Tuning the particle size and morphology of high energetic material nanocrystalsRaj KUMARa, Prem Felix SIRILa,*, Pramod SONIb
2015-07-02SchoolofBsicSciencesndAdvncedMterilsReserchCentreIndinInstituteofTechnologyMndiMndi175005HimchlPrdeshIndiTerminlBllisticsReserchLortorySector30Chndigrh160030IndiReceived21Mrch2015revised28July2015ccepted30July2015Avil
School of Bsic Sciences nd Advnced Mterils Reserch Centre, Indin Institute of Technology Mndi, Mndi, 175005, Himchl Prdesh, IndiTerminl Bllistics Reserch Lortory, Sector-30, Chndigrh, 160030, IndiReceived 21 Mrch 2015; revised 28 July 2015; ccepted 30 July 2015 Aville online■■■
Tuning the particle size and morphology of high energetic material nanocrystals
Raj KUMARa, Prem Felix SIRILa,*, Pramod SONIb
aSchool of Basic Sciences and Advanced Materials Research Centre, Indian Institute of Technology Mandi, Mandi, 175005, Himachal Pradesh, IndiabTerminal Ballistics Research Laboratory, Sector-30, Chandigarh, 160030, India
Received 21 March 2015; revised 28 July 2015; accepted 30 July 2015 Available online■■■
Abstract
Morphology controlled synthesis of nanoparticles of powerful high energetic compounds (HECs) such as 1,3,5-trinitro-1,3,5-triazinane (RDX) and 1,3,5,7-tetranitro-1,3,5,7-tetrazocane (HMX) were achieved by a simple solvent-antisolvent interaction (SAI) method at 70°C The effects of different solvents on particle size and morphology of the prepared nano-HECs were studied systematically. Particle size and morphology of the nano-HECs was characterized using field emission scanning electron microscopy (FE-SEM) imaging. X-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy studies revealed that RDX and HMX were precipitated in their most stable poly morphic forms, i.e.α and β, respectively. Thermogravimetric analysis coupled with differential scanning calorimetry (TGA-DSC) studies showed that the thermal response of the nanoparticles was similar to the respective raw-HECs. HEC nanoparticles with spherical and rod shaped morphology were observed under different solvent conditions. The mean particle size also varied considerably with the use of different solvents Copyright©2015, China Ordnance Society. Production and hosting by Elsevier B.V. All rights reserved.
Keywords:Nano-RDX; Nano-HMX; High energetic materials; Spherical nanoparticles; Nanorods; Morphology control
Abbreviations: RDX, 1,3,5-trinitro-1,3,5-triazinane; HMX, 1,3,5,7-tetranitro-1,3,5,7-tetrazocane; CL, 20-2, 4, 6, 8, 10, 12-hexanitro-2, 4, 6, 8, 10, 12-hexazaisowurtzitane; TATB, 1, 3, 5-triamino-2, 4, 6-trinitrobenzene; ICDD, The International Centre for Diffraction Data; HMXb, Bulk-HMX; HMXn, nano-HMX; Tm.p, melting point; Texo, exothermic peak temperature; Tendo, endothermic peak temperature;ΔH, enthalpy change; Tid, initial decomposition temperature; Tfd,final decomposition temperature;Δmass, mass loss; TBRL, Terminal Ballistics Research Laboratory; AC, acetone; EA, ethyl acetate; EN, ethanol; MN, methanol; DMSO, dimethyl sulfoxide; DMF, dimethylformamide; NMP, N-methylpyrrolidone.
E-mail address: prem@iitmandi.ac.in (P.F. SIRIL).
Peer review under responsibility of China Ordnance Society.
http://dx.doi.org/10.1016/j.dt.2015.07.002
2214-9147/Copyright©2015, China Ordnance Society. Production and hosting by Elsevier B.V. All rights reserved.
1. Introduction
High energetic materials (HEMs) are rich sources of energy stored in the form of chemical bonds [1]. They are thermodynamically unstable, but the kinetics of energy release can be controlled. They have found extensive use in explosives, rocket propellants and gas generators for automobile air bags [1,2]. Focus of research on HEMs has recently been to synthesize novel molecules with high energy density combined with insensitivity to hazardous stimul [1,2]. Unfortunately, the research and development of new HECs has been very slow. RDX and HMX, which were developed many decades ago, are still being used as the main explosives due to their technological-economical characteristics such as their ready availability in large scale [2] Powerful explosives such as CL-20 and octanitrocubane have much higher energetic performance than RDX and HMX [2,3]. But, their sensitivity to accidental stimuli is a matter of concern. Sensitivity of explosives is related to their chemica as well as physical characteristics [4]. The physical properties such as crystal size, shape, morphology, purity, inclusions and crystal defects can be altered to improve the performance of existing explosives [5,6]. Previous studies reported that thenovel behaviour in deflagration to detonation transition was observed with submicron particles [7]. A few studies have indicated that the particle size of explosives influences the impact sensitivity and maximum energy output from a detonation [8]. Thus, the preparation of micrometer or submicrometer sized solid particles is of great interest in explosives.
However, the limited production strategies are only available for making organic nanoparticles in general compared to the large array of methods that are available for the preparation of inorganic nanoparticles. Some of the methods for the preparation of sub-micron sized HEMs includes rapid crystallisation from solvent by the addition of antisolvent [9,10], sol-gel method [11,12], rapid expansion of supercritical solution (RESS) [13,14], mechanical milling [15-17] and aero-sol method [18]. An excellent review on the various methods for the preparation of nanoenergetic materials was published recently [19]. Unfortunately, many of these techniques proved to be less attractive in large scale production of organic nano-sized materials. Among various techniques for the reduction of particle size, the antisolvent precipitation process is a simple and effective technique to produce the nanosized particles by introducing the organic solution containing an active substance to the antisolvent (e.g. water) that is solvent-miscible under rapid mixing, which generates high supersaturation leading to fast nucleation rates [20-25]. Instantaneous precipitation occurs by a rapid desolvation of the hydrophobic active ingredient in the antisolvent medium [26-29]. The antisolvent may contain hydrophilic stabilizers such as polymers or surfactants. The hydrophilic stabilizer in the antisolvent gets adsorbed on the particle surface to inhibit particle growth [20-25]. We have recently prepared nano-HECs by a simple evaporation assisted solvent-antisolvent interaction (EASAI) method using acetone as solvent at 70°C [26,27]. The same method was also used to prepare nanodrugs [28,29]. It has been shown that the particle size can be controlled by varying a number of experimental parameters such as the concentration, ratio of solvent to antisolvent, temperature of the antisolvent during injection, stirring speed etc. Infact, a lot more experimental parameters such as ultrasonication, nozzle geometry, mixing rate, nature of solvent and nature of antisolvent also are known to affect the particle properties [30]. Although there has been some studies on the effect of many of these experimental parameters, only very few reports are there in the literature about the effects of different solvents on particle size and morphology of HECs. Here we demonstrate that particle size and even morphology of nano-HECs can be tuned by changing the solvent using the SAI method.
2. Materials and methods
2.1. Materials
RDX (98.2%) and HMX (99.1%) were prepared in an inhouse facility using Bachmann process [31]. Solvents, dimethylsulfoxide (DMSO), dimethyl formamide (DMF), ethyl acetate (EA), N-methyl-2-pyrrolidone (NMP), methanol (MN) and ethanol (EN) were purchased from Sigma Aldrich and used as received. Ultra-pure water (18.2 MΩ-cm) from double stage water purifier (ELGA PURELAB Option-R7) was used throughout the process of preparation. HPLC micro syringe was purchased from Hamilton, USA. Syringe filter with pore size of 0.22 μm was purchased from Millipore, USA. Whatman Anodisc®25 filter with pore size of 0.02 μm was purchased from Sigma Aldrich, India.
2.2. Preparation of RDX and HMX nanoparticles
Solution of HECs (5 mM) in different solvents was prepared by adding accurately weighted amount of HECs. The solution (100 μl, 5 mM) was quickly injected into water (25 ml) as antisolvent at 70°C under magnetic stirring using an HPLC micro-syringe to precipitate nanoparticles. The solution of HECs was always filtered using a syringe filter with pore size of 0.22 μm before injection to ensure that no particle was present in it. The nanoparticles were collected by filtration using Whatman Anodisc®25 filter membrane (diameter = 25 mm and pore size = 20 nm). The membrane was dried in an oven at 40°C for 24 h to collect the sample for further characterization.
2.3. Particle size and morphology
Accurate particle size and morphology of the prepared nanoparticles were observed using an FESEM (Zeiss FEI Quanta FEG 450 and Supra 55 VP model). The suspension of RDX and HMX nanoparticles in water was drop-coated on an 1 cm2glass slide and dried. The dried sample containing the glass slide was kept on a clean aluminum stub that was covered with carbon tape. The sample was subsequently sputter-coated with gold at 20 mA for 180 s before the FESEM observation. Particle size of more than 300 nanoparticles from different FESEM images that were taken from different regions of the sample was calculated in each experimental condition using Image J software.
2.4. FTIR spectroscopy
FTIR spectroscopy was performed using the Perkin Elmer FTIR emission spectrometer (Spectrum Two). The FTIR spectra of raw and nanoparticles of RDX and HMX were recorded in the frequency range from 4000 to 600 cm-1with a resolution of 4 cm-1. The samples were properly grounded with KBr powder and then pressed to obtain a suitably sized pellet for FTIR spectrum measurement. Pure KBr pellet was used for background correction.
2.5. Powder X-ray diffraction (XRD)
XRD patterns were recorded on a smart lab X-ray diffractometer (Rigaku, Japan) using Cu Kα radiation as X-ray source (λ= 0.15418 nm) at room temperature. The voltageand current applied were 45 kV and 100 mA, respectively. The samples were placed on a scorch tape and scanned at 2θ from 10°to 70°at a scan rate of 2°/min-1with a step size of 0.02°.
2.6. TGA-DSC
TGA-DSC analyses were carried out by using Netzsch STA 449 F1 Jupiter instrument. Small amount of the sample (2-3 mg) was taken in a standard alumina pan with an alumina lid with a pin hole at the middle. An empty crucible was used as reference. The samples were heated from room temperature to 500°C at a heating rate of 5°C/min-1under nitrogen atmosphere with the flow rates of 60 ml/min-1protective gas and 40 ml/min-1purge gas.
3. Results and discussion
The nanoparticles of RDX and HMX were precipitated immediately on the injection of their respective solution into antisolvent (water). Stirring was continued up to a few minutes after the injection to complete the mixing to precipitate smaller particles with narrow size distribution [30]. Precipitation of the HECs did not produce any turbidity due to the tiny size as well as the very low concentration of the particles. However, the particles could be detected using DLS. Precipitation was performed from a number of differen solvents in order to study the effects of solvents on the size and shape of nanoparticles. While varying the solvents, the previously optimized experimental conditions for obtaining small particles of RDX from acetone were used [26]. The experimental conditions were: temperature (70°C), solven to antisolvent ratio (1:250) and concentration (5 mM) [26] The particle sizes and morphologies of the prepared nano-RDX and nano-HMX were studied using FESEM imaging The representative FESEM images of nano-RDX and nano-HMX are given in Figs. 1 and 2, respectively. Average particle sizes were calculated from the FESEM images using Image J software. The average particle sizes that were calculated from FESEM images of solvents are listed in Table 1.
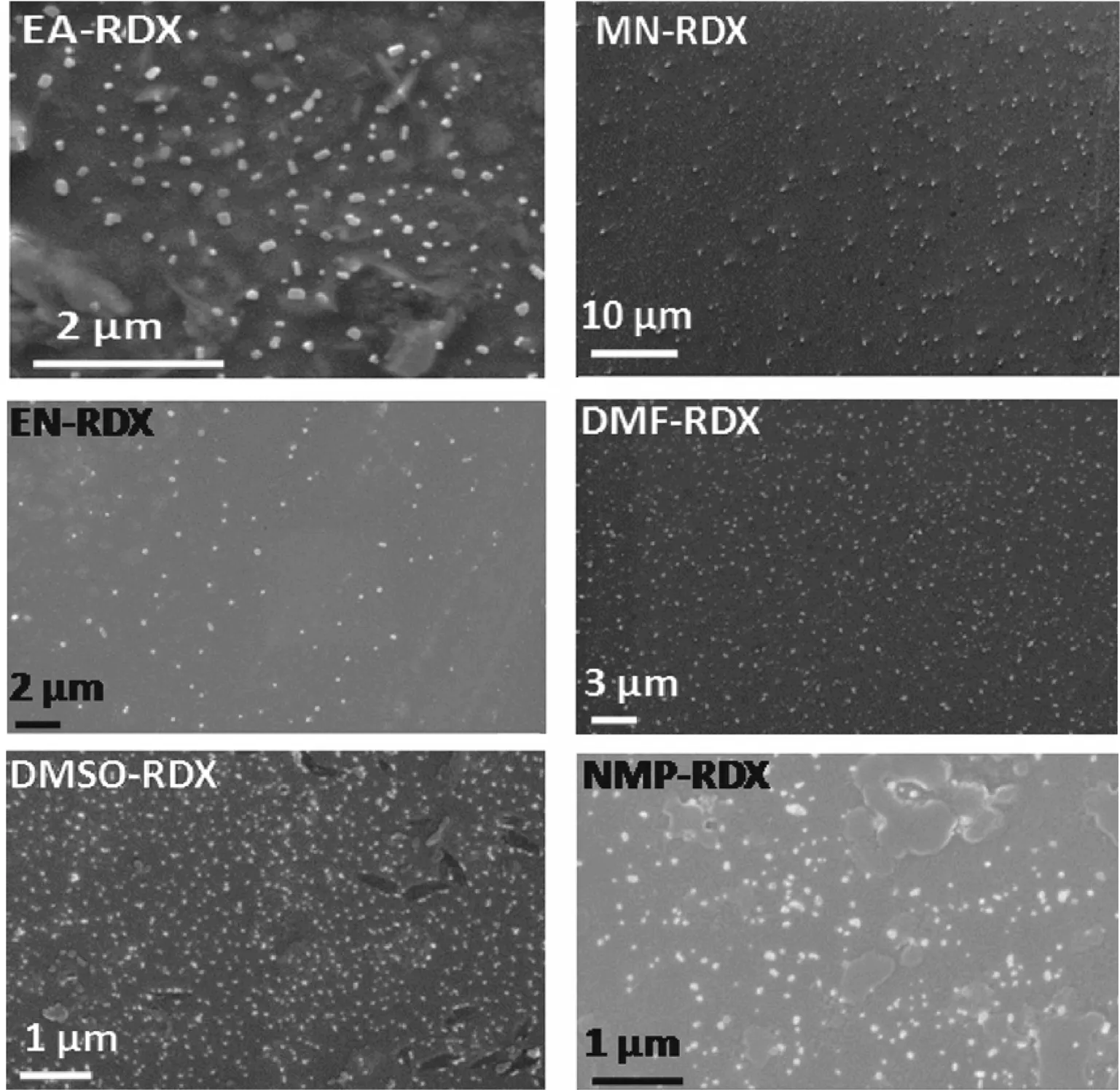
Fig. 1. FESEM images of nano-RDX in different solvent conditions.
3.1. Particle size and morphology
It is evident from Figs. 1 and 2 that the spherical nanoparticles with almost uniform particle sizes were formed inmost of the conditions. However, the cubical and rod shaped nanoparticles were formed when EA was used as the solvent. This is a significant observation as the control of particle morphology is usually difficult to achieve without any additives such as surfactants or polymers. The particle sizes of RDX and HMX nanoparticles prepared from EA as solvent are not listed in Table 1 as they were non-spherical. EA was the least polar among all the solvents used in the present study. The miscibility of EA is the least with the antisolvent (water), which may have resulted in significant reduction in nucleation and growth of particles. Slow nucleation and growth led to the growth of particles into relatively larger size.
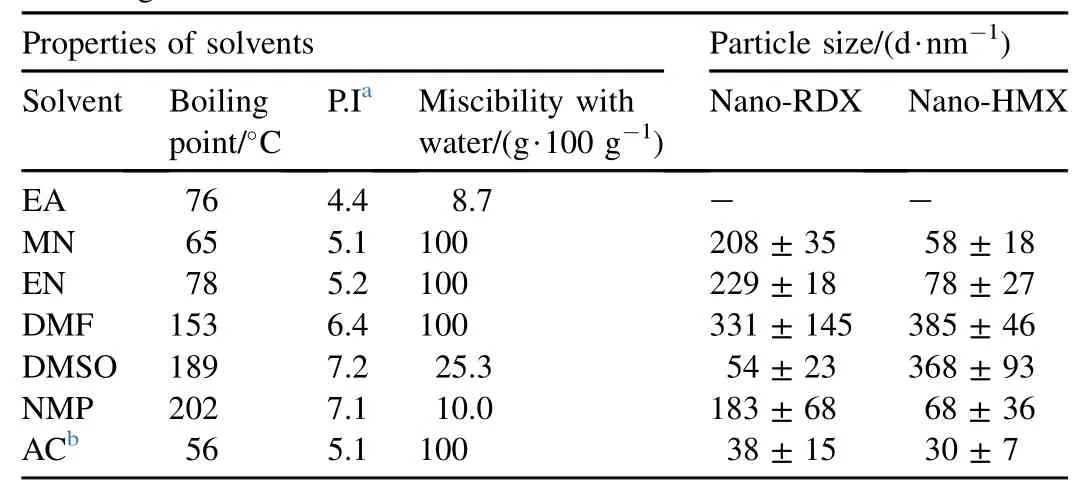
Table 1Particle size measured from FESEM images of nano-HECs that were precipitated from different solvents. Some physical properties of the solvents are also given.
From Table 1, it is clearly evident that the particle size depends on the nature of the solvent. Size of RDX nanoparticles varied in the range from 38±15 nm to 331±145 nm whereas size of HMX nanoparticles were in the range from 30±7 nm to 385±46 nm when they were precipitated from different solvents. Chung et al. [32] studied the effects of solvents on the particle size of low molecular weight organic compounds at 298 K. They concluded that both the solubility of compound and the polarity of solvent are the key factors determining particle size [32,33]. The boiling point and miscibility of solvent with the antisolvent seems to be the deciding factors in controlling the particle size of HMX nanoparticles with the exception of NMP. Among the solvents having high miscibility (100%) with water, the particle size decreases with decrease in boilingpoint. Reduction in particle size, when the precipitation is evaporation assisted using SAI method, has been consistently reported in Refs. [26-29]. Lower boiling point of the solvents results in evaporation-assisted precipitation that leads to the formation of large number of nuclei. Further growth of the nuclei into large particles is not possible, as substantial depletion of the HEC molecules in the solution takes place during nucleation.
Unfortunately, any such generalization was not possible for the observed variation in particle size with the change in solvents for nano-RDX. There was no clear trend in the data given in Table 1 to correlate the properties of the solvent with the particle size of nano-RDX. This is because the nucleation and growth at 70°C are expected to be an intricate interplay of a number of dynamic factors, such as miscibility of the solvent with the antisolvent, diffusivity, viscosity and solubility of HECs, in the mixture of solvent and antisolvent [33]. Unfortunately, many of these factors are not precisely known for the studied system to make any meaningful correlation.
3.2. FTIR
FTIR spectroscopy was used to determine the chemical compositions of RDX and HMX before and after the antisolvent precipitation. The FTIR spectra of raw-HECs are shown in Fig. 3 along with the spectra of the prepared nano-HECs. It is clearly evident from Fig. 3 that nano-RDX samples have similar IR bands as bulk-RDX. The major bands for the RDX samples were as follows: 1592 cm-1(νs NO2), 1270 cm-1(νs NO2+ν N-N), 1039 cm-1(ring stretching bands), 945 and 783 cm-1(δ NO2and γNO2) and 604 cm-1(τ+γ NO2). Two peaks were observed for all the RDX samples at 736 and 754 cm-1, as highlighted in Fig. 3. Peaks in the range of 700-760 cm-1are characteristic of α-polymorph form of RDX, and there are no peaks in this range for β-RDX [34]. Similarly, the major IR bands that were observed for the nano-HMX samples matched well with the raw-HMX [35,36]. The major IR bands for HMX samples were assigned as follows: 1564 cm-1(νs NO2), 1145 cm-1(νs NO2,ν ring), 964 cm-1and 946 cm-1(ring stretching bands), 830 and 761 cm-1of (δ and γ NO2), 625 and 600 cm-1(t +γ NO2). No peak was observed in the FTIR spectrum of HMX in the range of 700-750 cm-1as marked in Fig. 3. This indicates that all the HMX samples were in the β-polymorph form [37,38]. Additionally, it can be concluded that SAI method does not lead to any change in chemical structure or crystalline nature of RDX and HMX.
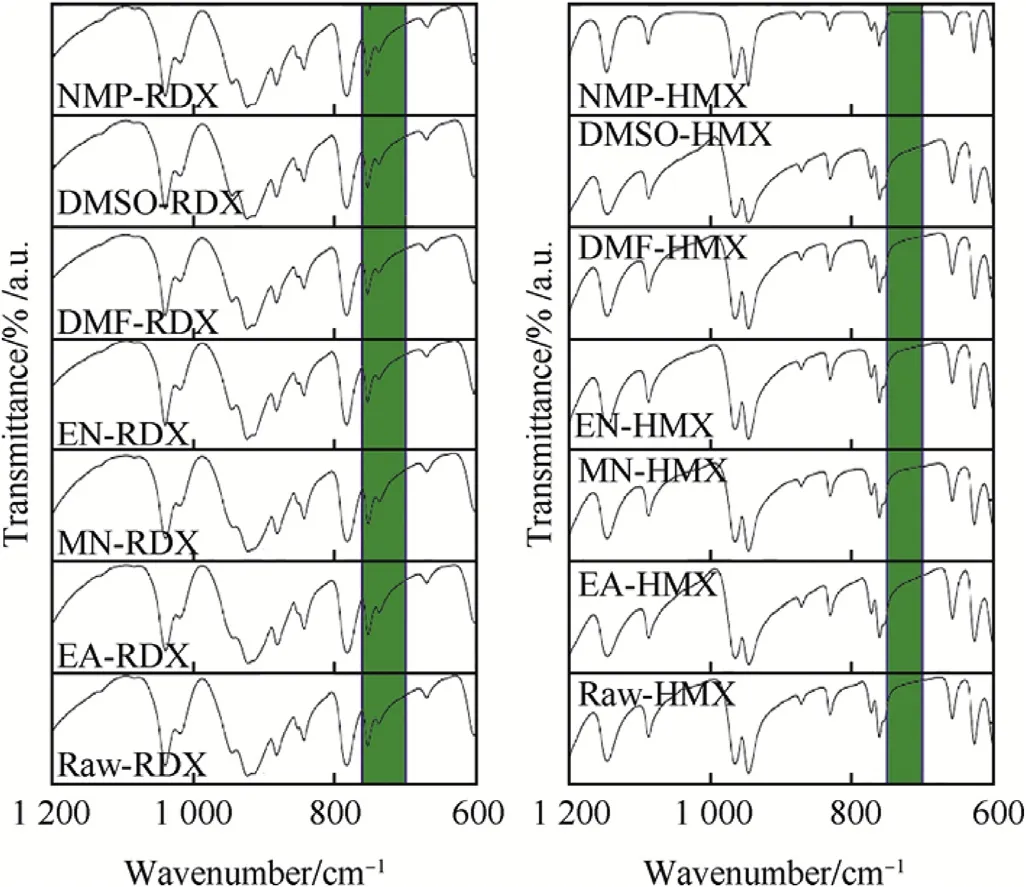
Fig. 3. FTIR spectra of prepared nano-HECs and raw-HECs.
3.3. Powder X-ray diffraction (XRD)
XRD was performed to ascertain the crystalline state of the nano-HECs. XRD patterns of the nano-HECs are shown in Fig. 4 along with the diffraction pattern of raw-HECs. The XRD patterns of the nano-HECs show the presence of numerous distinct peaks showing the crystalline nature of the samples. The XRD patterns of raw-HECs matched well with the nano-HECs. This clearly indicates that the HMX nanoparticles prepared by SAI method were also crystalline. The crystal structure of RDX is well known to have at least 5 different polymorphs:α,β,γ,δ and ε. The α-form is the most stable one at room temperature (orthorhombic, a = 1.3182, b = 1.1574 and c = 1.0709 nm) [39,40]. HMX crystals can exist in one of the four different polymorphs:α(orthorhombic),β(monoclinic),γ(monoclinic) and δ(hexagonal) phases. Among these,β-HMX is highly dense and is the most stable at room temperature (monoclinic, a = 0.65370, b = 1.10540 and c = 0.87018 nm) [41-43]. The XRD patterns of all the RDX and HMX samples showed similar patterns corresponding to α-RDX and β-HMX, respectively [8,39,44,45]. The diffraction peaks of all the RDX samples at 2θ= 13.1°, 13.4°, 15.4°, 17.4°, 17.8°, 20.4°, 22.0°, 25.4°and 29.3°confirmed that the RDX samples were in α-polymorphic form [JCPDS PDF#44-1618] [46]. The diffraction peaks of all the HMX samples at 2θ= 14.7°, 16.0°, 23.0°, 26.1°, 29.6°and 31.9°confirmed that the HMX samples were in β-polymorphic form [JCPDS PDF#44-1620] [47].

Fig. 4. XRD of prepared nano-HECs and raw-HECs.

Fig. 5. Thermal curves for nano-HECs and raw-HECs.
3.4. TGA-DSC
The thermal properties of energetic compounds are important characteristics as the initiation of HECs is intrinsically related to the thermal properties. TGA-DSC thermal curves of raw and nano-HECs are shown in Fig. 5. The phonomenological data of raw-HECs along with the prepared nano-HECs are summarized in Table 2. The overall thermal response of the nano-HECs was very much similar to that of the raw-HECs. RDX melts initially and then decomposes to give gaseous products. Melting of RDX was registered as an endothermic event at around 202°C in the DSC thermal curves. Rapid mass loss follows after melting. Almost 100% mass loss was observed in TGA thermal curves with a simultaneous exotherm in DSC thermal curves. These thermal events are typical of raw-RDX that was recorded under similar experimental conditions [48]. The DSC thermal curves of raw-HMX and the nano-HMX samples showed an endotherm at around 190°C. This corresponds to solid state phase transition from β to δ phase [49-52]. Exothermic thermal decomposition leading to almost complete mass loss was observed for all the HMX samples after 270°C. This result is also similar to the characteristic thermal response of HMX which is reported in Refs. [52,53].

Table 2Phenomenological TGA-DSC data of nano-HECs along with raw-HECs.
# Data from refs. [26,27].
4. Conclusions
The particle sizes and shapes of nano-RDX and nano-HMX can be controlled using SAI method by changing the solvent without affecting the chemical structure and crystal morphology. Particle morphology was spherical when RDX and HMX were precipitated from all the solvents studied using SAI method; except from EA. Poor miscibility of EA with water led to the formation of rod-shaped nanoparticles. Any meaningful correlation of solvent properties with the particle size of RDX was difficult to achieve. Overall thermal response of nano-RDX and nano-HMX was more or less similar to those of the raw-RDX and raw-HMX from which they were prepared. However, the boiling points and miscibilities of solvents with water emerged as the key factors controlling the particle size of HMX nanoparticles using SAI method.
Acknowledgements
Thanks are due to IIT Mandi for providing laboratory facilities. Financial assistance from ARMREB (DRDO) under grant No. ARMREB/CDSW/2012/149 and research fellowship to Mr. Raj Kumar from UGC are gratefully acknowledged. Mr. Sudhir Scarlch, NIT Hamirpur and Dr. Kenny Pandey, (Sophisticated Instrument Centre) IIT Indore are also thanked for FESEM imaging.
References
[1] Singh G, Singh CP, Siril PF. Recent research on thermolysis of energetic compounds. USA: Nova Science Publishers; 2008.
[2] Agrawal JP. High energy materials. Wiley VCH; 2010.
[3] Monteil-Rivera F, Paquet L, Deschamps S, Balakrishnan VK, Beaulieu C, Hawari J. Physico-chemical measurements of CL-20 for environmental applications: comparison with RDX and HMX. J Chromatogr A 2004;1025:125-32.
[4] Zeman S. Sensitivities of high energy compounds. In: Klapotke TM, editor. High energy density materials. Structure and Bonding, vol 125. NewYork: Springer-Verlag Berlin Heidelberg; 2007. p. 195-271. http:// dx.doi.org/10.1007/430_2006_052.
[5] Millar DI, Maynard-Casely HE, Allan DR, Cumming AS, Lennie AR, Mackay AJ, et al. Crystal engineering of energetic materials: co-crystals of CL-20. Cryst Eng Comm 2012;14:3742-9.
[6] Roberts CW, Hira SM, Mason BP, Strouse GF, Stoltz CA. Controlling RDX explosive crystallite morphology and inclusion content via simple ultrasonic agitation and solvent evaporation. Cryst Eng Comm 2011;13:1074-6.
[7] Siviour C, Gifford M, Walley S, Proud W, Field J. Particle size effects on the mechanical properties of a polymer bonded explosive. J Mater Sci 2004;39:1255-8.
[8] Yongxu Z, Dabin L, Chunxu L. Preparation and characterization of reticular nano-HMX. Propellants Explos Pyrotech 2005;30:438-41.
[9] Kr¨ober H, Teipel U. Crystallization of insensitive HMX. Propellants Explos Pyrotech 2008;33:33-6.
[10] van der Heijden AE, Creyghton YL, Marino E, Bouma RH, Scholtes GJ, Duvalois W, et al. Energetic materials: crystallization, characterization and insensitive plastic bonded explosives. Propellants Explos Pyrotech 2008;33:25-32.
[11] Tillotson T, Gash A, Simpson R, Hrubesh L, Satcher J, Poco J. Nanostructured energetic materials using sol-gel methodologies. J Non-Cryst Solids 2001;285:338-45.
[12] Tillotson T, Hrubesh L, Simpson R, Lee R, Swansiger R, Simpson L. Sol-gel processing of energetic materials. J Non-Cryst Solids 1998;225:358-63.
[13] Stepanov V, Elkina IB, Matsunaga T, Chernyshev AV, Chesnokov EN, Zhang X, et al. Production of nanocrystalline RDX by rapid expansion of supercritical solutions. Int J Energy Mater Chem Propu 2007; 6:75-87.
[14] Pourmortazavi SM, Hajimirsadeghi SS. Application of supercritical carbon dioxide in energetic materials processes: a review. Ind Eng Chem Res 2005;44:6523-33.
[15] Koch C. Synthesis of nanostructured materials by mechanical milling: problems and opportunities. Nanostruct Mater 1997;9:13-22.
[16] Liu J, Jiang W, Zeng J, Yang Q, Wang Y, Li F. Effect of drying on particle size and sensitivities of nano hexahydro-1,3,5-trinitro-1,3,5-triazine. Def Technol 2014;10:9-16.
[17] Liu J, Jiang W, Yang Q, Song J, Hao G, Li FS. Study of nano-nitramine explosives: preparation, sensitivity and application. Def Technol 2014;10:184-9.
[18] Prakash A, McCormick AV, Zachariah MR. Aero-sol-gel synthesis of nanoporous iron-oxide particles: a potential oxidizer for nanoenergetic materials. Chem Mater 2004;16:1466-71.
[19] Huang B, Cao M, Nie F, Huang H, Hu CW. Construction and properties of structure- and size-controlled micro/nanoenergetic materials. Def Technol 2013;9:59-79.
[20] Talawar M, Agarwal A, Anniyappan M, Gore G, Asthana S, Venugopalan S. Method for preparation of fine TATB (2-5 μ m) and its evaluation in plastic bonded explosive (PBX) formulations. J Hazard Mater 2006;137:1848-52.
[21] Chen H, Khemtong C, Yang X, Chang X, Gao J. Nanonization strategies for poorly water-soluble drugs. Drug Discov Today 2011;16:354-60.
[22] Cho E, Cho W, Cha K-H, Park J, Kim M-S, Kim J-S, et al. Enhanced dissolution of megestrol acetate microcrystals prepared by antisolvent precipitation process using hydrophilic additives. Int J Pharm 2010; 396:91-8.
[23] Matteucci ME, Hotze MA, Johnston KP, Williams RO. Drug nanoparticles by antisolvent precipitation: mixing energy versus surfactant stabilization. Langmuir 2006;22:8951-9.
[24] Thorat AA, Dalvi SV. Liquid antisolvent precipitation and stabilization of nanoparticles of poorly water soluble drugs in aqueous suspensions: recent developments and future perspective. Chem Eng J 2012;181:1-34.
[25] Zhao H, Wang J-X, Wang Q-A, Chen J-F, Yun J. Controlled liquid antisolvent precipitation of hydrophobic pharmaceutical nanoparticles in a microchannel reactor. Ind Eng Chem Res 2007;46:8229-35.
[26] Kumar R, Siril PF, Soni P. Preparation of nano-RDX by evaporation assisted solvent-antisolvent interaction. Propellants Explos Pyrotech 2014;39:383-9.
[27] Kumar R,Soni P,siril PF.Optimizedsynthesisof HMXnanoparticlesusing antisolvent precipitation method. J Energy Mater 2015;33(4):277-87.
[28] Kumar R, Siril PF. Ultrafine carbamazepine nanoparticles with enhanced water solubility and rate of dissolution. RSC Adv 2014;4:48101-8.
[29] Kumar R, Siril PF. Controlling the size and morphology of griseofulvin nanoparticles using polymeric stabilizers by evaporation-assisted solvent-antisolvent interaction method. J Nanopart Res 2015;17(256):1-11 (6).
[30] Sinha B, Mu¨ller RH, M¨oschwitzer JP. Bottom-up approaches for preparing drug nanocrystals: formulations and factors affecting particle size. Int J Pharm 2013;453:126-41.
[31] Bachmann W, Sheehan JC. A new method of preparing the high explosive RDX. J Am Chem Soc 1949;71:1842-5.
[32] Chung H-R, Kwon E, Oikawa H, Kasai H, Nakanishi H. Effect of solvent on organic nanocrystal growth using the reprecipitation method. J Cryst Growth 2006;294:459-63.
[33] Storm TD, Hazleton RA, Lahti LE. Some effects of solvent properties on nucleation. J Cryst Growth 1970;7:55-60.
[34] Karpowicz RJ, Sergio ST, Brill TB. beta.-Polymorph of hexahydro-1, 3, 5-trinitro-s-triazine. A Fourier transform infrared spectroscopy study of an energetic material. Ind Eng Chem Prod Res Devlop 1983;22:363-5.
[35] Liau Y-C, Kim E, Yang V. A comprehensive analysis of laser-induced ignition of RDX monopropellant. Combust Flame 2001;126:1680-98.
[36] da Costa Mattos E, Moreira ED, Diniz MF, Dutra RC, da Silva G, Iha K, et al. Characterization of Polymer-coated RDX and HMX particles. Propellants Explos Pyrotech 2008;33:44-50.
[37] Kim S-J, Lee B-M, Lee B-C, Kim H-S, Kim H, Lee Y-W. Recrystallization of cyclotetramethylenetetranitramine (HMX) using gas antisolvent (GAS) process. J Supercrit Fluids 2011;59:108-16.
[38] Lee B-M, Kim S-J, Lee B-C, Kim H-S, Kim H, Lee Y-W. Preparation of micronized β-HMX using supercritical carbon dioxide as antisolvent. Ind Eng Chem Res 2011;50:9107-15.
[39] Dreger ZA, Gupta YM. Raman spectroscopy of high-pressure-hightemperature polymorph of Hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (ε-RDX). J Phys Chem A 2010;114:7038-47.
[40] Dreger ZA, Gupta YM. Phase diagram of hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine crystals at high pressures and temperatures. J Phys Chem A 2010;114:8099-105.
[41] Yoo C-S, Cynn H. Equation of state, phase transition, decomposition of β-HMX (octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine) at high pressures. J Chem Phy 1999;111:10229-35.
[42] Bayat Y, Pourmortazavi SM, Iravani H, Ahadi H. Statistical optimization of supercritical carbon dioxide antisolvent process for preparation of HMX nanoparticles. J Supercrit Fluids 2012;72:248-54.
[43] Brand HV, Rabie RL, Funk DJ, Diaz-Acosta I, Pulay P, Lippert TK. Theoretical and experimental study of the vibrational spectra of the α,β, and δ phases of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine (HMX). J Phys Chem B 2002;106:10594-604.
[44] Sun J, Shu X, Liu Y, Zhang H, Liu X, Jiang Y, et al. Investigation on the thermal expansion and theoretical density of 1, 3, 5-trinitro-1, 3, 5-triazacyclohexane. Propellants Explos Pyrotech 2011;36:341-6.
[45] Li R, Wang J, Shen JP, Hua C, Yang GC. Preparation and characterization of insensitive HMX/graphene oxide composites. Propellants Explos Pyrotech 2013;38:798-804.
[46] The powder diffraction file, International centre for diffraction data, for RDX: 44-1618. provide the authou and the publishing year?
[47] The powder diffraction file, International centre for diffraction data, for HMX: 44-1620. provide the authou and the publishing year?
[48] Singh G, Felix SP, Soni P. Studies on energetic compounds: part 31. Thermolysis and kinetics of RDX and some of its plastic bonded explosives. Thermochim Acta 2005;426:131-9.
[49] Weese R, Maienschein J, Perrino C. Kinetics of the β→δ solid-solid phase transition of HMX, octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine. Thermochim Acta 2003;401:1-7.
[50] Brill T, Gongwer P, Williams G. Thermal decomposition of energetic materials. 66. Kinetic compensation effects in HMX, RDX, and NTO. J Phys Chem 1994;98:12242-7.
[51] Henson B, Smilowitz L, Asay B, Dickson P. The β-δ phase transition in the energetic nitramine octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine: thermodynamics. J Chem Phys 2002;117:3780-8.
[52] Bayat Y, Eghdamtalab M, Zeynali V. Control of the particle size of submicron HMX explosive by spraying in non-solvent. J Energy Mater 2010;28:273-84.
[53] Singh G, Felix SP, Soni P. Studies on energetic compounds part 28: thermolysis of HMX and its plastic bonded explosives containing estane. Thermochim Acta 2003;399:153-65.
* Corresponding author. Tel.: +91 1905 207040; fax: +91 1905 237942.
杂志排行
Defence Technology的其它文章
- Heat treatment optimization for tensile properties of 8011 Al/15% SiCp metal matrix composite using response surface methodology V. VEMBU*, G. GANESAN
- Hypersonic sliding target tracking in near space
- Microstructure, mechanical and corrosion behavior of high strength AA7075 aluminium alloy friction stir welds - Effect of post weld heat treatment P. Vijaya Kumara, G. Madhusudhan Reddyb, K. Srinivasa Raoc,*
- Optimal trajectory and heat load analysis of different shape lifting reentry vehicles for medium range application S. Tauqeer ul Islam RIZVI*, Lin-shu HE, Da-jun XU
- An experimental investigation of wire electrical discharge machining of hot-pressed boron carbide Ravindranadh BOBBILI*, V. MADHU, A.K. GOGIA
- Multi-layer protective armour for underwater shock wave mitigationA hmed HAWASS, Hosam MOSTAFA, Ahmed ELBEIH*
