根际促生菌Bacillussubtilis Y-IVI在香草兰上的应用效果研究
2015-06-15赵青云赵秋芳庄辉发朱自慧
赵青云, 赵秋芳, 王 辉, 王 华, 庄辉发,朱自慧
(1 中国热带农业科学院香料饮料研究所, 海南万宁 571533;2 农业部香辛饮料作物遗传资源利用重点实验室, 海南万宁 571737;3 海南省热带香辛饮料遗传改良与品质调控重点实验室, 海南万宁 571533)
根际促生菌BacillussubtilisY-IVI在香草兰上的应用效果研究
赵青云1,3, 赵秋芳1,2,3, 王 辉1,3, 王 华1,2, 庄辉发1,2,朱自慧1,3
(1 中国热带农业科学院香料饮料研究所, 海南万宁 571533;2 农业部香辛饮料作物遗传资源利用重点实验室, 海南万宁 571737;3 海南省热带香辛饮料遗传改良与品质调控重点实验室, 海南万宁 571533)
【目的】香草兰为多年生热带经济作物,随着种植年限的增加,植株长势弱,土壤有益微生物减少,土壤微生物区系失衡,严重制约了香草兰产业的可持续发展。枯草芽孢杆菌作为一种根际促生菌,被广泛应用于促进作物生长,改善土壤微生物环境。本文将枯草芽孢杆菌Y-IVI接种在有机肥上,生产了生物有机肥,并就该生物有机肥对香草兰生长的影响进行了研究。【方法】采用温室盆栽试验,调查施用根际促生菌枯草芽孢杆菌(Bacillussubtilis)Y-IVI及其经固体发酵制得的微生物有机肥料(Y-IVI: 3×108cfu/g)后,香草兰植株地上部及根系的生长状况,采用选择性培养基方法研究了Y-IVI在香草兰根际土壤中的定殖能力及对香草兰根茎腐病致病菌-尖孢镰刀菌数量的影响。【结果】施用Y-IVI及BIO 4个月后,香草兰根际土壤Y-IVI数量仍可达到106cfu/g土,二者无显著差异,在处理OF和对照中未检测到菌株Y-IVI。施用生物有机肥香草兰地上部干重和根系干重均显著高于对照,分别增加了63.1%和59.4%,与不接种Y-IVI的有机肥处理(OF)相比,地上部干重显著提高了43.2%,根系干重提高了18%,差异不显著;施用Y-IVI菌液的处理植株地上部干重和根系干重均高于对照,但无显著性差异;处理BIO根系直径、根系表面积和总体积与对照相比分别增加了41.9%、88.9%和80.4%,均显著高于对照,总根长与对照差异不显著;处理BIO根系表面积和总体积与有机肥处理OF相比分别显著增加了41.9%和30.8%,根系直径与OF相比增加了10.1%,差异不显著;处理Y-IVI根系直径与对照相比显著增加了25.5%,但根系表面积和总体积与对照差异不显著;与对照相比,施用BIO及Y-IVI的处理根际土壤尖孢镰刀菌数量分别明显降低了52.2%和41.8%,施用有机肥OF的处理降低了10%,差异不显著。【结论】Y-IVI可稳定定殖于香草兰根际土壤对其生长起有益作用,含促生菌Y-IVI的生物有机肥料比单独使用促生菌菌液可以更有效地减少根际土壤中尖孢镰刀菌数量,降低连作生物障碍。施用生物有机肥料比施用化肥和有机肥更有效地促进香草兰地上部及根系生长,因此,施用由根际促生菌枯草芽孢杆菌(Bacillussubtilis)Y-IVI制得的生物有机肥是解决香草兰连作生物障碍和提高收益的有效手段。
香草兰; 根际促生菌; 生物有机肥; 尖孢镰刀菌
香草兰(VanillaplanifoliaAmes.)是兰科香草兰属热带攀缘藤本香料植物,有“天然食品香料之王”的美誉。其鲜豆荚经过生香加工后含有250多种芳香成分,香气独特,被广泛用于调制各种高级香烟、名酒、茶叶,是各类高档食品和饮料的配香原料,在国际市场上供不应求[1]。另外香草兰还是用途广泛的天然药材,被列入美国、德国、英国的国家药典,有补肾、健胃、消胀、健脾之功效[2]。香草兰广泛分布于热带和亚热带地区,南北纬25°以内。在中国海南省及云南西双版纳地区均有栽培。
香草兰为多年生作物,在生产上以施用化肥和有机肥为主。随着种植年限的增加,植株长势变弱,土壤微生物区系失衡,土壤有益微生物数量减少,根茎腐病致病菌-尖孢镰刀菌数量增加,出现连作生物障碍问题[3],严重制约了香草兰产业的可持续发展。
近年来,根际促生菌成为国内外学者研究的热点之一,被广泛应用于促进作物生长,改善土壤微生物环境。Zaidi等[4]盆栽试验研究显示,土壤接种BacillussubtilisSJ-101可显著提高芥菜生物量,促进植物生长。Han等[5]从中国南方水稻根际土中分离到的菌株DelftiatsuruhatensisHR4具有固氮能力,对植株生长有促生作用,并且可作为生防菌株防治水稻纹枯病等常见病害。Karlidag等[6]在大田条件下研究了促生菌M3、 OSU-142、 FS01对苹果树生长的影响,结果表明,在移栽前用109cfu/mL的菌悬液浸根可刺激苹果树生长。Niranjan Raj[7]通过温室盆栽及大田试验比较了5种不同的促生菌对御谷的促生作用,结果表明土壤接种促生菌的处理种子发芽率,株高,植株鲜、干重,叶片面积,千粒重,籽粒数等均显著高于对照;开花期与对照相比提前了4-5 d。Ling等[8]通过大田和盆栽试验研究表明,施用由PaenibacilluspolymyxaSQR21制得的微生物肥料可显著提高西瓜产量,有效降低根际土壤尖孢镰刀菌的数量。Wang等[9]研究显示,施用由B.amyloliquefaciensW19制得的微生物肥料可显著提高香蕉植株干重,降低枯萎病发病率,改善土壤微生物环境。
促生菌在植物根际土壤中的成功定殖是其起促生作用的关键步骤[10-11]。Zhao等[12]研究表明,土壤接种B.subtilisY-IVI可显著促进甜瓜生长。施用由B.subtilisY-IVI制得的微生物肥料BIO可降低甜瓜根际土壤致病性真菌-尖孢镰刀菌的数量,提高植株干重,在施用BIO 60天后,Y-IVI在根际土壤仍可达到107cfu/g土[13]。本文采用的促生菌B.subtilisY-IVI由南京农业大学江苏省固体有机废弃物资源化高技术研究重点实验室提供,旨在通过盆栽试验研究施用Y-IVI及其制得的微生物肥料BIO对香草兰生长是否有促进作用,并采用选择性培养基的方法研究Y-IVI在香草兰根际土壤中的定殖能力及对香草兰根茎腐病致病菌-尖孢镰刀菌数量的影响,为解决香草兰种植中出现的长势弱和连作生物障碍问题提供理论依据。
1 材料与方法
1.1 试验材料与设计
供试土壤为沙壤土,养分含量为全氮0.72 g/kg、 速效磷13.00 mg/kg、 速效钾93.00 mg/kg、 有机质12.67 g/kg。试验盆钵采用周转箱(内尺寸600×420×165 mm),每盆装土10 kg。选取长势一致的健康香草兰作为供试苗。

试验设4个处理: 1)对照(CK,施用等量化肥); 2)施用牛粪堆肥(OF,300 g/盆);3)施用标记菌株Y-IVI菌悬液(接种浓度107cfu/g,土);4)施用微生物有机肥(BIO,300 g/盆)。该盆栽试验每盆栽种2株香草兰(1株用于测定生物量及根系扫描,另外1株用于根际土壤微生物数量测定),每处理重复4次,3次独立试验,共48盆。
1.2 测定项目及方法
1.2.1 植株生物量及根系形态 在香草兰移栽4个月后毁灭性采样,采用EPSON根系扫描仪扫描, WINRhizo软件分析根系各生长参数。根系及茎叶分别装入牛皮纸袋中,置烘箱杀青并烘至恒重,称量。
1.2.2 根际土壤标记菌株Y-IVI数量及尖孢镰刀菌数量 植株根从盆钵中拔出,轻轻抖掉土体土,黏附在根上的土连同根作为根际样品[15-16],带回实验室保存于4℃冰箱中保存。采用平板稀释涂布的方法,稀释至不同的浓度梯度,分别涂布在Fusariumoxysporum[17],Bacillusspp.[14-15]选择性培养基上,计数。
1.3 数据分析
数据均采用SPSS软件(SPSS 16.0)进行ANOVA方差分析和多重比较(LSD,P≤0.05)。
2 结果与分析
2.1 不同处理对香草兰植株生物量的影响
由图1A可知,施用生物有机肥的处理(BIO)香草兰地上部干重显著高于其他处理,与对照(CK)相比,提高了63.1%。施用牛粪有机肥的处理(OF)和接种促生菌Y-IVI的处理(Y-IVI)地上部干重分别比CK增加了13.8%和14.6%,但三者之间无显著性差异。处理BIO香草兰根系干重显著高于CK及处理Y-IVI,分别增加了59.4%和37.2%。处理BIO与OF之间差异不显著(图1B)。由此可见,施用促生菌Y-IVI及BIO均可促进香草兰地上部及根系生长,且BIO促生效果更好。
2.2 不同处理对香草兰根系生长的影响
从表1得出,CK总根长高于其他处理,与处理OF及BIO无显著差异,但显著高于处理Y-IVI。另外,处理OF、Y-IVI和BIO之间差异不显著。处理BIO根系表面积与其他处理差异显著,分别比CK、OF及Y-IVI增加了88.9%、41.6%和71.9%,并且,处理OF根系表面积显著高于CK及处理Y-IVI。处理BIO根系直径与CK及处理Y-IVI相比,分别显著提高了41.9%和12.9%,与处理OF无显著性差异。处理BIO根系体积显著高于其他处理,但处理OF与Y-IVI之间差异不显著,分别比CK增加了80.4%和31.1%。这说明施用促生菌Y-IVI或由其制得的BIO均可促进香草兰根系生长发育,且以施用BIO对培育香草兰发达的根系具有显著效果。
2.3 不同处理对香草兰根际土壤尖孢镰刀菌数量的影响
图2表明,处理Y-IVI和BIO根际土壤尖孢镰刀菌数量无显著性差异,但均显著低于CK和处理OF。处理BIO与CK、OF及Y-IVI相比,尖孢镰刀菌数量分别降低了52.6%、47.3%和19.0%。另外,处理OF与CK相比,土壤尖孢镰刀菌数量降低了10.4%,但二者差异不显著。这说明,施用Y-IVI及BIO均可显著降低香草兰根际土壤尖孢镰刀菌的数量,但OF处理对降低尖孢镰刀菌数量不显著。
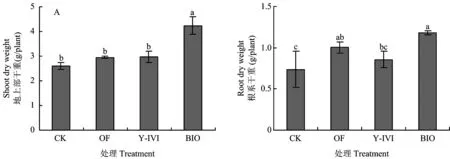
图1 不同处理对香草兰植株地上部及根系干重的影响Fig.1 Effects of different treatments on vanilla shoot and root dry weight[注(Note): CK—化肥Chemical fertilizer;OF—有机肥 manure;Y-IVI—枯草芽孢杆菌Y-IVI菌液(接种浓度107 cfu/g土)Inocula of Bacillus subtilis Y-IVI (107cfu/g, soil);BIO—接种Y-IVI菌液生物有机肥(Y-IVI, 3 × 108 cfu/g) Manure inoculated with Y-IVI (Y-IVI, 3 × 108 cfu/g). 图中柱上不同小写字母表示差异显著(P<0.05)Bars with different letters indicate significant differences (P<0.05).]
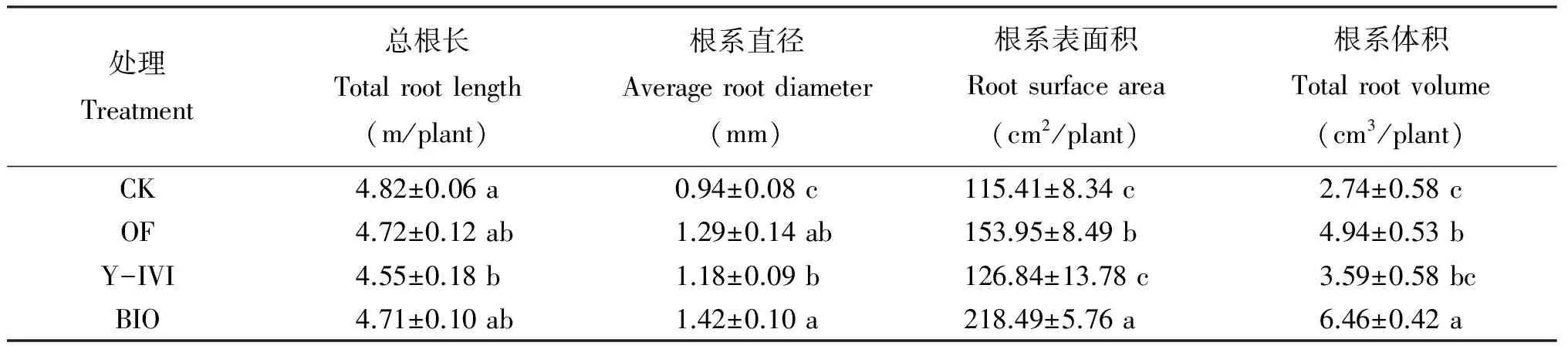
处理Treatment总根长Totalrootlength(m/plant)根系直径Averagerootdiameter(mm)根系表面积Rootsurfacearea(cm2/plant)根系体积Totalrootvolume(cm3/plant)CK4.82±0.06a0.94±0.08c115.41±8.34c2.74±0.58cOF4.72±0.12ab1.29±0.14ab153.95±8.49b4.94±0.53bY-IVI4.55±0.18b1.18±0.09b126.84±13.78c3.59±0.58bcBIO4.71±0.10ab1.42±0.10a218.49±5.76a6.46±0.42a
注(Note): 表中数据为3次独立试验的平均值±标准偏差, Data were expressed as mean±standard deviation (n=3); 数值后不同小写字母表示处理间差异显著(P<0.05)Values followed by different letters indicate significant differences (P<0.05).
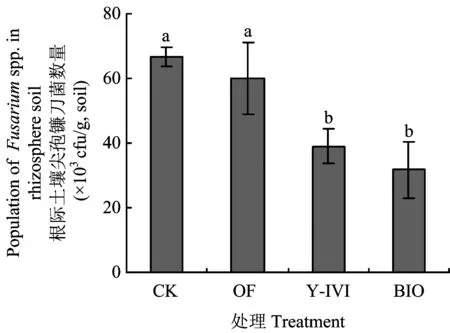
图2 不同处理对香草兰根际土壤尖孢镰刀菌数量的影响Fig.2 Effects of different treatments on Fusarium spp population in vanilla rhizosphere soil[注(Note): CK—化肥Chemical fertilizer;OF—有机肥 manure;Y-IVI—枯草芽孢杆菌Y-IVI菌液(接种浓度107 cfu/g土)Inocula of Bacillus subtilis Y-IVI (107cfu/g, soil);BIO—接种Y-IVI菌液生物有机肥(Y-IVI, 3 × 108 cfu/g) Manure inoculated with Y-IVI (Y-IVI, 3 × 108 cfu/g). 图中柱上不同小写字母表示差异显著(P<0.05)Bars with different letters indicate significant differences (P<0.05).]
2.4 促生菌Y-IVI在香草兰根际土壤的定殖能力
图3显示,在土壤施用Y-IVI及BIO 4个月后,处理Y-IVI和BIO根际土壤促生菌Y-IVI数量仍可达到106cfu/g土,但在处理CK及OF根际土壤中并未检测到促生菌Y-IVI。由此可见,Y-IVI可稳定定殖于根际土壤对香草兰生长起促进作用,并可抑制土壤中有害真菌尖孢镰刀菌的数量。
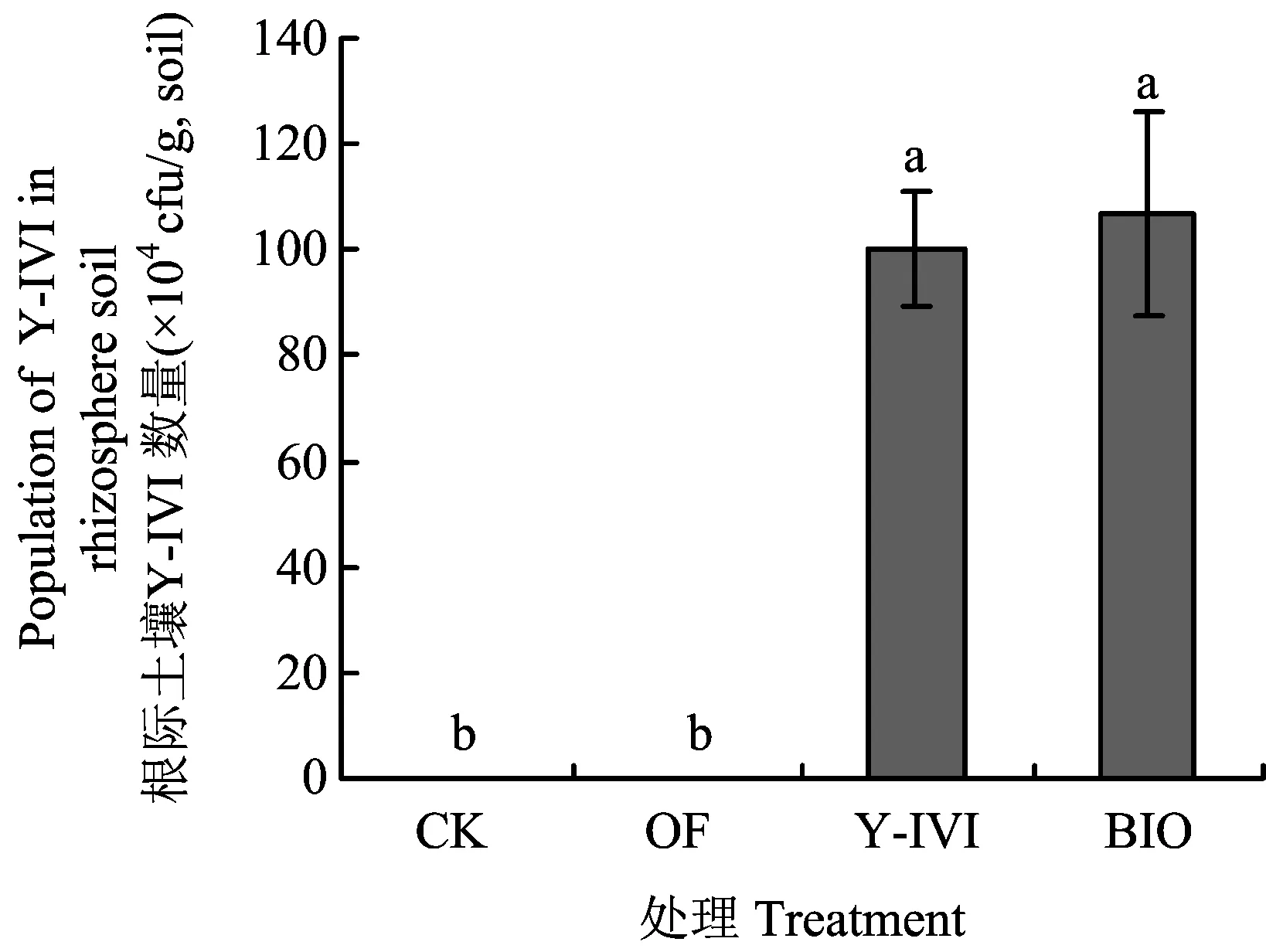
图3 促生菌Y-IVI在香草兰根际土壤的定殖能力Fig.3 Colonization ability of plant growth promotion rhizobacteria Y-IVI in vanilla rhizosphere soil[注(Note): CK—化肥Chemical fertilizer;OF—有机肥 manure;Y-IVI—枯草芽孢杆菌Y-IVI菌液(接种浓度107 cfu/g土)Inocula of Bacillus subtilis Y-IVI (107cfu/g, soil);BIO—接种Y-IVI菌液生物有机肥(Y-IVI, 3 × 108 cfu/g) Manure inoculated with Y-IVI (Y-IVI, 3 × 108 cfu/g). 图中柱上不同小写字母表示差异显著(P<0.05)Bars with different letters indicate significant differences (P<0.05).]
3 讨论与结论
化肥的大量施用对提高作物产量起到了重要的作用,但同时也带来了诸多问题,如化肥利用率降低,生态环境恶化,土壤微生物区系单一化等。根际促生菌在促进作物生长,防治植物病害,改善土壤微生物环境等方面具有重要的作用,在一定程度上可替代化肥、农药等应用于农业生产[12],减少有害成分对环境及人类健康造成危害,实现农业的可持续发展。另外,生物有机肥是由有益微生物菌群与有机肥结合形成的新型、高效、安全的生物有机肥料。它兼具了有机肥和微生物肥料的优点,可为微生物提供充足的养分,维持功能菌种活性,有效地提高肥料利用率,促进作物生长,改善土壤微生物环境[18-20]。促生菌及生物有机肥被广泛应用于瓜果蔬菜类经济作物以替代部分化肥及普通有机肥,在国内外促生菌及生物有机肥在香草兰上的应用研究鲜有报道。
本试验研究表明,施用促生菌B.subtilisY-IVI及由其制得的微生物肥料BIO可提高香草兰植株干重,促进根系生长,以施用BIO促生效果最显著,这与Zhao等[13]研究结果一致,表明B.subtilisY-IVI及其微生物肥料BIO可用于香草兰生产,以减少化肥施用量,改善香草兰生长状况。
芽孢杆菌是土壤微生态的优势种群之一,能形成具有较强抗逆能力的芽孢,有利于其在生物有机肥的生产及土壤环境中存活、定殖与繁殖[21]。微生物在根际成功定殖是其产生有益作用的先决条件[22]。Cao等[23]将B.subtilisSQR9与有机肥经固体发酵制得微生物有机肥,结果表明施用该肥料可显著抑制尖孢镰刀菌在根际土壤中的数量,降低黄瓜枯萎病发病率,其原因是B.subtilisSQR9可在植株根系及根际土壤稳定定殖。Zhang等[24]研究结果表明,施用由B.subtilisN11制得的微生物肥料可有效防治香蕉枯萎病,主要因为B.subtilisN11可在香蕉植株根系形成生物膜,稳定定殖于根系及根际土壤。Huang等[25]研究表明,施用由B.pumilusSQR-N43制得的微生物肥料20天后,B.pumilusSQR-N43在土壤中的数量并无显著下降,仍维持在108cfu/g土,对黄瓜立枯病防治率达到68%,显著高于施用普通有机肥的处理(9%)。Zhao等[12]盆栽试验结果显示,土壤接种B.subtilisY-IVI 30天后其在甜瓜根际土壤中的数量与接种时相比并未显著降低,仍可达到108cfu/g土。本文结果显示,土壤接种Y-IVI及施用微生物有机肥4个月后Y-IVI在香草兰根际土壤中的数量为106cfu/g土,说明Y-IVI亦可稳定定殖在香草兰根际土壤对其生长起促进作用。
部分微生物兼具促生和生防的作用。Ren等[26]研究指出,PaenibacilluspolymyxaC5可提高烟草植株干重,并可明显降低烟草黑胫病发病率。施用由B.amyloliquefaciens制得的生物有机肥可提高植株干重,有效防治番茄青枯病[27]。Wang等[28]盆栽试验结果显示,施用由B.amyloliquefaciensW19制得的生物肥料可有效防治香蕉枯萎病,提高植株地上部及地下部干重。Zhao等[29]盆栽及大田试验结果表明,施用由芽孢杆菌制得的微生物肥料后,甜瓜枯萎病发病率显著降低,植株生物量及甜瓜产量显著提高。本文结果表明,土壤接种Y-IVI及施用BIO的处理香草兰根际土壤尖孢镰刀菌数量显著低于对照及施用普通有机肥的处理,Y-IVI及BIO对降低香草兰根茎腐病有一定的作用。Y-IVI可产生脂肽类物质抑制有害真菌尖孢镰刀菌,改善土壤微生物环境,并可产生铁载体,吲哚乙酸等促生类物质促进甜瓜生长[12-13]。由此可见,B.subtilisY-IVI可作为广谱促生菌及生防菌应用于香草兰生产,以减少化肥施用量,促进香草兰生长,改善土壤微生物环境,缓解其种植中出现的植株长势弱,连作生物障碍问题。笔者拟从Y-IVI是否可在香草兰根际形成生物膜和分泌可调节植物生长的活性物质等方面进一步研究其作用机理。
[1] 王庆煌, 宋应辉, 陈封宝, 等. 香草兰高产栽培技术研究[J]. 热带农业科学, 1994, 2: 50-57. Wang Q H, Song Y H, Chen F Betal. Study on the high-yield cultivation of vanilla[J]. Tropical Agricultural Science, 1994, 2: 50-57.
[2] Gerasimov A V, Gornova N V, Rudometova N Vetal. Determination of vanillin and ethylvanillin in vanilla flavorings by planar (Thin-Layer) chromatography[J]. Journal of Analytical Chemistry, 2003, 58 (7): 677-684.
[3] 赵青云, 王辉, 王华, 等. 种植年限对香草兰生理指标及土壤微生物区系的影响[J]. 热带作物学报, 2012, 33(9): 1562-1567. Zhao Q Y, Wang H, Wang Hetal. Effects of planting period on vanilla physiological indices and rhizosphere soil microbial community structure[J]. Chinese Journal of Tropical Crops, 2012, 33(9): 1562-1567.
[4] Zaidi S, Usmani S, Singh B Retal. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea[J]. Chemosphere, 2006, 64: 991-997.
[5] Han J G, Sun L, Dong X Zetal. Characterization of a novel plant growth-promoting bacteria strain Delftia tsuruhatensis HR4 both as a diazotroph and a potential biocontrol agent against various plant pathogens[J]. Systematic and Applied Microbiology, 2005, 28: 66-76.
[6] Karlidag H, Esitken A, Turan Metal. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple[J]. Scientia Horticulturae, 2007, 114: 16-20.
[7] Niranjan Raj S, Deepaka S A, Basavarajua Petal. Comparative performance of formulations of plant growth promoting rhizobacteria in growth promotion and suppression of downy mildew in pearl millet[J]. Crop Protection, 2003, 22: 579-588.
[8] Ling N, Xue C, Huang Q Wetal. Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt[J]. Biocontrol, 2010, 55: 673-683.
[9] Wang B B, Yuan J, Zhang Jetal. Effects of novel bioorganic fertilizer produced by Bacillus amyloliquefaciens W19 on antagonism of Fusarium wilt of banana[J]. Biology and Fertility of Soils, 2013, 49(4): 435-446.
[10] Bolwerk A, Lagopodi A L, Lugtenberg B J. Visualization of interactions between a pathogenic and a beneficial Fusarium strain during biocontrol of tomato foot and root rot[J]. Molecular Plant-microbe Interactions, 2005, 18: 710-721.
[11] Chatterton S, Jayaraman J, Punja Z K. Colonization of cucumber plants by the biocontrol fungus Clonostachys rosea f. catenulate[J]. Biological Control, 2008, 46: 267-278.
[12] Zhao Q Y, Shen Q R, Ran Wetal. Inoculation of soil by Bacillus subtilis Y-IVI improves plant growth and colonization of the rhizosphere and interior tissues of muskmelon (CucumismeloL.)[J]. Biology and Fertility of Soils, 47: 507-514.
[13] Zhao Q Y, Ran W, Wang Hetal. Biocontrol of Fusarium wilt disease in muskmelon with Bacillus subtilis Y-IVI[J]. BioControl, 2013, 58: 283-292.
[14] Turner J T, Backman P A. Factors relating to peanut yield increases after seed treatment with Bacillus subtilis[J]. Plant Disease, 1991, 75: 347-353.
[15] Kinsella K, Schulthess C P, Morris T Fetal. Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere[J]. Soil Biology and Biochemistry, 2009, 41: 374-379.
[16] Pang Y D, Liu X G, Ma Y Xetal. Induction of systemic resistance, root colonization and biocontrol activities of the rhizospheric strain of Serratia plymuthica are dependent on N-acyl homoserine lactones[J]. European Journal of Plant Pathology, 2009, 124: 261-268.
[17] Muslim A, Horinouchi H, Hyakumachi M. Biological control of Fusarium wilt of tomato with hypovirulent binucleate Rhizoctonia in greenhouse conditions[J]. Mycoscience, 2003, 44: 77-84.
[18] 梅新兰, 赵青云, 谭石勇, 等. 辣椒疫病拮抗菌株筛选、鉴定及其防效[J]. 应用生态学报, 2010, 21(10): 2652-2658. Mei X L, Zhao Q Y, Tan S Yetal. Screening,identification,and biocontrol effect of antagonistic bacteria against Phytophthora capsici[J]. Chinese Journal of Applied Ecology, 2010, 21(10): 2652-2658.
[19] Luo J, Ran W, Hu J et al. Application of bio-organic fertilizer significantly affected fungal diversity of soils[J]. Soil Science Society of America Journal, 2010, 74: 2039-2048.
[20] Ling N, Zhang W W, Tan S Yetal. Effect of the nursery application of bioorganic fertilizer on spatial distribution of Fusarium oxysporum f. sp. niveum and its antagonistic bacterium in the rhizosphere of watermelon[J]. Applied Soil Ecology, 2012, 59: 13-19.
[21] 陈中义, 张 杰, 黄大昉, 等. 植物病害生防芽孢杆菌抗菌机制与遗传改良研究[J]. 植物病理学报, 2003, 33(2): 97-103. Chen Z Y, Zhang J, Huang D Fetal..Research progress on antimicrobial mechanism and genetic engineering of Bacillus for plant diseases biocontrol[J]. Acta Phytopathologica Sinica, 2003, 33(2): 97-103.
[22] Weller D M. Biological control of soilborne pathogens in the rhizosphere with bacteria[J]. Annual Review of Phytopathology, 1988, 26: 379-407.
[23] Cao Y, Zhang Z Z, Ling Netal. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots[J]. Biology and Fertility of Soils, 2011, 47: 495-506.
[24] Zhang N, Wu K, He Xetal. A new bioorganic fertilizer can effectively control banana wilt by strong colonization with Bacillus subtilis N11[J]. Plant and Soil, 2011, 344: 87-97.
[25] Huang X Q, Zhang N, Yong X Yetal. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43[J]. Microbiology Research, 2012, 167: 135-143.
[26] Ren X L, Zhang N, Cao M Hetal. Biological control of tobacco black shank and colonization of tobacco roots by a Paenibacillus polymyxa strain C5[J]. Biology and Fertility of Soils, 2012, 48: 613-620.
[27] Wei Z, Yang X M, Yin S Xetal. Efficacy of Bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field[J]. Applied Soil Ecology, 2011, 48: 152-159.
[28] Wang B B, Yuan J, Zhang Jetal. Effects of novel bioorganic fertilizer produced byBacillusamyloliquefaciens W19 on antagonism ofFusariumwilt of banana[J]. Biology and Fertility of Soils, 2013, 49: 435-447.
[29] Zhao Q Y, Dong C X, Yang X Metal. Biocontrol ofFusariumwilt disease for Cucumis melo melon using bio-organic fertilizer[J]. Applied Soil Ecology, 2011, 47: 67-75.
Beneficial effects of plant growth promoter rhizobacteria on vanilla (VanillaplanifoliaAmes.) growth
ZHAO Qing-yun1,3, ZHAO Qiu-fang1,2,3, WANG Hui1,3, WANG Hua1,2, ZHUANG Hui-fa1,2, ZHU Zi-hui1,3
(1InstituteofSpiceandBeverage,CATAS,Wanning,Hainan571533,China; 2KeyLaboratoryofGeneticResourcesUtilizationofSpiceandBeverageCrops,MinistryofAgriculture,Wanning,Hainan571533,China; 3HainanProvincialKeyLaboratoryofGeneticImprovementandQualityRegulationforTropicalSpiceandBeverageCrops,Wanning,Hainan571533,China)
【Objectives】Vanilla(VanillaplanifoliaAmes.) is one of tropical perennial crop. The elongation of growth period often leads to weak plant, reduced population of soil beneficial microbes, increased harmful microbes and unbalance of the soil microbial community structure. We hope this can provide theoretical basis for solving the problems of weak vanilla plant growth and continuous cropping biological obstacles. The main objectives of this study were to study the effect of one of recognized root growth promoter, rhizobacteriaBacillussubtilisY-IVI on ameliorate soil microbial community structure and the proper way of its application. 【Methods】 A solid fermented bioorganic fertilizer (BIO) was prepared by inoculatingBacillussubtilisY-IVI into cattle manure and fermented 5 days inside greenhouse, the final active Y-IVI content is 3 × 108cfu/g. A pot experiment in green house was carried out with chemical fertilizer as control, the effects of application of Y-IVI inocula, biofertilizer and cattle manure on the plant growth of both aboveground part and root were investigated Selective mediums method was used to study the colonization ability of Y-IVI in vanilla rhizosphere soil and their influence on the pathogen number of rhizome rot disease (Fusariumoxysporum). 【Results】 The application of BIO could obviously increase vanilla plant above ground and root dry weight by 63.1% and 59.4% compared with control, and significantly increased the plant above ground dry weight by 43.2% compared with cattle manure. The single application of Y-IVI binocular does not have the significant effect on plant above ground and root dry weight. The application of BIO significantly increases the average root diameter, root surface area and total root volume by 41.9%, 88.9% and 80.4%, compared with control, but not the total root length; root surface area and total root volume of BIO treatment were obviously increased by 41.9% and 30.8% compared with manure, respectively, while average root diameter merely increased by 10.1%; average root diameter of Y-IVI treatment was significantly increased by 25.5% compared with control, but root surface area and total root volume had no significant difference. Application of BIO and Y-IVI significantly decreased population ofFusariumoxysporumin rhizosphere soil by 52.2% and 41.8%, compared with control, respectively, and organic fertilizer treatment merely decreased by 10%; population of Y-IVI remained about 106cfu/g rhizosphere soil after application of BIO and Y-IVI 4 mouths. No Y-IVI was detected in organic fertilizer treatment and control. 【Conclusions】 Inoculation of Y-IVI solid fermented bio-organic fertilizer BIO could promote vanilla plant above ground and root growth, significantly decreaseF.oxysporumnumber in rhizosphere soil; Y-IVI can successfully colonize in rhizosphere soil to have sound effects on vanilla growth. The BIO can apply to vanilla culture to reduce chemical fertilizer and organic fertilizer application, the number ofF.oxysporum, and decrease continuous cropping obstacles.
vanilla (VanillaplanifoliaAmes.); plant growth promotion rhizobacteria; bio-organic fertilizer;Fusariumoxysporum
2014-02-09 接受日期: 2014-09-23
国家自然科学青年基金项目(31201683);海南省自然科学基金项目(312032)资助。
赵青云(1983—),女,河南驻马店人,博士,助理研究员,主要从事土壤微生物与生物肥料研究。E-mail: qingyun_022@163.com
S144; S573
A
1008-505X(2015)02-0535-06
