两个基于羧基-水分子链构筑的超分子镉配合物的合成、晶体结构和荧光性质
2015-06-01唐李志鹏杨明炜程美令
唐李志鹏 杨明炜 程美令*, 刘 琦*,,2
(1常州大学石油化工学院,江苏省绿色催化材料与技术重点实验室,常州213164)
(2南京大学配位化学国家重点实验室,南京210093)
两个基于羧基-水分子链构筑的超分子镉配合物的合成、晶体结构和荧光性质
唐李志鹏1杨明炜1程美令*,1刘 琦*,1,2
(1常州大学石油化工学院,江苏省绿色催化材料与技术重点实验室,常州213164)
(2南京大学配位化学国家重点实验室,南京210093)
以5-甲基-3-吡唑甲酸(H2MPCA)为主配体,在螯合配体菲咯琳(phen)或2,2′-联吡啶(2,2′-bpy)的存在条件下,与醋酸镉在常温下反应得到2个基于氢键构筑的羧基-水分子链的超分子化合物,[Cd(HMPCA)2(2,2′-bpy)]·2H2O(1)和[Cd(HMPCA)2(phen)]· 2.5H2O(2),并通过元素分析、红外光谱、热重分析、X-射线衍射等对其结构进行了表征。结构分析表明,在化合物1和2中,单核镉的配合物和游离水分子通过氢键及π…π堆积作用形成了三维超分子结构,在此过程中,游离水和羧基构筑的链状水分子簇起着非常重要的作用。此外,我们还研究了化合物1和2的热重和荧光性质。
镉;水分子簇;荧光;5-甲基-3吡唑甲酸;晶体结构
0 Introduction
The construction of supramolecular complexes has attracted continuous research interest in the last decades because of their fascinating structures and potential applications as functional materials[1-4].As an important part of supramolecular system,the water clusters or aggregates have provided a longstanding fascination for chemists.Besides,the water molecules can be interacted with the counter anions,deprotonated carboxyl groups,or halogen through hydrogen bonds[5-6]. Although diverse water clusters,aggregates,chains or even sheets have been reported,the unique watercarboxylate chains based supramolecular complexes still quite rare[7].Generally speaking,the structural diversity of such complexes is always depended on many factors,such as metal ions,pH value,template agents,metal-ligand ratio,counteranions[8-10].Without a doubt,among these factors,the rational design and reasonable use of the characteristic ligands occupies the capital.The length,rigidity,coordination modes, functional groups or substituent of organic ligands have consequential effect on the final structures[11]. Moreover,the N ancillary ligands,also have great influence on the assembly of supramolecular complexes. For example,2,2′-bipyridine(2,2′-bpy)and 1,10-phenanthroline(phen),as the chelate N ligands,can adjust the coordination structures by occupied the terminal position[12-13],also provide the C-H…π and π…π interactions for assembling the complex into high-dimensional supramolecular networks[14].
The5-methyl-1H-pyrazole-3-carboxylicacid (H2MPCA),containingbothcarboxylgroupand pyrazole ring,not only can coordinate with metal ions, but also can act as an acceptor and/or donor in hydrogen bond interactions,which play an important role in the formation of the supramolecular structures. These considerations inspired us to explore new supramolecular complexes with H2MPCA and chelating N-donors 2,2′-bpy or phen.Herein,we report the syntheses,crystal structures of two supramolecular complexes[Cd(HMPCA)2(2,2′-bpy)]·2H2O(1)and [Cd(HMPCA)2(phen)]·2.5H2O(2)basedonwatercarboxylate hydrogen bonded chains.Additionally,the thermal and luminescent properties of two complexes were also investigated.
1 Experimental
1.1 Materials and methods
Allsolventsandreagentswerepurchased commercially and were used as received.H2MPCA was prepared following the literature method[15].The elemental analysis(C,H and N)was performed on a Perkin-Elmer 2400 Series II element analyzer.FTIR spectra were recorded on a Nicolet 460 spectrophotometer in the form of KBr pellets.The luminescent spectra were recorded at room temperature on a SahimadzuRF-5301PCfluorescencespectrofluorometer.Powder X-ray diffraction(PXRD)determinations were performed on an X-ray diffractometer (D/max 2500 PC,Rigaku)with Cu Kα radiation(λ= 0.154 06 nm).Thermogravimetric analysis(TGA)experiments were carried out on a Dupont thermal analyzer from room temperature to 800℃under N2atmosphere at a heating rate of 10℃·min-1.Singlecrystal X-ray diffraction measurements of the 1 and 2 were carried out with a Bruker Smart Apex II CCD diffractometer at 293(2)K.
1.2 Synthesis
1.2.1 Synthesis of[Cd(HMPCA)2(2,2′-bpy)]·2H2O(1)
To a solution containing H2MPCA(0.025 2 g, 0.20 mmol)and 2,2′-bpy(0.015 6 g,0.10 mmol)in MeOH(4 mL)was added a solution of Cd(OAc)2· 4H2O(0.026 6 g,0.1 mmol)in deionized water(5 mL). The resulting solution was stirred for one hour and filteredtostandatroomtemperature.Colorless crystals of 1(0.075 5 g,68%)suitable for singlecrystal X-ray diffraction analysis were obtained after a week.Anal.Calcd.for C20H22N6O6Cd(%):C,43.30;H, 3.97;N,15.15.Found(%):C,43.68;H,3.95;N, 15.53.IR(KBr pellet,cm-1):3 400(s),3 173(w),3 077 (w),2 981(m),2 924(m),2 848(m),1 610(vs),1 486 (s),1 440(m),1 335(m),1 278(s),1 201(w),1 134 (w),1 020(m),810(w),753(w),696(w),629(w),563 (m),439(w).
1.2.2 Synthesis of[Cd(HMPCA)2(phen)]·2.5H2O(2)
2 was synthesized by a method similar to that of 1,except that 2,2′-bpy(0.015 6 g,0.10 mmol)was replaced by phen(0.018 0 g,0.10 mmol),and colorless crystals of 2 suitable for X-ray diffraction analysis were obtained with yield:0.044 1 g(75%).Anal. calcd.for C44H46N12O13Cd2(%):C,44.95;H,3.94;N, 14.30.Found(%):C,44.80;H,4.15;N,13.67.IR (KBr pellet,cm-1):3 492(s),3 134(w),3 048(w),2 924 (m),2 858(m),1 610(vs),1 525(s),1 400(m),1 335 (m),1 267(s),1 219(w),1 144(w),1 096(m),1 048 (m),848(w),810(w),714(s),640(w),563(m),430(w).
1.3 X-ray crystallography
The single-crystal X-ray diffraction was performed on a Bruker Smart Apex II CCD diffractometer at 293(2)K.Intensities of reflections were measured using graphite-monochromatizedMo Kαradiation(λ= 0.071 073 nm).The structure was solved by direct methods using the SHELXS program of the SHELXTL package and refined with SHELXL[16].Anisotropic thermal factors were assigned to all the non-hydrogen atoms.H atoms attached to C were placed geometrically and allowed to ride during subsequent refinement with an isotropic displacement parameter fixed at 1.2 times Ueqof the parent atoms.H atoms bonded to O or N atoms were first located in difference Fourier maps and then placed in the calculated sites and included in the refinement.Crystallographic data parameters for structuralanalysesaresummarizedinTable1. Selected bond lengths and angles for them are listed in Table 2 and Table 3.And the hydrogen bonds are given in the Table S1.
CCDC:1011422,1;1011423,2.
2 Results and discussion
2.1 Crystal structure of[Cd(HMPCA)2(2,2′-bpy)]·2H2O(1)
The single-crystal X-ray diffraction analysisreveals that complex 1 crystallizes in monoclinic P21/cspace group.As shown in Fig.1 ,the asymmetric unit of1consistsofahalfofcrystallographically independent Cdanion,one HMPCA-ligand,half of a 2,2′-bpy ligand,and two halves of lattice water molecules.Each Cdion locates in a distorted octahedral geometry,hexa-coordinated by two oxygen atoms and two nitrogen atoms from two HMPCA-, another two nitrogen atoms from one 2,2′-bpy ligand. The Cd-O bond distance is 0.233 6(4)nm,and the Cd-N bond distances are 0.233 6(4)nm and 0.231 7(4) nm,respectively.Besides,the bond angles around Cdare in the range of 70.4(2)°~141.7(2)°,similar with those in the reported complex[Cd(cbba)2(mbix)]n(Hcbba=2-(4′-chlorine-benzoyl)-benzoic acid,mbix= 1,3-bis(imidazol-1-ylmethyl)-benzene)[17].

Table1 Crystal structure parameters of complexes 1 and 2

Table2 Selected bond lengths(nm)and bond angles(°)for 1
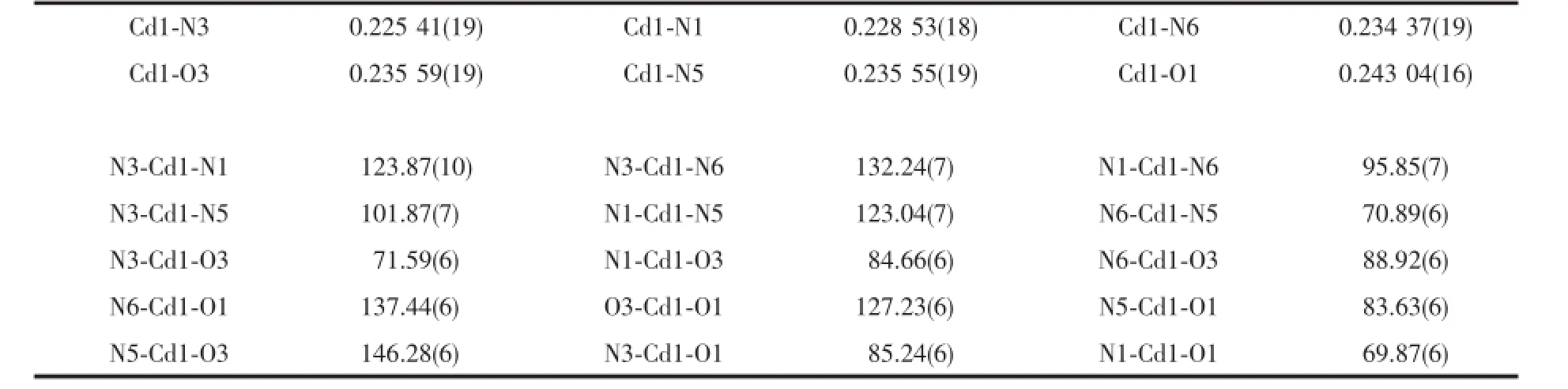
Table3 Selected bond lengths(nm)and bond angles(°)for 2

Fig.1 Molecular structure of complex 1
HMPCA-and 2,2′-bpy both act as chelating ligands in the formation of complex 1,making the higher dimensional coordination polymers impossible. But,misfortune may be an actual blessing,such zero dimensional structures give a way to systematic study of the non-covalent interactions in the supramolecular structures,especially the hydrogen bonds based on water cluster or chain.For complex 1,each independent unit interacts with the neighboring ones through π…π stacking interactions of pyrazole rings Cg1(containing N1,N2,C2,C3,and C4 atoms),with the Cg1…Cg1ii(Symmetry code:ii1-x,1-y,-z)distance 0.387 2(8) nm,finally forming a 1D chain along a axis(Fig.2 ). The dihedral angle between the two packing pyrazole rings is 0.002(6)°,which indicating the two pyrazole rings are paralleled with each other.These 1D chains are extended into a 2D sheet(Fig.3 )connected by four O-H…O strong hydrogen-bonding interactions between lattice water molecules and carboxyl oxygen atoms and two N-H…O hydrogen bonds as well as two weak C-H…O hydrogen bonds(Table S1).Asemphasized in Fig.3 ,in the 2D layer structure,there is an interestingly loop-stick chain constructed by two lattice water molecules and the carboxyl groups in HMPCA-ion though O-H…O hydrogen bonds(O4i-H4Xi…O1 0.264 2(3)nm,O3-H3Y…O2ii0.268 9(11) nm,O3-H3X…O4iii0.321 0(15)nm,O3-H4Y…O1iv0.303 9(11)nm,Symmetry codes:i1-x,y,1/2-z;ii-x, 1-y,-z;iii-1+x,y,z;iv1+x,1-y,1/2+z).Between the 2D sheets,the C8 atom in the phen ligand is involved in hydrogen bonds with the lattice water molecule O4, forming a 3D supramolecular network.

Fig.2 π…π stacking 1D chain in complex 1
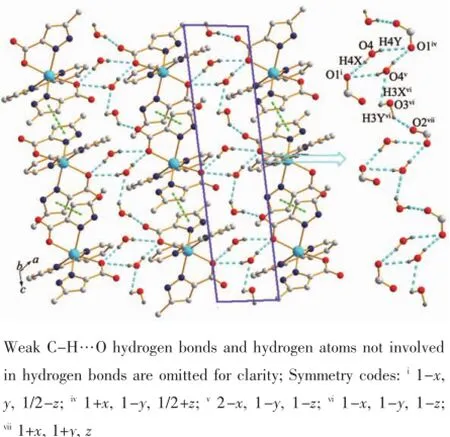
Fig.3 2D sheet of complex 1 constructed from π…π interactions and strong O(N)-H…O hydrogen bonds
2.2 Crystal structure of[Cd(HMPCA)2(phen)]· 2.5H2O(2)
When the 2,2′-bpy was replaced by phen,a stronger conjugated system makes the π…π stacking between phen ligands form more easily.Compared with 1,complex 2 crystallizes in the same crystal system,but with the C2/c space group.The asymmetric unit of 2 contains one crystallographically independent Cdion,two HMPCA-ligands,one phen ligand,and two and a half of lattice water molecules(Fig.4 ).Each Cdion shows a distorted{CdO2N4}octahedral geometry,completed by four N atoms from one phen ligand and two different HMPCA-ligands,and two O atoms from two HMPCA-ligands.The bond lengths of Cd-N are in the range of 0.225 41(19)~0.235 5(19) nm,and the Cd-O distances are 0.243 04(16)and 0.235 59(19)nm,respectively.The bond angles around Cdare in the range of 69.87(1)°~146.27(5)°,similar with those in 1.

Fig.4 Molecular structure of complex 2
Differentfromthatincomplex1,thetwo neighboring units in complex 2 are interacted with each other through N-H…O hydrogen bond(N4-H4…O1i0.284 4(3)nm,Symmetry code:i1/2-x,3/2-y, -z),and formed a dimmer.And then the dimmers connect with each other through the π…π stacking of phen ligands,with the Cg1…Cg2v(Cg refers to the ring centroid of the pyridyl of the phen ligand,Cg1 containing N5,C11,C12,C13,C14 and C22 atoms and Cg2 holding N6v,C17v,C18v,C19v,C20v,C21vatoms,Symmetry code:v-x,2-y,-z)distance of 0.390 3(9)nm,forming a 1D chain along c axis.The dihedral angle between the two interacted pyridyl ring is 29.29(7)°.These 1D chains are further expanded to a 2D sheet through the C-H…π interactions(C1-H1…π 0.350 4(5)nm)(Fig.5 ).It is noteworthy that thereis an unprecedented 1D loop-loop water-carboxylate hydrogen bonded chain in complex 2,in which a 20-mermberd ring are constructed from six lattice water moleculesandtwocarboxylategroupsthrough hydrogen bonds(O5-H5X…O3 0.291 0(2)nm,O6-H6X…O5ii0.287 4(3)nm,O6-H6Y…O7 0.269 0(3) nm,O7-H7X…O2iii0.286 6(3)nm,Symmetry code:iix, -1+y,z;iii1/2-x,-1/2+y,1/2-z)(Fig.6 ).Besides,the weak C-H…O hydrogen bonds further stabilized the 3D structure.
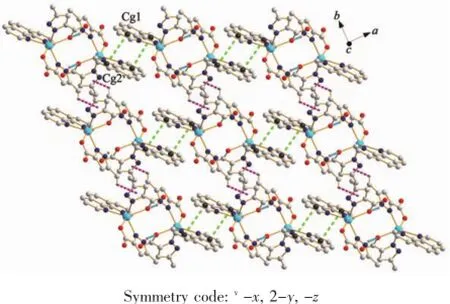
Fig.5 2D sheet constructed by non-covalent interactions view along c axis in complex 2
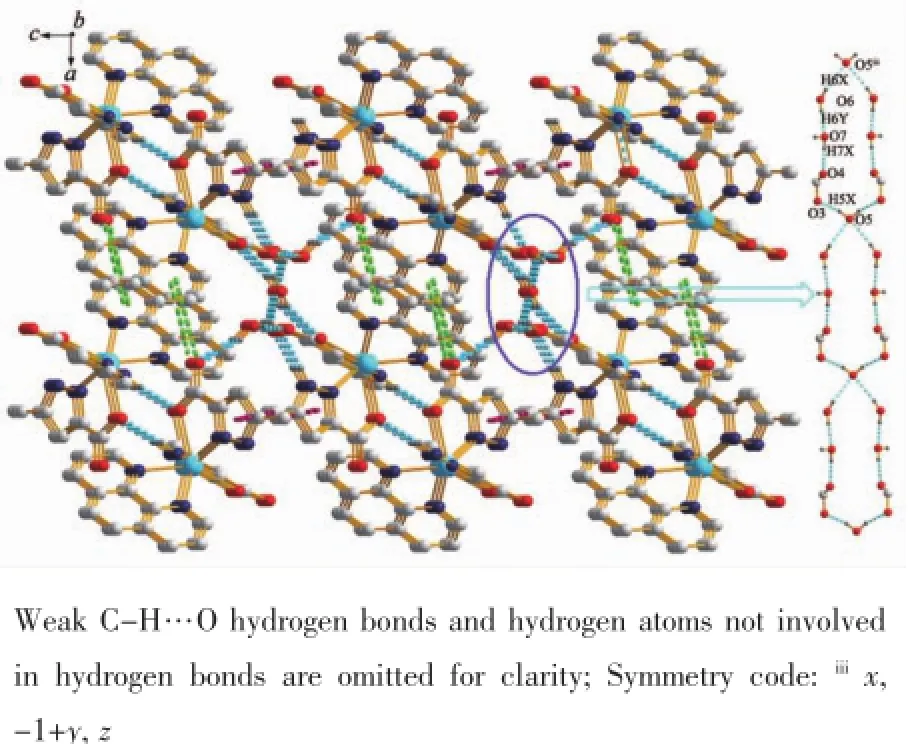
Fig.6 3D supermolecular structure of 2
2.3 Infrared spectrum
As shown in the Fig.S1,the absence of absorption peaks around 1 730 cm-1show that all carboxyl groups are deprotonated[17].Strong peaks at 1 601 cm-1(1),1 610 cm-1(2)and 1 440 cm-1(1),1 400 cm-1(2) maybe assignedto the νas(OCO)andνs(OCO) stretching vibration of HMPCA-ligand.A strong peak around 3 600~3 000 cm-1is assigned as characteristic peak of OH vibration,indicating that water molecules exist in 1 and 2.
2.4 Thermogravimetric analysis
In order to examine the thermal stability of 1 and 2,the thermogravimetric analyses were carried out from ambient temperature up to 800℃,and the results reveal that both of them possess three main loss stages(Fig.S2).For compound 1,the first weight loss of 6.52%between 95℃and 121.2℃is attributed to the loss of two water molecules(Calcd.6.49%).The second degradation stage is in the range 171~255℃with mass loss of 27.33%,corresponding to the loss of a 2,2′-bpy molecule(Calcd.28.15%),leaving a framework[Cd(HMPCA)2].The decompositionof [Cd(HMPCA)2]startsabove327℃,andfinally degrades to CdO with a loss of 43.37%(Calcd. 42.22%).For compound 2,the first weight loss of 7.18%which occurred from 105 to 179℃,corresponds to the release of two water molecules(Calcd.7.65%). Between 207℃and 303℃there is a 26.85%weight loss,corresponding to the loss of a phen molecule (Calcd.27.55%).The decomposition of[Cd(HMPCA)2] starts above 353℃,and finally degrades to CdO with a loss of 44.05%(Calcd.43.92%).
2.5 Powder X-ray diffraction
In order to check the phase purity of this complex,the PXRD patterns of title complexes were checked at room temperature.As shown in Fig.7 ,the peak positions of the simulated and experimental PXRD patterns are in agreement with each other, demonstrating the good phase purity of the complexes. The dissimilarities in intensity may be due to the preferredorientationofthecrystallinepowder samples.
2.6 Photoluminescent properties
Manycoordinationcomplexeshavebeen extensively studied due to their potential applications as luminescent materials.The solid-state luminescent properties of two complexes and the ligands were investigated at room temperature(Fig.8 ).The free H2MPCAliganddisplaysluminescencewithan emission maximum at 440 nm(λex=331 nm)[18].And two complexes exhibit blue fluorescence with theemission maximal peaks at 379 nm(for 1)and 373 nm(for 2)upon excitation at 331 nm.It is noteworthy that the emission bands of complexes 1 and 2 are similar to that of the free ancillary N donors in terms of position and band shape,which indicates these emission should also originate from theπ→π* transitions of the ancillary N ligand[19].

Fig.7 PXRD patterns of 1 and 2
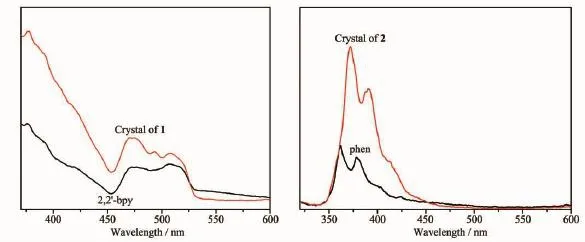
Fig.8 Solid-state emission spectra of two complexes and ancillary ligands at room temperature
3 Conclusions
Two3Dcadmiumsupramolecularcomplexes were constructed from H2MPCA and cadmium acetate in the presence of N-donor 2,2′-bpy or phen.In complex 1,the lattice water molecules interacted with carboxylate groups of HMPCA-ligand and formed a loop-stick water-carboxylate hydrogen bonded chain, while in 2,the lattice water molecules and carboxylate groups constructed another loop-loop water-carboxylate hydrogen bonded chain.Structural analysis revealed that the non-covalent bonds,including O(C,N)-H…O hydrogen bonds,C-H…π interaction and π…πinteraction,play an important role in the formation of the 3D supramolecular architectures of complexes.
[1]Zhang J P,Zhang Y B,Lin J B,et al.Chem.Rev.,2012, 112:1001-1033
[2]Zhang X T,Sun D,Li B,et al.Cryst.Growth Des.,2012,12: 3845-3848
[3]Evans O,Lin W B.Acc.Chem.Res.,2002,35:511-522
[4]Liu Q,Yu L L,Wang Y,et al.Inorg.Chem.,2013,52(6):2817 -2822
[5]Luo G,Xiong H,Sun D,et al.Cryst.Growth Des.,2011,11: 1948-1956
[6]Hao H,Sun D,Liu F,et al.Cryst.Growth Des.,2011,11: 5475-5482
[7]Sun D,Xu H,Yang C,et al.Cryst.Growth Des.,2010,10: 4642-4649
[8]Du M,Jiang X J,Zhao X J.Chem.Commun.,2005:5521-5523
[9]Zhang X T,Fan L M,Sun Z,et al.Cryst.Growth Des.,2013,13:792-803
[10]Zhang Q L,Feng G W,Zhang Y Q,et al.RSC Adv.,2014, 4:11384-11392
[11]Fan L M,Zhang X T,Zhang W,et al.CrystEngComm,2014, 16:2144-2157
[12]WANG Zhi-Yong(王志勇),WANG Wei-Ming(王卫民),LI Gang(李刚).Chinese J.Inorg.Chem.(无机化学学报),2014, 30:366-370
[13]Shi X,Zhu G S,Wang X H,et al.Cryst.Growth Des.,2005, 5:341-346
[14]Zhang X T,Fan L M,Zhao X,et al.CrystEngComm,2012, 14:2053-2061
[15]BAO Jin-Ting(包金婷),CHENG Mei-Ling(程美令),LIU Qi (刘琦),et al.Chinese J.Inorg.Chem.(无机化学学报),2013, 29:1504-1512
[16]Sheldrick G M.SHELXS-97,Program for Crystal Structure Refinement,University of Göttingen,Göttingen,Germany, 1997.
[17]LI Guo-Feng(李国峰),LI Xiu-Mei(李秀梅),JI Jian-Ye(纪建业),et al.Chinese J.Inorg.Chem.(无机化学学报),2014, 30:1947-1953
[18]HAN Wei(韩伟),CHENG Mei-Ling(程美令),LIU Qi(刘琦), et al.Chinese J.Inorg.Chem.(无机化学学报),2012,28: 1997-2004
[19]Fan L M,Zhang X T,Li D,et al.CrystEngComm,2013,15: 349-355
Syntheses,Crystal Structures and Luminescent Properties of Two Cadmium Supramolecular ComplexesBased on Water-Carboxylate Chains
TANG Li-Zhi-Peng1YANG Ming-Wei1CHENG Mei-Ling*,1LIU Qi*,1,2
(1School of Petrochemical Engineering and Jiangsu Key Labroratory of Advanced Catalytic Materials and Technology,Changzhou University,Changzhou,Jiangsu 213164,China)
(2State Key Labroratory of Coordination,Nanjing University,Nanjing 210093,China)
Two cadmium supramolecular complexes,[Cd(HMPCA)2(2,2′-bpy)]·2H2O(1)and[Cd(HMPCA)2(phen)] ·2.5H2O(2)(H2MPCA=5-methyl-1H-pyrazole-3-carboxylic acid,2,2′-bpy=2,2′-bipyridine,phen=1,10-phenanthroline),have been synthesized and characterized by elemental analysis,IR spectra and X-ray diffraction analysis. In 1 and 2,the mononuclear units are expanded to 3D supramolecular structures via O(C,N)-H…O hydrogen bonds,C-H…π interaction and π…π interaction.More interestingly,the water-carboxylate hydrogen bonded chains constructed by lattice water molecules and carboxylate groups play important role in the assembly of two supramolecular complexes.Besides,the thermal and luminescent properties of two complexes were also investigated.CCDC:1011422,1;1011423,2.
cadmium;water cluster;luminescent property;5-methyl-1H-pyrazole-3-carboxylic acid;crystal structure
O614.24+2
A
1001-4861(2015)03-0603-08
10.11862/CJIC.2015.070
2014-10-14。收修改稿日期:2014-12-12。
国家自然科学基金(No.20971060;21101018);江苏省高校自然科学研究面上项目(No.13KJB150001);南京大学配位化学国家重点实验室开放课题资助项目。
*通讯联系人。E-mail:liuqi62@163.com,chengmeiling01@163.com,Tel:0519-86330185;会员登记号:S060018987P。
