Endophytic fungi of wild legume Sesbania bispinosa in coastal sand dunes and mangroves of the Southwest coast of India
2015-06-09•
•
ORIGINAL PAPER
Endophytic fungi of wild legume Sesbania bispinosa in coastal sand dunes and mangroves of the Southwest coast of India
Suvarna J.Shreelalitha1•Kandikere R.Sridhar2
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Evaluation of 450 surface sterilized tissue segments of a seasonal wild legume,Sesbania bispinosa(Jacq.),of coastal sand dunes and mangroves of southwest India yielded 546 isolates comprising 39 endophytic fungi with six dominant taxa(Aspergillus f l avus,Aspergillus niger,Cladosporium tenuissimum,Fusarium moniliforme,Penicillium chrysogenumand morpho sp.1).A consortium of saprophytic,pathogenic and toxigenic fungi exists as endophytes inS.bispinosa.Number of segments colonized, number of isolates obtained,species richness and diversity were higher inS.bispinosain mangroves compared to coastal sand dunes.Seeds yielded more fungal isolates,but species richness and diversity were low.In spite of low fungal colonization in root segments,the diversity was high.Up to 30–40%endophytic fungi ofS.bispinosadiffered between coastal sand dunes and mangroves revealing partial host-and habitat-specif i city.AsS.bispinosais extensively used as green manure and forage in southwest India,further studies especially on the bioactive compounds of its endophytic fungi might broaden its range of uses.In addition to conventional morphological techniques,molecular tools would provide precise insight on the endophytic fungi of coastal sand dunes and mangroves.
Legumes⋅Sesbania bispinosa⋅Endophytic fungi⋅Coastal sand dunes⋅Mangroves
Introduction
The Indian subcontinent consists of 10 biogeographic zones in 25 provinces encompassing more than 400 biomes (Mehta 2000).Coastal habitats have been considered one of the major biogeographic zones supporting a wide range of f l ora,fauna and microbes.The coastal sand dunes along the southwest coast of India harbor a variety of wild legumes,which are nutritionally and agriculturally valuable(Rao and Meher-Homji 1985;Rao and Sherieff 2002). About 25 species of legumes are known from the mid-and hind-dunes of coastal sand dunes of this region(Bhagya and Sridhar 2009).Similar to coastal sand dunes,mangroves of the Indian subcontinent also support a wide variety of legumes(Rao and Suresh 2001;Kathiresan and Rajendran 2005).The Nethravathi mangroves in southwest India are endowed with several mangrove associates along with wild legumes of economic importance(Rao and Suresh 2001).They consist of seasonal to perennial herbs, shrubs,under-shrubs,climbers and woody plants.About 14 wild legumes have been identif i ed in the Nethravathi mangrove with dominance ofCanavalia cathartica,Derris trif l orum,Sesbania bispinosaandS.speciosa(Anita 2010). Many wild legumes f i x nitrogen symbiotically with rhizobia in coastal sand dunes as well as mangroves(Arun and Sridhar 2004).They are common in different tidal ranges of coastal sand dunes or mangroves and extend toagricultural f i elds where they improve soil fertility and provide green manure and fodder for livestock,sources of food and traditional medicine.S.bispinosain southwest India is self-dispersed,widely distributed in coastal sand dunes and mangroves and extends to agricultural f i elds (e.g.paddy,sugarcane and vegetable)and plantations(e.g. coconut and areca)(Bhat 2003).We compared the assemblage and diversity of endophytic fungi in f i ve tissues selected from f i ve different organs(leaf,stem,root,tender pods and seeds)ofS.bispinosagrown on the coastal sand dunes and mangroves of southwest India.The major objectives of this study were to determine whether the endophytic fungi ofS.bispinosais host-or habitat-specif i c and to compare our results with earlier studies.
Materials and methods
Plant species and processing
Five mature plants ofS.bispinosa(Jacq.)W.F.Wight growing about 50 m apart in coastal sand dunes(hinddunes)of Someshwara(12°47′N,74°52′E)and Nethravathi mangroves(12°50′N,74°51′E)were chosen during postmonsoon season(November–December 2010).Plants were uprooted,transferred to the laboratory and processed within 4–5 h of collection.Four tissue pieces(leaf,stem,root and tender pod)from each plantwere excised into nine segments each of one cm length(4×5×9=180 segments)and nine dry seeds per plant(9×5=45 seeds)were randomly chosen.To remove the extraneous matter,tissue segments and seeds were rinsed in distilled water and blotted.They were surface sterilized according to Tayloretal.(1999)with a slight modif i cation(sequence of95%ethanol,1 min;6% sodium hypochlorite,5 min;95%ethanol,0.5 min;followed by three rinses in sterile distilled water).
Fungal identif i cation
Surface sterilized tissue segments and seeds were placed on 1.5%potato dextrose agar(PDA)medium(HiMedia Laboratories Pvt.Ltd.,Mumbai,India)amended with antibiotic(tetracycline,250 mg L-1;Sigma Chemical Co., St.Louis,Missouri).The plates were incubated (27±2°C)up to 4 weeks at 12 h light and dark regimes. Tissues and seeds were periodically screened for growth of mycelia or development of colonies on the medium or on the tissue or seeds.Actively advancing mycelial portions were transferred aseptically to fresh antibiotic-free PDA medium.Fungi were identif i ed based on colony characteristics and spore morphology using standard monographs and taxonomic keys(Ellis 1971,1976,Carmichael et al. 1980;Ellis and Ellis 1987;Cai et al.2006;Bhat 2010).
Data analysis
The percent colonization frequency(CF,%),total colonization frequency(TF,%)and relative abundance(RA,%) of each fungus in different tissues/seeds were calculated:

whereNis the number of segments of each tissue colonized,the number of segments of all tissue colonized,Tthe total segments of each tissue screened,andis the total segments all tissue screened.Diversity,Simpson;Shannon)(Magurran 1988)and evennessPielou’s) (Pielou 1975)of fungal taxa on each tissue and all tissues were calculated:



whereais total number of fungal taxa in tissue 1,bthe total number of fungal taxa in tissue 2,andcis number of fungal taxa common to tissues 1 and 2.
Results
The incubated plates were periodically screened for growth of fungi from the edges of the segments embedded on the medium under low-power microscope.No symptoms of projection of mycelia or fruiting of fungi were seen up to1 week of incubation.This indicated the eff i ciency of surface sterilization in destruction of surface mycelia and spores,which resulted in growth of any fungi present in the interior of the tissues.Although the number of sampled host plants and the number of assessed segments of different organs from each host were low for assessment of the diversity of endophytic fungi,the sample sizes provided reasonable results for comparison of endophytic fungi between habitats and between tissue segments of host plant organs.
Coastal sand dune plants
Out of a total of 225 segments examined,88%yielded 227 isolates(Table 1).The number of segments colonized as well as the number of isolates were highest in seeds(44 and 69)followed by leaves(43 and 62).However,the number of core-group taxa was highest in roots(4 vs.3 in other tissues),but the expected number of species out of 30 random segments was lowest(21 in roots vs.25–27 in other tissues) (Fig.1).Simpson and Shannon diversities and Pielou’s evenness were higher in root than in other tissues(Simpson: 0.889 vs.0.825–0.850;Shannon:3.359 vs.2.757–3.238; Pielou’s evenness,0.937 vs.0.809–0.875).A total of 26 taxa were recovered with a maximum of 16 taxa in leaves. The total frequency of occurrence ofP.chrysogenumwas highest(20.2%)followed by morpho sp.1(19.1%)and they belonged to core-group taxa in all tissues.A.f l avus,A.niger,C.tenuissimumandF.moniliformewere also the core-group taxa in at least one of the tissues.A total of 10 taxa was conf i ned to one of the tissues assessed(exclusive species)with a maximum of four taxa each in leaves and roots(leaf:Aspergillus sydowii,A.tamarii,Paecilomycessp.andRhizopussp.;root:Codinaea assamica,Colletotrichum dematium,Fusarium solaniandPhialophorasp.;pod:Penicllium citrinumandPestalotiopsis neglecta) (Table 2).Fungal similarity among the tissues ranged from 29%(root vs.pod)to 80%(stem vs.seed)(Table 3). Morpho sp.1 did not sporulate on the PDA and it also failed to sporulate on the malt extract agar(MEA).
Mangrove plants
In mangrove plants,out of 225 segments,91.6%yielded 319 isolates(Table 1).All tested seeds yielded endophytic fungi and the number of isolates(130 vs.35–70%)as well as core-group taxa were higher in seeds than in other tissues(5 vs.2–3).The expected number of species was higher in pods than in other tissues(29 vs.23–25)(Fig.1). Simpson diversity was higher in roots than in other tissues (0.897 vs.0.775–0.884),while Shannon diversity was higher in leaf segments(3.652 vs.2.453–3.564 in other tissues).Pielou’s evenness was higher in roots than in other tissues(0.912 vs.0.816–0.876).A total of 31 taxa were recovered with a maximum of 18 taxa in leaves.The total frequency of occurrence ofP.chrysogenumwas highest(22.4%),which was a core-group taxon in all tissues. BesidesP.chrysogenum,f i ve other fungal taxa(A.f l avus, A.niger,C.tenuissimum,Fusarium moniliformeand morpho sp.1)belonged to the core-group at least in one of the tissues(Table 4).A total of 12 taxa were conf i ned to one type of tissue with a maximum of four taxa each in leaves and roots(leaf:Chaetomium globosum,Curvularia prasadii,P.citrinumandTrichoderma harzianum;stem:Eurotium chevalieri;root:C.assamica,Rhizopussp.,Scytalidium lignicolaandTrichoderma hamatum;pod:Aspergillus ochraceus;seed:Aspergillus oryzaeandCladosporium psoraleae).The fungal similarity among tissues was 27%(root vs.pod)with a maximum between leaf and stem(67%)(Table 3).

Table 1 Fungal assemblage,species richness and diversity of endophytic fungi of S.bispinosa of coastal sand dunes and mangroves in the Southwest coast of India

Fig.1 Rarefaction curves of endophytic fungi in different tissues of S.bispinosa in coastal sand dunes and mangroves with rarefaction curves of endophytic fungi in coastal sand dunes and mangroves irrespective of tissues(number of segments vs.expected number of species,E(t))
Comparison of habitats
In both habitats,more seeds were colonized by endophytic fungi than were other tissues(44–45 vs.36–44)and seeds yielded more fungal isolates(69–130 vs.35–70 in other tissues)(Table 1).However,the total number of taxa in seeds was lower than in leaves(10–14 vs.16–18).In both habitats,species richness was highest in leaves followed by roots,seeds,stem and pods.The exclusive species were more numerous and more consistent in all tissues of mangroves compared to the coastal sand dunes.For the f i ve tissues examined,mangrove habitat showed higher expected number of species than coastal sand dunes (Fig.1).However,the overall expected number of species out of 265 segments on random assessment was higher in coastal sand dunes than in mangroves(196 vs.172 taxa). Simpson and Shannon diversities were higher in mangroves than in coastal sand dunes(Simpson:0.906 vs. 0.889;Shannon:3.974 vs.3.749).Overall,18 fungal taxa were common to coastal sand dunes and mangroves (Tables 2,4).P.chrysogenumwas the most frequent and was a core-group taxon in both habitats.The top six taxa of the core-group that were common to both habitats were:A. fl avus,A.niger,C.tenuissimum,F.moniliforme,P. chrysogenumand morpho sp.1.The exclusive species of tissues in coastal sand dunes and mangroves were not common except forC.assamicain roots(Tables 2,4). Sørensen’s similarity showed least similarity between roots and pods in both habitats(27-29%),while stem vs.pods (53%)and roots vs.seeds(55%)showed almost equal similarity between the habitats(Table 3).
Discussion
In the coastal sand dunes of southwest India,Beena et al. (2000)surveyed endophytic fungi of root segments of three dominant plant species(Ipomoea pes-caprae,Launaea sarmentosaandPolycarpea corymbosa)employing plating and damp-incubation techniques.Seena and Sridhar(2004) evaluated endophytic fungi in three age groups(seeds, seedlings and mature plants)and f i ve tissue classes(root, stem,leaf,seed coat and cotyledon)of two coastal sand dune wild legumes(Canavalia catharticaandCanavalia maritima)of southwest India.Foliar endophytic fungi of many mangrove plant species likeAvicennia marina,Avicennia off i cinalis,Bruguiera cylindrica,Kandelia candel,Rhizophora apiculata,Rhizophora mucronata,Sonneratia caseolarisandSuaeda maritimawere studied in southeast India(Suryanarayanan et al.1998;Suryanarayanan and Kumaresan 2000;Kumaresan and Suryanarayanan 2001, 2002).Endophytic fungi of bark,woody tissues and leaves ofK.candelwere studied at Maipo Marshes nature reserve in Hong Kong(Pang et al.2008).Segments of plant organs including stem,pods,seeds,roots and rhizomes of mangrove species have been studied for endophytic fungi (Ananda and Sridhar 2002;Maria and Sridhar 2003;Anita and Sridhar 2009;Anita et al.2009).Based on the above literature and the present study,the following sub-sections compare endophytic fungi of plant species in different maritime habitats.

Table 2 Colonization frequency(CF,%)of endophytic fungi in f i ve tissues of S.bispinosa in coastal sand dunes(out of 45 segments of each tissue)

Table 3 Sørensen’s similarity coeff i cient(Cs,%)of endophytic fungi in tissues of S.bispinosa in coastal sand dunes and mangroves
Dominant endophytes
In our study,the core-group fungi(A.f l avus,A.niger,C. tenuissimum,F.moniliforme,P.chrysogenumand morpho sp.1)seem to render major defense inS.bispinosaof coastal sand dunes as well as mangroves.Except forC. tenuissimumandF.moniliforme,the others were top coregroup taxa inS.bispinosain southwest India mangroves (Anita et al.2009).Similarly,the mangrove landraceC. catharticaalso supportedA.f l avus,A.niger,P.chrysogenumand morpho sp.1 as core-group taxa(Anita and Sridhar 2009).However,C.catharticaof coastal sand dunes showed single species dominance byC.globosum(Seena and Sridhar 2004).In the current study,C.globosumwas restricted to stem and pods ofS.bispinosain coastal sand dunes,but was conf i ned to leaves in mangroves.In coastal sand dunes non-legumes(L.sarmentosaandP.corymbosa),C.globosumwas a dominant coregroup taxon(Beena et al.2000).Occurrence ofC.tenuissimum,P.chrysogenumand morpho sp.1 as common coregroup taxa in seedsS.bispinosaindicates the possibility of their vertical transmission.
The present study showed multiple species dominance in all tissues ofS.bispinosa.Such dominance is common in many mangrove species(e.g.Acanthus ilicifolius,Acrostichum aureum,A.off i cinalis,Lumnitzera recemosa,R. mucronata,Sesbania bispinosaandS.caseolaris)(Kumaresan and Suryanarayanan 2001;Ananda and Sridhar2002;Maria and Sridhar 2003;Anita et al.2009).However, single species dominance was also seen in foliar tissues of several mangrove species(e.g.Avicennia marina,B.cylindrica,R.apiculata,R.mucronataandS.maritima)(Suryanaraynan et al.1998;Suryanarayanan and Kumaresan 2000; Kumaresan and Suryanarayanan 2001).Similar to mangroves,S.bispinosain the present study showed maximum diversity of endophytic fungi in roots(with the exception of Shannon diversity)(Anita et al.2009).The onset of senescence in hostplantsmay facilitate the endophyticcore-group taxa to dominate as saprophytes to decompose the host tissues.However,to prove this notion,additional experiments are necessary to isolate such fungi in dead host tissues.
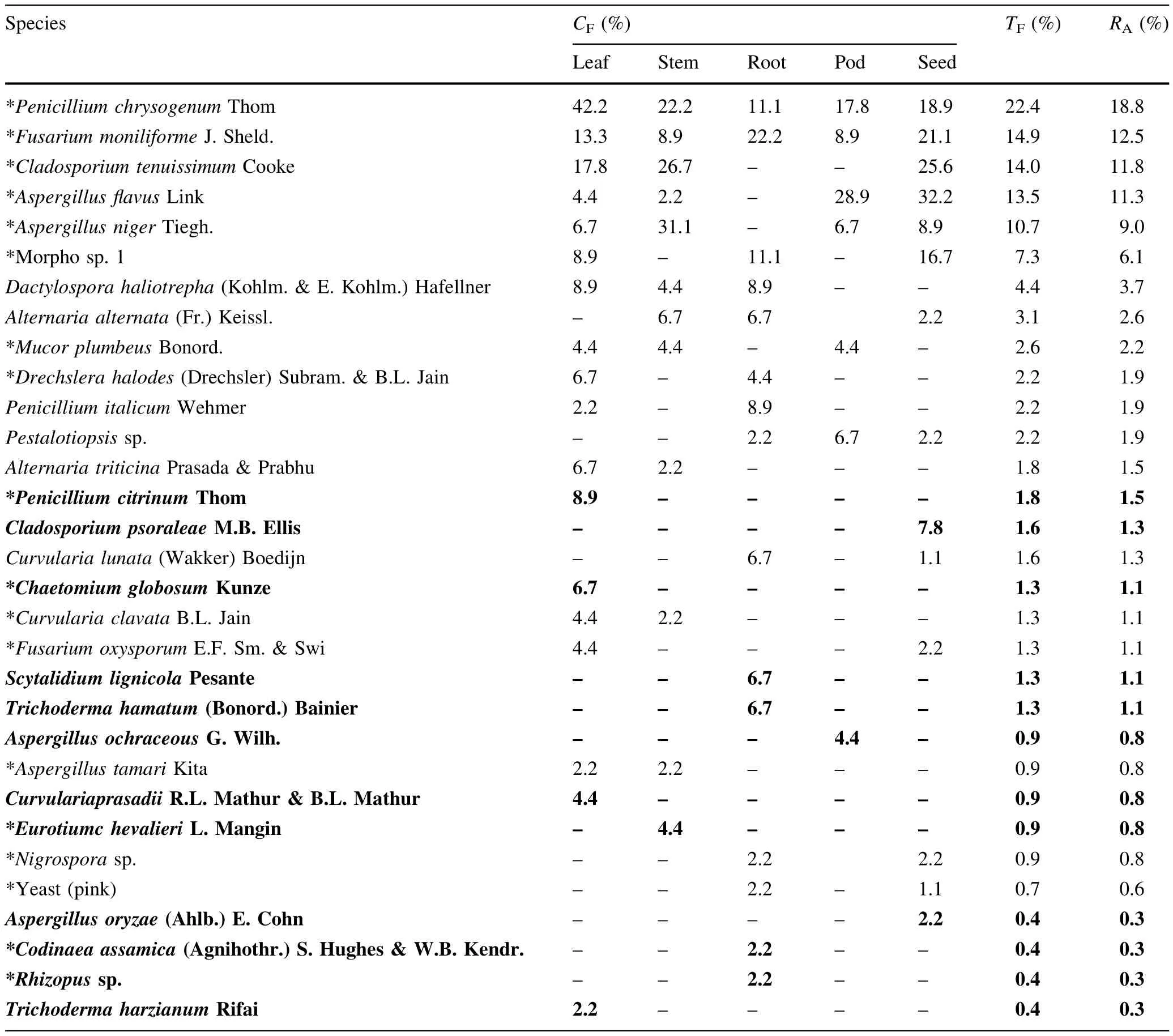
Table 4 Colonization frequency(CF,%)of endophytic fungi in f i ve tissues of S.bispinosa in mangroves(out of 45 segments of each tissue)
Terrestrial and marine fungi
Decomposing coastal sand dune and mangrove litter are reported to support more ascomycetes than mitosporic fungi(Kohlmeyer and Volkmann-Kohlmeyer 1991).That the tissues ofS.bispinosain our study had a majority of terrestrial mitosporic fungi corroborates earlier studies on foliar and root endophytes(Suryanarayanan et al.1998; Beena et al.2000;Kumaresan and Suryanarayanan 2001, 2002;Ananda and Sridhar 2002;Maria and Sridhar 2003; Seena and Sridhar 2004).Almost all endophytic fungi recovered in the current study were typical terrestrial taxa (with the exception ofDactylospora haliotrephain leaves, stem and roots in mangroves).The typical mangrove fungusD.haliotrephawas also dominant in the sterilized roots ofS.bispinosain mangroves(Anita et al.2009).Kallichroma tethyswas recorded from the unsterilized stem, leaves and pods ofS.bispinosa,which was core-group taxon in leaves(Anita et al.2009).BesidesD.haliotrephaandK.tethys,another marine fungusCumulospora marinawas endophytic in roots ofA.ilicifolius(Maria and Sridhar 2003).Legumes likeC.catharticaandC.maritimaalso supported the marine fungusHalosarpheiasp.in coastal sand dunes(Seena and Sridhar 2004).Up to 13%of endophytes were marine fungi(Monodictys pelagica,Periconia prolif i ca,Verruculina enaliaandZalerion maritimum)in roots of coastal sand dune plants(Ipomoea pescaprae,L.sarmentosaandPolycarpaeacorymbosa) (Beena et al.2000).However,no mycorrhizal fungi were recorded in association with roots ofSesbaniain either habitat.The saline condition prevailing in the studied habitats might have hindered their colonization or they did not sporulate in the culture medium and were therefore unidentif i able without molecular characterization.
Saprophytic,pathogenic and toxigenic fungi
Several endophytic fungi are known as saprotrophs or opportunisticpathogens(e.g.C.globosumandPaecilomycesvarioti)(Ananda and Sridhar 2002;Seena and Sridhar 2004; Arnold etal.2007;Naik etal.2007;Hyde and Soytong 2008; Vega et al.2008).A.f l avus,A.niger,Fusarium oxysporumandP.chrysogenumwere the top core-group taxa in sterilized as wellasunsterilized tissue segments ofS.bispinosain mangroves(Anita et al.2009).Except forF.oxysporum,the others were dominant endophytes in our study,indicating their role as saprophytes on plant senescence.The endophytic fungal assemblage ofS.bispinosain our study could bedivided into three groups,viz.saprophytes,pathogensand toxigens(saprophytes:Aspergillus niger,A.oryzae,A. tamarii,C.globosum,C.assamica,E.chevalieri,Mucor plumbeus,Penicillium chrysogenum,P.citrinum,P.italicumandT.hamatum;pathogens:Alternaria alternata,A. longipes,A.triticina,Colletotrichum dematium,Curvularia clavata,C.lunata,C.prasadii,Drechslera halodes,F. moniliforme,F.oxysporumandF.solani;toxigens:A.f l avus,A.ochraceusandT.harzianum).
Azevedo et al.(2000)reviewed the literature on endophytic entomopathogenic fungi.Webber(1981)showed that elm trees are protected against beetles(Physocnemum brevilineum)by an entomopathogenic endophytic fungusPhomopsis oblonga.Widely distributed endophytic entomopathogenic fungi includeBeauveriaspp.,Paecilomycess pp.andVerticillium lecanii(Petrini 1981;Bills and Polishook 1991;Ananda and Sridhar 2002;Vega et al.2008). These fungi are known to protect host plants from insect herbivory and also to increase host plant biomass(Waller et al.2005;Tejesviet al.2007).Among these fungi,Paecilomycessp.was recovered in our study exclusively from the leaf tissues ofS.bispinosain coastal sand dunes.
Endophytic fungi are the major source of novel bioactive compounds of medicinal value and biological control (Strobel 2003;Hyde and Soytong 2008;Jones et al.2008; Suryanarayanan et al.2010).They are known to produce 2-3 fold higher herbicidal metabolites compared to phytopathogenic and soil fungi(Schulz et al.1999).Many endophytic fungi recorded in our study are potential producers of bioactive metabolites.For example,Chaetomiumproduces chaetocyclinones,chaetoglobosins,chaetomin, chaetoquadrins,chaetospiron,f l avipin,orsellides and oxaspirodion(Sekita et al.1976;Chitwood 2002;Lo¨esgen et al.2007;Suryanarayanan et al.2010).In addition,some endophytic fungi also produce compounds that mimic plant hormones(Hyde and Soytong 2008).
Conclusions
Surface sterilized tissue segments ofS.bispinosaof coastal sand dunes and mangroves of southwest India yielded 39 endophytic fungi with dominance by six taxa(A.f l avus,A. niger,C.tenuissimum,F.moniliforme,P.chrysogenumandmorpho sp.1).A consortium of identi fi ed and unidenti fi ed saprophytic,pathogenic and toxigenic fungi existed as endophytes inS.bispinosain coastal sand dunes and mangroves.Besides conventional fungal identi fi cation, molecular approaches are necessary to follow the endophytic nature of fungi more precisely.Seeds yielded more fungal isolates than species,revealing limited vertical transmission of their endophytic fungi.Only one typical mangrove fungus(Dactylospora haliotrepha)was associated with leaves,stem and roots of mangroveS.bispinosa. Up to 30–40%the endophytic fungal composition of coastal sand duneS.bispinosadiffered from that of mangroveS.bispinosaand other coastal sand dune plant species,indicating their partial host-and habitat-speci fi city.S. bispinosabeing is an important green manure and forage legume of southwest India,further insight on its endophytic fungi and production of bioactive metabolites will be bene fi cial in future.
AcknowledgmentsThe authors are grateful to Mangalore University for permission to carry out this study in the Department of Biosciences.One of us(SJS)acknowledges the University Grants Commission,New Delhi,India for the award of RMSMS fellowship under the scheme Research Fellowship in Sciences for Meritorious Students.K.R.Sridhar acknowledges the award of UGC-BSR Faculty Fellowship by the University Grants Commission,New Delhi,India. We thank Dr.S.Shishupala for helpful suggestions regarding pathogenic fungi.The authors are indebted to the editor and anonymous referees for suggestions to improve the presentation of this paper.
Ananda K,Sridhar KR(2002)Diversity of endophytic fungi in the roots of mangrove species on the west coast of India.Can J Microbiol 48:871–878
Anita DD(2010)Microbiological and nutritional studies on the legumes of Nethravathi mangroves,Southwest Coast of India. PhD Dissertation,Biosciences.Mangalore University,Mangalore,p 121
Anita DD,Sridhar KR(2009)Assemblage and diversity of fungi associated with mangrove wild legumeCanavalia cathartica. Trop Subtrop Agroecosyst 10:225–235
Anita DD,Sridhar KR,Bhat R(2009)Diversity of fungi associated with mangrove legumeSesbania bispinosa(Jacq.)W.Wight (Fabaceae).Livest Res Rural Dev 21:67.http://www.lrrd.org/ lrrd21/5/cont2105.htm
Arnold AE,Henk DA,Eells RL,Lutzoni F,Vilgalys R(2007) Diversity and phylogenetic aff i nities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia 99:185–206
Arun AB,Sridhar KR(2004)Symbiotic performance of fast-growing rhizobia isolated from the coastal sand dune legumes of west coast of India.Biol Fertil Soils 40:435–439
Azevedo JL,Maccheroni W Jr,Pereira JO,De Arau´jo WL(2000) Endophytic microorganisms:a review on insect control and recent advances on tropical plants.Electron J Biotechnol 3:40–65
Beena KR,Ananda K,Sridhar KR(2000)Fungal endophytes of three sand dune plant species of west coast of India.Sydowia 52:1–9
Bhagya B,Sridhar KR(2009)Ethnobiology of coastal sand dune legumes of southwest India.Indian J Tradit Knowl 9:611–620
Bhat KG(2003)Flora of Udupi.Indian Naturalist,Udupi,p 913
Bhat DJ(2010)Fascinating microfungi(Hyphomycetes)of Western Ghats—India.Broadway Publishers,Panaji,p 221
Bills GF,Polishook JD(1991)Microfungi fromCarpinus caroliniana.Can J Bot 69:1477–1482
Cai L,Hyde KD,Tsui CKM(2006)Genera of freshwater fungi. Fungal Diversity Research Series#18.Fungal Diversity Press, Hong Kong,p 261
Carmichael JW,Kendrick WB,Conners IL,Sigler L(1980)Genera of Hyphomycetes.The University of Alberta Press,Edmonton, p 386
Chao A,Chazdon RL,Colwell RK,Shen T-J(2005)A new statistical approach for assessing similarity of species composition with incidence and abundance data.Ecol Lett 8:148–159
Chitwood DJ(2002)Phytopathological based strategies for nematode control.Annu Rev Phytopathol 40:221–249
Ellis MB(1971)Dematiaceous Hyphomycetes.Commonwealth Mycological Institute,Kew,p 608
Ellis MB(1976)More dematiaceous Hyphomycetes.Commonwealth Mycological Institute,Kew,p 507
Ellis MB,Ellis JP(1987)Microfungi on land plants:an identi fi cation handbook.Croom Helm,London,p 818
Hyde KD,Soytong K(2008)The fungal endophyte dilemma.Fungal Divers 33:163–173
Jones EBG,Stanley SJ,Pinruan U(2008)Marine endophyte sources of new chemical natural products:a review.Bot Mar 51:163–170
Kathiresan K,Rajendran N(2005)Coastal mangrove forests mitigated tsunami.Estuar Coast Shelf Sci 65:601–606
Kohlmeyer J,Volkmann-Kohlmeyer B(1991)Illustrated key to the fi lamentous higher marine fungi.Bot Mar 34:1–61
Kumaresan V,Suryanarayanan TS(2001)Occurrence and distribution of endophytic fungi in a mangrove community.Mycol Res 105:1388–1391
Kumaresan V,Suryanarayanan TS(2002)Endophyte assemblage in young,mature and senescent leaves ofRhizophora apiculata: evidence for the role of endophytes in mangrove litter degradation.Fungal Divers 9:81–91
Lo¨esgen S,Schlo¨erke O,Meindl K,Herbst-Irmer R,Zeeck A(2007) Structure and biosynthesis of chatocyclinones,new polyketides produced by and endosymbiotic fungus.Eur J Org Chem 2007:2191–2196
Ludwig JA,Reynolds JF(1988)Statistical ecology:a primer on methods and computing.John Wiley and Sons,New York,p 337
Magurran AE(1988)Ecological diversity and its measurement. Princeton University Press,New Jersey,p 192
Maria GL,Sridhar KR(2003)Endophytic fungal assemblage of two halophytes from west coast mangrove habitats,India.Czech Mycol 55:241–251
Mehta R(2000)WWF—India.In:Singh S,Sastry ARK,Mehta R, Uppal V(eds)Setting biodiversity conservation priorities for India.Wildlife Institute of India,Mangalore,pp 245–266
Naik BS,Shashikala J,Krishnamurthy YL(2007)Study on the diversity of endophytic communities from rice(Oryza sativaL.) and their antagonistic activitiesinvitro.Microbiol Res 164:90–296
Pang K-L,Vrijmoed LLP,Goh TK,Plaingam N,Jones EBG(2008) Fungal endophytes associated withKandelia candel(Rhizophoraceae)in Mai Po Nature Reserve,Hong Konga.Bot Mar 51:171–178
Petrini O(1981)Endophytische Pilze in Epiphytischen Araceae, Bromeliaceae und Orchidiaceae.Sydowia 34:135–148
Pielou FD(1975)Ecological diversity.Wiley InterScience,New York,p 165
Rao TA,Meher-Homji VM(1985)Strand plant communities of the Indian sub-continent.Proc Indian Acad Sci 94:505–523
Rao TA,Sherieff AN(2002)Coastal ecosystem of the Karnataka State,India II—Beaches.Karnataka Association for the Advancement of Science,Bangalore,p 192
Rao TA,Suresh PV(2001)Coastal ecosystems of the Karnataka State,India—I.Mangroves.Karnataka Association for the Advancement of Science,Bangalore,p 320
Schulz B,Ro¨mmert A-K,Dammann U,Aust H-J,Strack D(1999) The endophyte-host interaction:a balanced antagonism?Mycol Res 103:1275–1283
Seena S,Sridhar KR(2004)Endophytic fungal diversity of 2 sand dune wild legumes from the southwest coast of India.Can J Microbiol 50:1015–1021
Sekita S,Yoshihira K,Natori S,Kuwano H(1976)Structures of chaetoglobisins C,D,E and F,cytotoxic indole-3-yl-(13) cytochalasans fromChaetomium globosum.Tetrahedron Lett 17:1351–1354
Strobel GA(2003)Endophytes as sources of bioactive products. Microbes Infect 5:535–544
Suryanarayanan TS,Kumaresan V(2000)Endophytic fungi of some halophytes from an estuarine mangrove forest.Mycol Res 104:1465–1467
Suryanarayanan TS,Kumaresan V,Johnson JA(1998)Foliar fungal endophytes from two species of the mangroveRhizophora.Can J Microbiol 44:1003–1006
Suryanarayanan TS,Thirunavukkarasu N,Govindarajulu MB,Sasse F,Jansen R,Murali TS(2010)Fungal endophytes and bioprospecting.Fungal Biol Rev 23:9–18
Taylor JE,Hyde KD,Jones EBG(1999)Endophytic fungi associated with the temperate palm,Trachycarpus fortunei,within and outside its natural geographic range.New Phytol 142:335–346
Tejesvi MV,Kini KR,Prakash HS,Subbiah V,Shetty HS(2007) Genetic diversity and antifungal activity of species ofPestalotiopsisisolated as endophytes from medicinal plants.Fungal Divers 24:37–54
Vega FE,Posada F,Catherine Aime M,Pava-Ripoll M,Infante F, Rehner SA(2008)Entomopathogenic fungal endophytes.Biol Control 46:72–82
Waller F,Achatz B,Baltruschat H,Fodor J,Becker K,Fischer M, Heler T,Huckelhoven R,Neumann C,von Wettstein D,Franken P,Kogel K(2005)The endophytic fungusPiriformospora indicareprograms barley to salt-stress tolerance,disease resistance,and higher yield.Proc Natl Acad Sci 102:13386–13391
Webber J(1981)A natural control of Dutch Elm disease.Nature 292:449–451
10 May 2013/Accepted:9 June 2014/Published online:21 July 2015
Project funding:University Grants Commission,New Delhi,India.
The online version is available at http://www.springerlink.com
Corresponding editor:Zhu Hong
✉Kandikere R.Sridhar
kandikere@gmail.com
1Department of Biotechnology,St.Aloysius College, Mangalore 575 003,Karnataka,India
2Department of Biosciences,Mangalore University, Mangalagangotri 574 199,Karnataka,India
杂志排行
Journal of Forestry Research的其它文章
- Drone remote sensing for forestry research and practices
- Life cycle environmental impact assessment of biochar-based bioenergy production and utilization in Northwestern Ontario, Canada
- Growth rates of Eucalyptus and other Australian native tree species derived from seven decades of growth monitoring
- Effect of f i rst thinning and pruning on the individual growth of Pinus patula tree species
- The inf l uence of selective cutting of mixed Korean pine(Pinus koraiensis Sieb.et Zucc.)and broad-leaf forest on rare species distribution patterns and spatial correlation in Northeast China
- Modeling forest f i res in Mazandaran Province,Iran
