonates,Ketones and Alkyl Acetates[J].Journal of Chemical Thermodynamics,2006,38(6):651-661.
[26]Kurnia K A,Ariwahjoedi B,Abdul Mutalib M I,et al.Density and Excess Molar Volume of the Protic Ionic Liquid Bis(2-hydroxyethyl)ammonium Acetate with Alcohols[J].Journal of Solution Chemistry,2011,40(3):470-480.
[27]Iulian O,Ciocirlan O.Volumetric Properties of Binary Mixtures ofTwo 1-Alkyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquids with Molecular Solvents[J].Journal of Chemical Engineering Data,2012,57(10):2640-2646.
[28]MacGregor W S.The Chemical and Physical Properties of DMSO[J].Annals of the New York Academy of Sciences,1967,141 (1):3-12.
[29]Akerlof G.Dielectric Constants of Some Organic Solvent-Water Mixtures at Various Temperatures[J].Journal of American Chemical Society,1932,54(11):4125-4139.
[30]Arce A,Rodil E,Soto A.Physical and Excess Properties for Binary Mixtures of 1-Methyl-3-Octylimidazolium Tetrafluoroborate,[OMIM][BF4],Ionic Liquid with Different Alcohols[J].Journal of Solution Chemistry,2006,35(1):63-78.
[31]Kurnia K A,Taib M M,Abdul Mutalib M I,et al.Densities,Refractive Indices and Excess Molar Volumes for Binary Mixtures of Protic Ionic Liquids with Methanol at T=293.15 to 313.15 K[J].Journal of Molecular Liquids,2011,159(3):211-219.
[32]Anouti M,Vigeant A,Jacquemin J,et al.Volumetric Properties,Viscosity and Refractive Index ofthe Protic Ionic Liquid,Pyrrolidinium Octanoate in Molecular Solvents[J].Journal of Chemical Thermodynamics,2010,42(7):834-845.
[33]Every H,Bishop A G,Forsyth M,et al.Ion Diffusion in Molten Salt Mixtures[J].Electrochimica Acta,2000,45(8/9):1279-1284.
O631.4
A
1672-5220(2015)03-0406-05
date:2013-12-18
National Natural Science Foundation of China(No.51273041)
*Correspondence should be addressed to ZHANG Yu-mei,E-mail:zhangym@dhu.edu.cn
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Effect of Sludge Retention Time on the Fate of Proteins and Polysaccharides in AAO Process
- Compressive Strength Estimation for the Fiber-Reinforced Polymer(FRP)-Confined Concrete Columns with Different Shapes Using Artificial Neural Networks
- Force-Based Quadrilateral Plate Bending Element for Plate Using Large Increment Method
- Effects of 1,7-Bromine Substitution at Bay Area on Self-assembly Behavior and Photo Physical Properties of Perylene Diimide
- Theoretical and Experimental Analyses of Poisson Ratios for Plain-Woven Fabrics
- Dynamic Engaging Characteristics of Wet Clutch in Automatic Transmission
