丹参经肝动脉灌注对大鼠移植性肝癌的治疗及其肝功能保护作用研究
2015-07-07魏明琴蒋建霞
魏明琴,蒋建霞
(1.湖北医药学院附属襄阳医院 肿瘤科,湖北 襄阳 441000;2.南京医科大学,江苏 南京 210000)
丹参经肝动脉灌注对大鼠移植性肝癌的治疗及其肝功能保护作用研究
魏明琴1,蒋建霞2
(1.湖北医药学院附属襄阳医院 肿瘤科,湖北 襄阳 441000;2.南京医科大学,江苏 南京 210000)
目的 观察丹参经肝动脉灌注对大鼠移植性肝癌的治疗作用,并研究丹参在抗肿瘤的同时对移植性肝癌大鼠肝功能的保护作用。方法 选用Sprague-Dawley雄性大鼠150只,并成功建立Walker-256移植性肝癌模型120只。所有造模大鼠随机分为对照组、丝裂霉素组和丹参组,每组40只。正常饲养10 d后,取各组大鼠血液检测血清ALT、AST和ALP水平,剖腹测量计算移植性瘤体体积。同时行经胃十二指肠动脉至肝脏固有动脉插管灌注治疗:对照组大鼠灌注2 mL的注射用生理盐水,丝裂霉素组大鼠灌注丝裂霉素(2.5 mg/kg),丹参组灌注丹参(1.6 g/kg)。灌注1周后,取血液检测ALT,AST和ALP水平,测量计算瘤体体积,并计算肿瘤增长率和抑制率。同时处死所有大鼠,取出瘤体,显微镜下观察肿瘤坏死范围并判断坏死程度。结果 灌注治疗后,对照组、丝裂霉素组和丹参组大鼠分别剩余37只、37只和38只。丝裂霉素组和丹参组肿瘤体积及增长率均明显小于对照组(P<0.05),丝裂霉素组和丹参组肿瘤体积及增长率无明显差异。丝裂霉素组和丹参组对移植性肝癌肿瘤的抑制率分别为22.32%和26.38%,差异无统计学意义。灌注后,丹参组和丝裂霉素组大鼠中度坏死明显高于对照组,丝裂霉素组和丹参组轻、中、重肿瘤坏死分布无显著性差异。灌注后,丹参组和丝裂霉素组大鼠血清ALT,AST和ALP的水平均明显低于对照组,差异有统计学意义(P<0.01,P<0.05);丹参组上述指标显著低于丝裂霉素组,差异具有统计学意义(P<0.05)。结论 丹参经肝动脉灌注对大鼠移植性肝癌有良好的抑制作用,其抑瘤活性与常用抗肿瘤药物丝裂霉素的活性相当。在抑制肿瘤的同时对移植性肝癌大鼠的肝功能也具有较好的保护作用。
丹参;肝动脉灌注;移植性肝癌;肝功能保护
肝癌是一种十分常见的恶性消化道肿瘤,其病死率高、危害性大,已经位居世界肿瘤性疾病的第3位,且有逐年升高的趋势[1-4]。中国是肝癌的高发区,其发患者数约占全世界总发病数的一半,而其死亡率也位居世界之首。目前,治疗肝癌的首选方法是进行手术切除,但是大多数的患者在确诊为肝癌时已经错过了最佳的手术时机[5]。由于肝脏供血主要来自肝动脉,且肝癌血供中有90%来自肝动脉[6]。所以,对于不能进行手术切除治疗的患者,目前主要采用经肝动脉灌注化疗或经肝动脉栓塞化疗[7]。丹参作为一味传统中药,归心、肝经,有祛瘀止痛,活血通经之功效[8]。近年来的研究表明丹参具有十分广泛的药理活性,如抑制血小板凝聚、扩张冠状动脉等功效[9-10]。此外,丹参能够显著抑制多种肿瘤细胞的增殖。为了进一步研究丹参在治疗肝癌和肝功能保护方面的特殊作用,本研究建立了大鼠移植性肝癌模型,并对肝癌造模小鼠进行丹参肝动脉灌注治疗,以观察丹参对肝癌的治疗效果及肝功能保护作用。
1 材料与方法
1.1 实验试剂 注射用丹参粉针剂(哈药集团中药二厂,国药准字Z10970093);注射用丝裂霉素粉针剂(浙江海正药业股份有限公司,国药准字H33020786);注射用生理盐水(四川科伦药业股份有限公司,国药准字H51021157);75%医用乙醇(衡阳原野实业有限公司,湘卫消证字第0009号);2%戊巴比妥钠注射液用注射用生理盐水自行配制。
1.2 实验动物 Sprague-Dawley雄性大鼠150只,体质量210~250 g,皮下荷瘤SD小鼠(Walker-256瘤株),均购自中国科学院斯莱克实验动物中心(SCXK京2011-0012),购回后适应性饲养1周再用于试验。本实验遵循《实验动物保护条例》,对实验动物进行严格的检验检疫和饲养管理。
1.3 方法
1.3.1 大鼠移植性肝癌造模:将荷瘤SD小鼠处死,经75%乙醇消毒后,取出瘤体,挑选无出血损坏的肿瘤组织,切割成0.5 mm2见方的瘤块备用。用2%戊巴比妥钠注射液(45 mg/kg)对150只大鼠进行腹腔麻醉,然后以仰卧位固定于超净台,剪掉腹部毛发,用75%乙醇消毒手术区后,于腹部中央剪开皮肤,暴露出肝脏,采用肝内隧道植入法植入准备好的Walker-256瘤块。肝脏复位,腹部缝合。共有120只大鼠造模成功。
1.3.2 实验分组及给药:将造模成功的120只大鼠随机分为对照组、丝裂霉素组和丹参组,共3组,每组各40只。正常饲养10 d后,所有大鼠用2%戊巴比妥钠注射液(45 mg/kg)行腹腔麻醉,取血备用,并再次切开腹部,暴露出肝脏,测量肿瘤表面最大和最小径。同时行经胃十二指肠动脉至肝脏固有动脉插管灌注治疗。对照组大鼠灌注2 mL的注射用生理盐水,丝裂霉素组大鼠灌注丝裂霉素(2.5 mg/kg),丹参组灌注丹参(1.6 g/kg)。灌注完成后,缝合切口,并涂抹抗生素药膏。正常饲养1周后,2%戊巴比妥钠行腹腔注射麻醉,再次取血,并测量肿瘤表面最大和最小径。最后处死所有实验大鼠,取出瘤体,在显微镜下观察3组大鼠肿瘤坏死程度。
1.3.3 测定移植肿瘤的体积及其生长率:体积(V)=肿瘤最大径(cm)×肿瘤最小径(cm)2/2;增长率(GR)=治疗后体积/治疗前体积;生长抑制率=(GR1-GR2)/GR1×100%。
1.3.4 肿瘤坏死程度观察:实验结束时,处死所有实验大鼠,取出瘤体,显微镜下观察3组大鼠肿瘤坏死程度,按照轻、中、重程度划分。因术后死亡,各组大鼠剩余数量分别为对照组37只,丝裂霉素组37只,丹参组38只。
1.3.5 大鼠肝功能检测:2次所取大鼠血液经离心后,取出上清液,用自动生化仪检测丙氨酸氨基转移酶(ALT)、天门冬酸氨基转移酶(AST)水平、碱性磷酸酶(ALP)的水平。
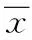
2 结果
2.1 大鼠移植性肝癌肿瘤体积、增长率及抑制率比较 由表1可知,丝裂霉素组和丹参组肿瘤体积及增长率均明显小于对照组(P<0.05),丝裂霉素和丹参组肿瘤体积及增长率无明显差异。丝裂霉素组和丹参组对移植性肝癌肿瘤抑制率的差异无统计学意义(见表1)。
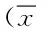
表1 各组大鼠肝癌肿瘤体积、增长率及抑制率比较±s)Tab.1 Comparison of the volume,growth rate and inhibition rate of ±s)
*P<0.05,与对照组相比较,compared with control group
2.2 大鼠移植性肝癌肿瘤坏死程度比较 由表2可知,灌注治疗后,丹参组和丝裂霉素组大鼠中度坏死高于对照组。丝裂霉素组和丹参组轻,中,重肿瘤坏死分布无显著性差异。
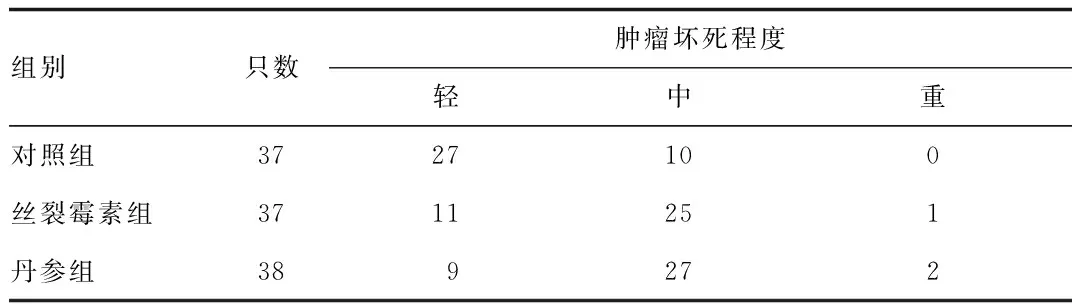
表2 各组大鼠肿瘤坏死程度比较Tab.2 Comparison of the degree of tumor necrosis among groups
2.3 大鼠血清中ALT,AST和ALP的测定比较 由表3可知,灌注治疗后,丹参组和丝裂霉素组大鼠血清ALT,AST和ALP的水平均明显低于对照组,差异有统计学意义(P<0.01,P<0.05);丹参组上述指标显著低于丝裂霉素组,差异具有统计学意义(P<0.05,见表3)。
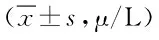
表3 各组大鼠血清中ALT,AST和ALP的水平比较Tab.3 Comparison of the serum ALT,AST and ALP levels among ±s,μ/L)
*P<0.05,**P<0.01,与对照组比较,compared with control group;△P<0.05,与丝裂霉素组比较,compared with mitomycin group
3 讨论
有研究表明[11],不能接受手术治疗的肝癌患者,采用化疗药物经肝动脉灌注,可直达肝癌组织,导致肝癌组织坏死,而对正常的肝组织损伤较小。近年来,已有丝裂霉素、顺铂等化疗药物经肝动脉灌注治疗并取得理想疗效的报道[12-13]。此外,传统中药丹参具逆转肝病纤维化、诱导肝癌细胞分化、促进肝癌细胞凋亡等作用。
本研究采用丹参经肝动脉灌注疗法来治疗大鼠移植性肝癌。研究发现,丹参经肝动脉灌注对于大鼠移植性肝癌有明显的治疗作用,能够显著抑制移植性瘤体的增长,其抑瘤活性与常用抗肿瘤药物丝裂霉素的活性相当。ALT主要存在于肝细胞浆内,AST主要分布于肝细胞线粒体内,ALP也主要分布在肝细胞内[14]。正常情况下,血清中ALT、AST和ALP的水平很低,只有当肝细胞受到损伤坏死时,ALT、AST和ALP才会释放进入血液中,导致其血清水平升高。因此检测血清ALT、AST和ALP水平可以判断肝功能[15]。本研究发现经丹参灌注治疗后,大鼠血清ALT,AST和ALP的水平均明显降低,表明丹参在抗肿瘤的同时,对移植性肝癌大鼠具有良好的肝保护作用。
[1] Gandhi S,Khubchandani S,Iyer R.Quality of life and hepatocellular carcinoma[J].J GastrointestOncol,2014,5(4):296-317.
[2] Kanda T,Jiang X,Yokosuka O.Androgen receptor signaling in hepatocellular carcinoma and pancreatic cancers[J].World J Gastroenterol,2014,20(28):9229-9236.
[3] Anwar SL,Lehmann U.DNA methylation,microRNAs,and their crosstalk as potential biomarkers in hepatocellular carcinoma[J].World J Gastroenterol,2014,20(24):7894-7913.
[4] 应希慧,纪建松,涂建飞,等.原发性肝癌合并骨转移45例临床分析[J].中华全科医学,2014,12(8):1346-1347.
[5] 李照,高杰,孙馨,等.肝癌肝移植术后骨转移患者的手术治疗[J].中华普通外科杂志,2013,28(3):193-195.
[6] Huang H,Deng M,Jin H,et al.Reduced hepatic arterial perfusion impairs the recovery from focal hepatic venous outflow obstruction in liver-resected rats[J].Transplantation,2014,97(10):1009-1018.
[7] Forster MR,Rashid OM,Perez MC,et al.Chemosaturation with percutaneous hepatic perfusion for unresectable metastatic melanoma or sarcoma to the liver:a single institution experience[J].J SurgOncol,2014,109(5):434-439.
[8] 郝文慧,赵文文,陈修平.丹参酮类抗肿瘤作用与机制研究进展[J].中国药理学通报,2014,30(8):1041-1044.
[9] 王新荣,赵苏.丹参酮ⅡA磺酸钠对肺心病患者血流变及血脂的影响[J].中国生化药物杂志,2011,31(3):237-238.
[10] 冯旭霞,何亚.丹参酮ⅡA磺酸钠对急性心肌梗死溶栓后缺血再灌注损伤的防护作用[J].中国生化药物杂志,2012,32(4):460-462.
[11] Altomonte J,Braren R,Schulz S,et al.Synergistic antitumor effects of transarterial viroembolization for multifocal hepatocellular carcinoma in rats[J].Hepatology,2008,48(6):1864-1873.
[12] Agarwala SS,Panikkar R,Kirkwood JM.Phase I/II randomized trial of intrahepatic arterial infusion chemotherapy with cisplatin and chemoembolization with cisplatin and polyvinyl sponge in patients with ocular melanoma metastatic to the liver[J].Melanoma Res,2004,14(3):217-222.
[13] Bagliani A,Ierardi AM,Caspani B,et al.Hypoxic liver perfusion with mitomycin-C for treating multifocal metastases and unresectable primary tumours:a single-centre series of 42 patients[J].Radiol Med,2011,116(8):1239-1249.
[14] Kukuk GM,Schaefer SG,Fimmers R,et al.Hepatobiliary magnetic resonance imaging in patients with liver disease:correlation of liver enhancement with biochemical liver function tests[J].Pharm Biol,2014,15(1):1-8.
[15] Wagener G.Assessment of hepatic function,operative candidacy,and medical management after liver resection in the patient with underlying liver disease[J].Semin Liver Dis,2013,33(3):204-212.
(编校:谭玲,王俨俨)
Efficacy of salvia miltiorrhiza on transplanted hepatoma in rats via hepatic artery perfusion and its protection of liver function
WEI Ming-qin1,JIANG Jian-xia2
(1.Department of Oncology, Xiangyang Hospital, Affiliated Hospital of Hubei University of Medicine, Xiangyang 441000, China; 2.Nanjing Medical University, Nanjing 210000, China)
ObjectiveTo observe the therapeutic effect of salvia miltiorrhiza on transplanted hepatoma in rats via hepatic artery perfusion therapy and research the liver protection of salvia miltiorrhiza.Methods150 Sprague-Dawley rats were selected to build Walker-256 transplanted hepatoma model with successful modeling of 120 rats.All rats were randomly divided into control group,mitomycin group,salvia miltiorrhiza group,and 40 rats in each group.After 10 days normal feeding,bloods of all rats were collected to detect levels of ALT,AST and ALP.Tumor volumes were measured at the same time.Hepatic arterial infusion therapy: 2 mL saline was injected into control group,mitomycin (2.5 mg/kg) was injected into mitomycin group and salvia miltiorrhiza (1.6 g/kg) was injected into salvia miltiorrhiza group.After a week,bloods were collected to detect levels of ALT,AST,ALP and tumor volumes were measured.The tumor growth rate and inhibition rate were calculated.At the same time,all rats were killed and tumor were removed.The scope of tumor necrosis and degree of tumor necrosis was observed and compared.ResultsThere were 37 rats left in control group,37 rats left in mitomycin group and 38 rats left in salvia miltiorrhiza group after perfusion.The tumor volume and growth rate of mitomycin group and salvia miltiorrhiza group were significantly smaller than those of control group(P<0.05),that had no significant difference between mitomycin group and salvia miltiorrhiza group.The tumor inhibition rate of mitomycin group and salvia miltiorrhiza group were 22.32% and 26.38%,and there was no statistical difference.After perfusion,the moderate degrees of tumor necrosis in mitomycin group and salvia miltiorrhiza group were higher than that in control group,and there were no significant difference in mitomycin group and salvia miltiorrhiza group in mild,moderate,and severe degrees of tumor necrosis.The levels of ALT,AST and ALP of mitomycin group and salvia miltiorrhiza group were lower than those of control group (P<0.01,P<0.05); the above indexes were significantly in salvia miltiorrhiza group lower than those in mitomycin group(P<0.05). ConclusionSalvia miltiorrhiza could inhibit transplanted hepatoma in rats via hepatic artery perfusion therapy and protect the liver function.
salvia miltiorrhiza; hepatic artery perfusion; transplanted hepatoma; protection of liver function
国家自然科学基金(81101800)
魏明琴,女,本科,主治医师,研究方向:肺癌,E-mail:qch1821460051@163.com。
R969
A
1005-1678(2015)02-0042-03
