血清尿酸和踝臂指数联合检测对慢性肾病的筛检作用
2015-07-12赵苗苗
赵苗苗, 张 杰, 李 觉
(同济大学医学院心肺血管中心,上海 200092)
·临床研究·
血清尿酸和踝臂指数联合检测对慢性肾病的筛检作用
赵苗苗, 张 杰, 李 觉
(同济大学医学院心肺血管中心,上海 200092)
目的 了解血清尿酸(serum uric acid, SUA)和踝臂指数(ankle brachial index, ABI)单独与慢性肾病(chronic kidney disease, CKD)的关系。方法 本研究选取北京和上海8所大学附属医院年龄≥35岁具有动脉硬化高危因素的内科住院患者3732例,将其中具有完整基线资料的3557例作为研究对象。以logistic回归分析评估SUA和ABI与CKD的关系;以ROC曲线探讨二者联合检测对CKD筛检的应用价值。结果 logistic回归分析调整性别、年龄和其他变量后,SUA四分位分组,以Q1为参照,Q2、Q3、Q4的调整OR值及95%CI分别为2.274(95%CI,1.696~3.049),3.770(95%CI,2.833~5.015)和8.532(95%CI,6.435~11.312)。以正常对照组为参照,PAD临界组、轻度至中度PAD组、重度PAD组的调整OR值及95%CI分别为1.499(95%CI,0.932~2.411),1.996(95%CI,1.206~3.306)和2.636(95%CI,1.641~4.236)。在全人群、男性和女性中SUA和ABI联合筛检CKD,ROC曲线下AUC分别为0.731、0.734和0.755,相对应的SUA单独筛检ROC曲线下AUC分别为0.702、0.698和0.741。结论 SUA水平升高是CKD的独立危险因素,ABI越低发生CKD的可能性越大。在高危人群中,SUA和ABI联合检测对CKD具有重要的筛检价值。
血清尿酸; 踝臂指数; 联合筛检价值; 慢性肾病; 中国住院患者
近年来,全球慢性肾病(chronic kidney disease, CKD)的发生逐年上升,已成为严重危害人类健康的公共卫生问题。中国各地区因生活方式和经济水平的差异,CKD发生情况有所不同[1-4]。有研究[5]显示,我国成年人群中CKD的患病率为10.8%,据此估计我国现有成年CKD患者大约1.2亿人。大多数CKD患者早期没有明显症状,因此,早期对CKD进行筛检尤其对高危人群是有效预防和减缓CKD进程及降低一系列并发症风险的重要措施。
国内外临床及流行病学调查结果显示血清尿酸(serum uric acid, SUA)水平在CKD发生发展过程中发挥着重要的作用[6-7]。踝臂指数(ankle brachial index, ABI)是踝收缩压与上臂收缩压的比值,临床上常用以筛选外周动脉疾病(peripheral arterial disease, PAD)。有研究[8-9]表明在CKD患者中PAD的发生率显著升高,且异常的ABI低值或高值是CKD的独立危险因素[10-12]。
目前还没有文献报道SUA和ABI联合检测对CKD的筛检作用。本研究目的在于了解高危人群中SUA和ABI单独与CKD的关系,同时探讨二者联合检测对CKD的筛检价值。
1 资料与方法
1.1 一般资料
连续收集2004年7月至2005年1月上海和北京地区8所大学附属医院内科住院患者为研究对象。入选标准同时具备下列条件: 年龄≥35岁,且具有动脉硬化危险因素(包括糖尿病、高血压、血脂紊乱、脑卒中史、冠心病史和吸烟史)。排除标准: 年龄和动脉硬化危险因素不符合入选条件者,严重心、肝、肾功能衰竭者。本次研究经同济大学伦理委员会批准,每位研究对象在被调查前均经知情同意。
1.2 调查方法
基线资料的收集采用问卷调查的方法,每位患者测量ABI的同时,由经过培训的流行病学专业人员收集研究对象的一般情况、生活方式、疾病史等信息;血生化样品均在早晨空腹至少12h的情况下采集;ABI值的测量统一使用5MHz手持超声探头,(仪器型号: EliteModel.100R),血压计袖带气囊宽10cm,长40cm,测量时研究对象采取仰卧位。ABI值为胫后动脉或足背动脉收缩压与前臂收缩压的最高值之比,本研究取左右ABI中的较小值,ABI≤0.9定义为PAD。本研究删除ABI>1.4的数据,以减少假阴性[13]。按照美国PAD患者治疗指南,根据ABI水平的不同,将研究对象分为4组: 重度PAD组(ABI: ≤0.40)、轻度至中度PAD组(ABI: 0.41~0.90)、临界组(ABI: 0.91~0.99)、正常对照组(ABI: 1.00~1.40)。
1.3 诊断标准
正常嘌呤饮食状态下,非同日2次空腹SUA水平男性≥416μmol/L,女性≥357μmol/L,即可诊断为高尿酸血症(hyperuricemia, HUA)。
根据美国临床实践指南(K/DOQI)标准,肾脏损伤或肾功能下降持续≥3个月诊断为CKD[14]。肾脏损伤表现为病理性异常、影像学异常或肾脏损伤指标异常(血或尿成分异常)。应用简化MDRD公式估算肾小球滤过率(GFR)[21],即GFR[ml/(min·1.73m2)]=186×SCr-1.154×(年龄,岁)-0.203×0.742(女性)。以估算GFR<60ml/(min·1.73m2)定义为肾功能下降。
1.4 统计学处理

2 结 果
2.1 一般情况
共调查高危患者3732例,其中3557例具有完整的基线资料,进入统计分析。研究对象的平均年龄为(66.96±11.12)岁,男性1885例(53%)。共有CKD患者841例(23.6%),HUA患者797例(22.4%),PAD患者927例(26.1%)。
2.2 基线特征
CKD组患者的年龄、女性构成比、TG、SBP、SUA、糖尿病史、冠心病史及脑卒中史均高于非CKD组,而ABI值前者低于后者,差异均有统计学意义,见表1。
表1 CKD组和正常组的基线特征比较Tab.1 Baseline characteristics of subjects with CKD and without CKD ±s,n(%)]
1mmHg=0.133kPa
2.3 CKD的危险因素
以CKD为因变量,基线分析中差异有统计学意义的因素为自变量,拟合多因素logistic回归模型。结果显示高龄、女性、SBP升高、HUA、PAD、糖尿病史和冠心病史是CKD的独立危险因素,见表2。
2.4 SUA和ABI与CKD的关系
CKD患病率随着SUA水平的升高有增加的趋势;随着ABI水平的降低,CKD患病率逐渐升高。SUA四分位1~4组的CKD患病率及不同ABI亚组的CKD患病率见图1,各组间患病率的差异有统计学意义(P<0.001)。采用多因素logistic回归分析,调整年龄、性别、TG、SBP、糖尿病史、冠心病史和脑卒中史后,不同SUA水平组与第一组相

表2 CKD相关危险因素的多因素logistic回归分析Tab.2 Multiple logistic regression analysis for risk factors associated with CKD
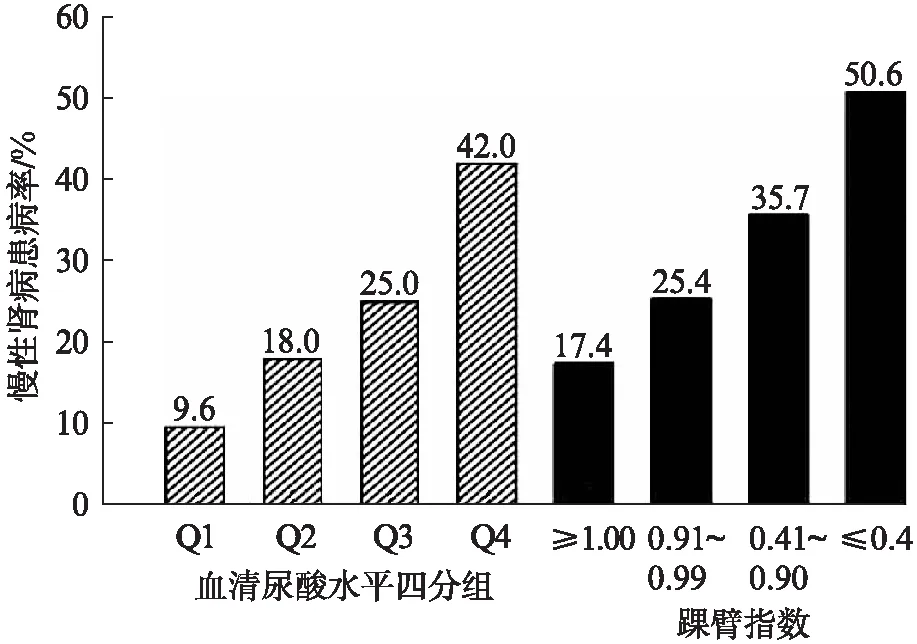
图1 不同SUA水平组和ABI亚组的CKD患病率Fig.1 Prevalence of CKD by quartile of SUAlevels and ABI subgroups
比较及不同ABI组与正常对照组比较的分析结果见图2。

图2 不同SUA水平组和ABI亚组发生CKD的OR值及95%CIFig.2 Adjusted ORs and 95%CI for CKD of SUAlevels and ABI subgroups
2.5 SUA和ABI联合检测对CKD的筛检作用
采用ROC曲线对SUA和ABI联合检测对CKD进行筛检与SUA单独筛检CKD进行比较。结果显示在全人群中二者联合筛检ROC曲线下面积(area under the curve, AUC)为0.731(95%CI,0.711~0.751),SUA单独筛检AUC为0.702(95%CI,0.682~0.722);在男性中联合筛检AUC为0.734(95%CI,0.705~0.763),SUA单独筛检AUC为0.698(95%CI,0.668~0.727);在女性中联合筛检AUC为0.755(95%CI,0.728~0.781),SUA单独筛检AUC为0.741(95%CI,0.714~0.767),差异均有统计学意义(P<0.001),见图3。
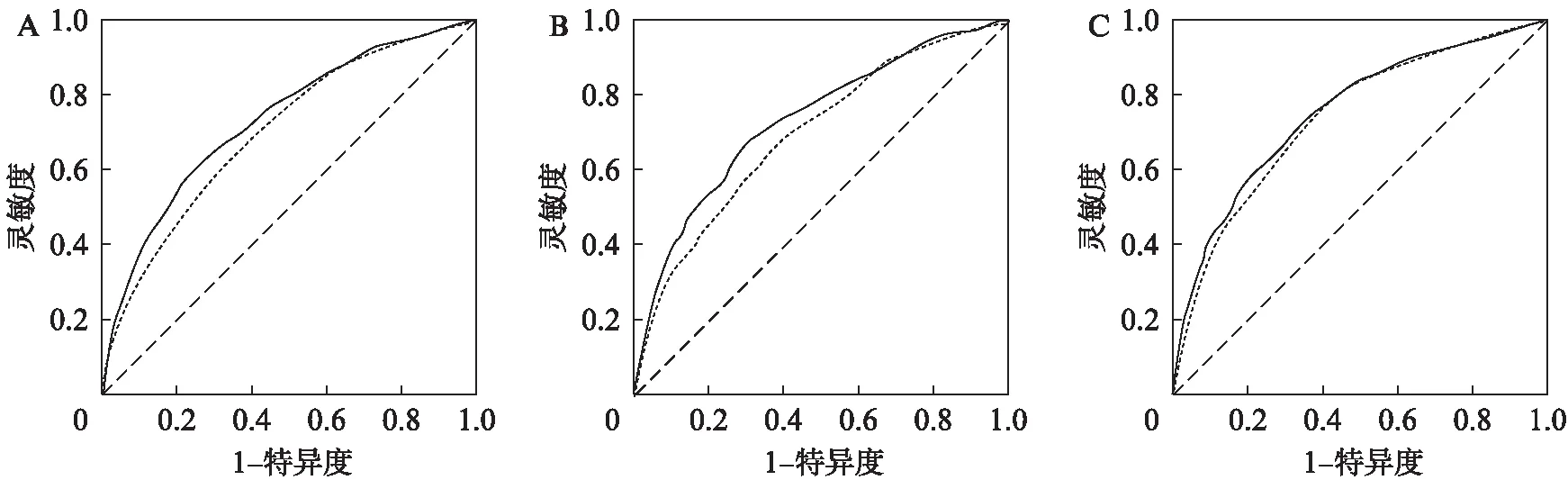
图3 全人群(A)、男性(B)和女性(C)中SUA和ABI联合筛检与SUA单独筛检CKD的ROC曲线Fig.3 ROC curves for CKD in all population(A), males(B)and females(C)by using SUA and ABI compared to SUA aloneA: 全人群;B: 男性;C: 女性
3 讨 论
CKD目前已成为全球威胁健康的公共卫生问题,给家庭、社会带来了巨大的经济负担和精神负担。近年来,肾脏病学界关注重点由慢性肾衰竭及其替代治疗前移至CKD早期。本研究结果显示SUA水平升高是CKD的独立危险因素,ABI越低发生CKD的可能性越大。在高危人群中,SUA联合ABI对CKD具有重要的筛检价值。
大多数CKD患者早期没有症状或症状较轻,因此早期对CKD进行筛检可以及早地发现和治疗、进而延缓或阻止疾病的进程,降低治疗费用,为患者家人及社会减轻疾病负担。有针对性的CKD筛查其成本效益与乳腺癌、宫颈癌和结直肠癌筛查的成本效益大体相似。目前全球范围内开展了一些有针对性的CKD筛检项目。2007年澳大利亚在社区高危人群中进行CKD筛检,目标人群包括年龄>50岁,患有糖尿病、高血压或一级肾衰竭患者[15]。2010年新南威尔士在高危住院患者中推行Kidney Health Check(KHC)计划[16],通过对CKD的早期筛检延缓其发生发展。
本研究选择了具有动脉硬化危险因素的内科住院患者为目标人群,目标人群中CKD患病率为23.6%,远远超过一般人群。ABI值的测量因其无创性及操作方便的特点,适合在大人群的筛检项目中应用。本研究首次在高危住院患者中应用SUA和ABI联合检测对CKD进行筛检,发现在全人群、男性和女性中二者联合筛检ROC曲线下AUC分别为0.731(95%CI: 0.711~0.751)、0.734(95%CI: 0.705~0.763)和0.755(95%CI: 0.728~0.781),相对应的SUA单独筛检ROC曲线下AUC为0.702(95%CI: 0.682~0.722)、0.698(95%CI: 0.668~0.727)和0.741(95%CI: 0.714~0.767),差异有统计学意义。研究结果表明SUA联合ABI对CKD具有较强的预测价值,此结果为CKD的筛检提供理论依据。
本研究属于横断面研究,SUA及ABI与CKD的因果关系有待于前瞻性研究进一步加以证实。综上所述,SUA水平越高发生CKD的可能性越大,低ABI值增加了CKD发生的风险。SUA联合ABI在高危人群中对CKD具有较强的筛检价值。因此高危人群可以同时检测SUA及ABI值对CKD进行筛检,以做到早发现、早预防、早治疗。
[1] Zhou Y, Sun Q, Ruan XN, et al. Epidemiology of chronic kidney disease in adults of Pudong New Area, Shanghai [J]. Chin J Nephrol, 2011,27(7): 504-510.
[2] Zhang L, Zhang P, Wang F, et al. Prevalence and factors associated with CKD: a population study from Beijing [J]. Am J Kidney Dis, 2008,51(3): 373-384.
[3] Wang H, Zhang L, Lv J. Prevention of the progression of chronic kidney disease: practice in China[J]. Kidney Int Suppl, 2005, 94: S63-S67.
[4] Chen W, Liu Q, Wang H, et al. Prevalence and risk factors of chronic kidney disease: a population study in the Tibetan population[J]. Nephrol Dial Transplant, 2011,26(5): 1592-1599.
[5] Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey[J]. Lancet, 2012,379(9818): 815-822.
[6] Ryoo JH, Choi JM, Oh CM, et al. The association between uric acid and chronic kidney disease in Korean men: a 4-year follow-up study[J]. J Korean Med Sci, 2013,28(6): 855-860.
[7] Bose B, Badve SV, Hiremath SS, et al. Effects of uric acid-lowering therapy on renal outcomes: a systematic review and meta-analysis[J]. Nephrol Dial Transplant, 2014,29(2): 406-413.
[8] Traynor J, Mactier R, Geddes CC, et al. How to measure renal function in clinical practice[J]. BMJ, 2006,333(7571): 733-777.
[9] O’Hare AM, Glidden DV, Fox CS, et al. High prevalence of peripheral arterial disease in persons with renal insufficiency: results from the National Health and Nutrition Examination Survey 1999-2000[J]. Circulation, 2004,109(3): 320-323.
[10] Foster MC, Ghuman N, Hwang SJ, et al. Low ankle-brachial index and the development of rapid estimated GFR decline and CKD[J]. Am J Kidney Dis, 2013,61(2): 204-210.
[11] Dong X, Wu D, Jia C,et al. Low ankle-brachial index is associated with early-stage chronic kidney disease in type 2 diabetic patients independent of albuminuria[J]. PLoS One, 2014,9(10): e109641.
[12] Liu H, Shi H, Yu J, et al. Is chronic kidney disease associated with a high ankle brachial index in adults at high cardiovascular risk?[J]. J Atheroscler Thromb, 2011,18(3): 224-230.
[13] McDermott MM, Greenland P, Liu K, et al. The ankle brachial index is associated with leg function and physical activity: the Walking and Leg Circulation Study[J]. Ann Intern Med, 2002,136(12): 873- 883.
[14] Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidnev Disease: Improving Global Outcomes (KDIGO)[J]. Kidnev Int, 2005,67(6): 2089-2100.
[15] Mathew TH, Corso O, Ludlow M, et al. Screening for chronic kidney disease in Australia: A pilot study in the community and workplace[J]. Kidney Int Suppl, 2010,116: S9-16.
[16] NSW Health. Kidney health check: promoting the early detection & management of chronic kidney disease[M]. Sydney: NSW Health,2010.
Combined measurement of serum uric acid and ankle brachial index for screening of chronic kidney disease
ZHAOMiao-miao,ZHANGJie,LIJue
(Heart, Lung and Blood Vessel Center, Medical College, Tongji University, Shanghai 200092, China)
Objective To evaluate the combined measurement of serum uric acid (SUA) and ankle brachial index (ABI) for screening of chronic kidney disease (CKD). Methods A total of 3732 inpatients aged over 35 years and at high risk of atherosclerosis were enrolled from eight university-affiliated hospitals in Beijing and Shanghai. Complete baseline data were obtained from 3557 of them. Logistic regression analysis was applied to assess the association of SUA and ABI with CKD and ROC curves were performed to evaluate the combined measurement of SUA and ABI for screening of CKD, compared to using SUA alone. Results After multivariable adjustment for age, gender, and other confounders, patients with the second, third and fourth quartile of SUA value had theORs of 2.274 (95%CI, 1.696-3.049), 3.770 (95%CI, 2.833-5.015) and 8.532(95%CI, 6.435-11.312) respectively, when the lowest quartile used as reference. Compared to the individuals with an ABI≥1.00, theORs for patients with ABI: 0.91-0.99, 0.41-0.90,≤0.40 were 1.499 (95%CI, 0.932-2.411),1.996(95%CI, 1.206-3.306) and 2.636(95%CI,1.641-4.236) after multivariable adjustment. AUCs of ROC curves for combined SUA and ABI to screen for CKD were 0.731, 0.734, and 0.755 in all population, males and females, respectively; while the corresponding AUCs for SUA alone were 0.702, 0.698 and 0.741, respectively. Conclusion A high SUA level is independently associated with a high risk of CKD. There is an inverse association between ABI and CKD. The combined measurement of SUA and ABI can be more effective for screening of CKD among the people at high risk.
serum uric acid; ankle brachial index; combined screening value; chronic kidney disease; Chinese hospital inpatients
10.16118/j.1008-0392.2015.04.015
2015-03-19
上海市气象与健康重点实验室开放基金(QXJK201404)
赵苗苗(1989—),女,硕士研究生.E-mail: mmz_1989@163.com
李 觉.E-mail: jueli@tongji.edu.cn
R 692
A
1008-0392(2015)04-0075-05
