基于两个取代四甲基环戊二烯基配体的金属羰基化合物的合成及晶体结构
2015-06-01马志宏李素贞韩占刚郑学忠
樊 冬 马志宏 李素贞 韩占刚 郑学忠 林 进*,
(1河北师范大学化学与材料科学学院,石家庄050024)
(2河北医科大学基础医学院,石家庄050017)
(3河北工业职业技术学院,石家庄050091)
基于两个取代四甲基环戊二烯基配体的金属羰基化合物的合成及晶体结构
樊 冬1马志宏2李素贞3韩占刚1郑学忠1林 进*,1
(1河北师范大学化学与材料科学学院,石家庄050024)
(2河北医科大学基础医学院,石家庄050017)
(3河北工业职业技术学院,石家庄050091)
配体[C5Me4HR][R=4-BrPh(1),(MeC5H3N)CH2(2)]分别与Mo(CO)6,Ru3(CO)12和Fe(CO)5在二甲苯中加热回流,得到了6个双核配合物trans-[η5-C5Me4R]2Mo2(CO)6(3,4),trans-[(η5-C5Me4R)Ru(CO)(μ-CO)]2(5,6)和trans-[η5-(C5Me4R)Fe(CO)(μ-CO)]2(7,8)。通过元素分析、红外光谱、核磁共振氢谱对配合物的结构进行了表征,并用X-射线单晶衍射法测定了配合物3,5,6和8的结构。
环戊二烯;金属羰基化合物;X-射线衍射;结构
0 Introduction
The cyclopentadienyl groups have been among the most important ligands in organometallic chemistry, and group 6 and 8 metal carbonyl dimers with cyclopentadienyl-type ligands have been intensively investigated as a class of organometallic compounds. Cyclopentadienyl metal carbonyl complexes have been received much attention because of their potential utility as catalysts in many organic catalytic reactions[1-4].The steric and electronic factors of cyclopentadienyl ring substituents have great influence on catalytic activity,thussubtlechangesincyclopentadienyl ligand substitution can have profound consequences on chemistry reactivity.The changes can be easily tailored by replacement of both the cyclopentadienyl fragmentandanionicancillaryligands.Ligand modification not only opens access to construct new compounds,but also has the most profound effect on catalyst performance[5-6].Investigations in our laboratory focusing on the chemistry of group 6 and 8 metallocenes have demonstrated the importance of cyclopentadienyl substituent effects[7-12].On going our works to gain a deeper understanding of the steric and electric influences of substituents on the molecular structures and reactions of the corresponding cyclopentadienyl binuclear metal carbonyl complexes,herein, we reported the syntheses and characterization of a series of dinuclear metal carbonyl complexes bearing the substituted tetramethylcyclopentadienyl ligands.
1 Experimental
1.1 General considerations
Schlenkandvacuumlinetechniqueswere employed for all manipulations of air-and moisturesensitive compounds.All solvents were distilled from appropriate drying agents under an atmosphere of nitrogen prior to use.1H NMR spectra were recorded on a Bruker AV 500 instrument,while IR spectra were recorded as KBr disks on a FT IR 8900 spectrometer.X-ray measurements were made on a Bruker Smart APEX diffractometer with graphite monochromated Mo Kα(λ=0.071 073 nm)radiation.Elemental analyses were performed on a Vario ELⅢanalyzer. The ligand precursors C5Me4HR(R=4-BrPh(1), (MeC5H3N)CH2(2))were synthesized according to the literature[13-14].Mo(CO)6,Fe(CO)5and Ru3(CO)12were purchased from J&K Scientific Ltd and used without further purification,other reagents were purchased from commercial suppliers.
1.2 Synthesis of trans-[η5-C5Me4(4-BrC6H4)]2Mo2(CO)6(3)
A solution of ligand precursor 1(0.832 g,3 mmol) and Mo(CO)6(0.794 g,3 mmol)in 30 mL of xylene was refluxed for 10 h.After removal of solvent under reduced pressure,the residue was chromatographed on an alumina column using petroleum ether/CH2Cl2(6∶1,V/V)as eluent.The only red band was eluted and collected.After vacuum removal of the solvents from the above eluate,the residue was recrystallized from n-hexane/CH2Cl2(1∶2,V/V)at room temperature to give complex 3 as dark-red crystals(Yield:0.733 g, 53.5%).m.p.169~171℃(dec.).Anal.Calcd.(%)for C36H32Br2Mo2O6:C,47.39;H,3.54.Found(%):C, 47.28;H,3.53.1H NMR(500 MHz,CDCl3):δ 7.53(d, 4H,J=8.0 Hz C6H4),7.22(d,4H,J=8.0 Hz C6H4), 1.98(s,12H,C5Me4),1.93(s,12H,C5Me4),IR(KBr, νCO/cm-1):1 944(s),1 926(s),1 898(s).
1.3 Synthesis of trans-[η5-C5Me4CH2(MeC5H3N)]2Mo2(CO)6(4)
Complex 4 was prepared in the same way as 3. The reaction of ligand precursor 2 with Mo(CO)6in xylene refluxing 10 h afforded complex 4 as dark red crystals in 43.6%yield.m.p.127~129℃(dec.).Anal. Calcd.(%)for C38H40Mo2N2O6:C,56.17;H,4.96;N, 3.45.Found(%):C,56.21;H,4.99;N,3.41.1H NMR (500 MHz,CDCl3):δ 7.54(t,2H,J=7.5 Hz,Py-H), 7.04(d,2H,J=7.5 Hz,Py-H),6.87(d,2H,J=7.5 Hz, Py-H),3.77(s,4H,-CH2-),2.53(s,6H,Py-CH3),2.00 (s,12H,C5Me4),1.99(s,12H,C5Me4).IR(KBr,νCO/ cm-1):1 932(s),1 892(s),1 871(s).
1.4 Synthesis of trans-[(η5-C5Me4(4-BrC6H4))Ru (CO)(μ-CO)]2(5)
Complex 5 was prepared in the same way as 3. The reaction of ligand precursor 1 with Ru3(CO)12in xylene refluxing 10 h afforded complex 5 as yellow crystals in 60.7%yield.m.p.216℃.Anal.Calcd.(%) for C34H32Br2Ru2O4:C,47.12;H,3.72.Found(%):C, 47.15;H,3.73.1H NMR(500 MHz,CDCl3):δ 7.55(d, 4H,J=8.0 Hz,C6H4),7.38(d,4H,J=8.0 Hz,C6H4), 1.85(s,12H,C5Me4),1.77(s,12H,C5Me4),IR(KBr,νCO/cm-1):1 930(s),1 747(s).
1.5 Synthesis of trans-[(η5-C5Me4CH2(MeC5H3N)) Ru(CO)(μ-CO)]2(6)
Complex 6 was prepared in the same way as 3. The reaction of ligand precursor 2 with Ru3(CO)12inxylene refluxing 10 h afforded complex 6 as yellow crystals in 62.7%yield.m.p.178℃.Anal.Calcd.(%) for C36H40N2Ru2O4:C,56.38;H,5.26;N,3.65.Found (%):C,56.35;H,5.27;N,3.69.1H NMR(500 MHz, CDCl3):δ 7.48(t,2H,J=7.5 Hz,Py-H),6.98(d,2H, J=7.5 Hz,Py-H),6.79(d,2H,J=8.0 Hz,Py-H),3.88 (s,4H,-CH2-),2.53(s,6H,Py-CH3),1.93(s,12H,C5Me4), 1.84(s,12H,C5Me4).IR(KBr,νCO/cm-1):1 921(s), 1 756(s).
1.6 Synthesis of trans-[(η5-C5Me4(4-BrC6H4))Fe (CO)(μ-CO)]2(7)
Complex 7 was prepared in the same way as 3. The reaction of ligand precursor 1 with Fe(CO)5in xylene refluxing 10 h afforded complex 7 as dark red crystals in 45.6%yield.m.p.173℃.Anal.Calcd(%). for C34H32Br2Fe2O4:C,52.62;H,4.16.Found(%):C, 52.61;H,4.18.1H NMR(500 MHz,CDCl3):δ 7.54(d, 4H,J=8.0 Hz,C6H4),7.35(d,4H,J=8.0 Hz,C6H4), 1.88(s,12H,C5Me4),1.71(s,12H,C5Me4),IR(KBr,νCO/ cm-1):1 928(s),1 768(s).
1.7 Synthesis of trans-[(η5-C5Me4CH2(MeC5H3N)) Fe(CO)(μ-CO)]2(8)
Complex 8 was prepared in the same way as 3. The reaction of ligand precursor 2 with Fe(CO)5in xylene refluxing 10 h afforded complex 8 as dark redcrystals in 45.2%yield.m.p.147℃.Anal.Calcd.(%) for C36H40Fe2N2O4:C,63.92;H,5.96;N,4.14.Found (%):C,63.90;H,5.97;N,4.09.1H NMR(500 MHz, CDCl3):δ 7.44(t,2H,J=7.5 Hz,Py-H),6.98(d,2H, J=7.5 Hz,Py-H),6.73(d,2H,J=7.5 Hz,Py-H),3.76 (s,4H,-CH2-),2.51,(s,6H,Py-CH3),1.73(s,12H, C5Me4),1.68(s,12H,C5Me4).IR(KBr,νCO/cm-1): 1 922(s),1 758(s).
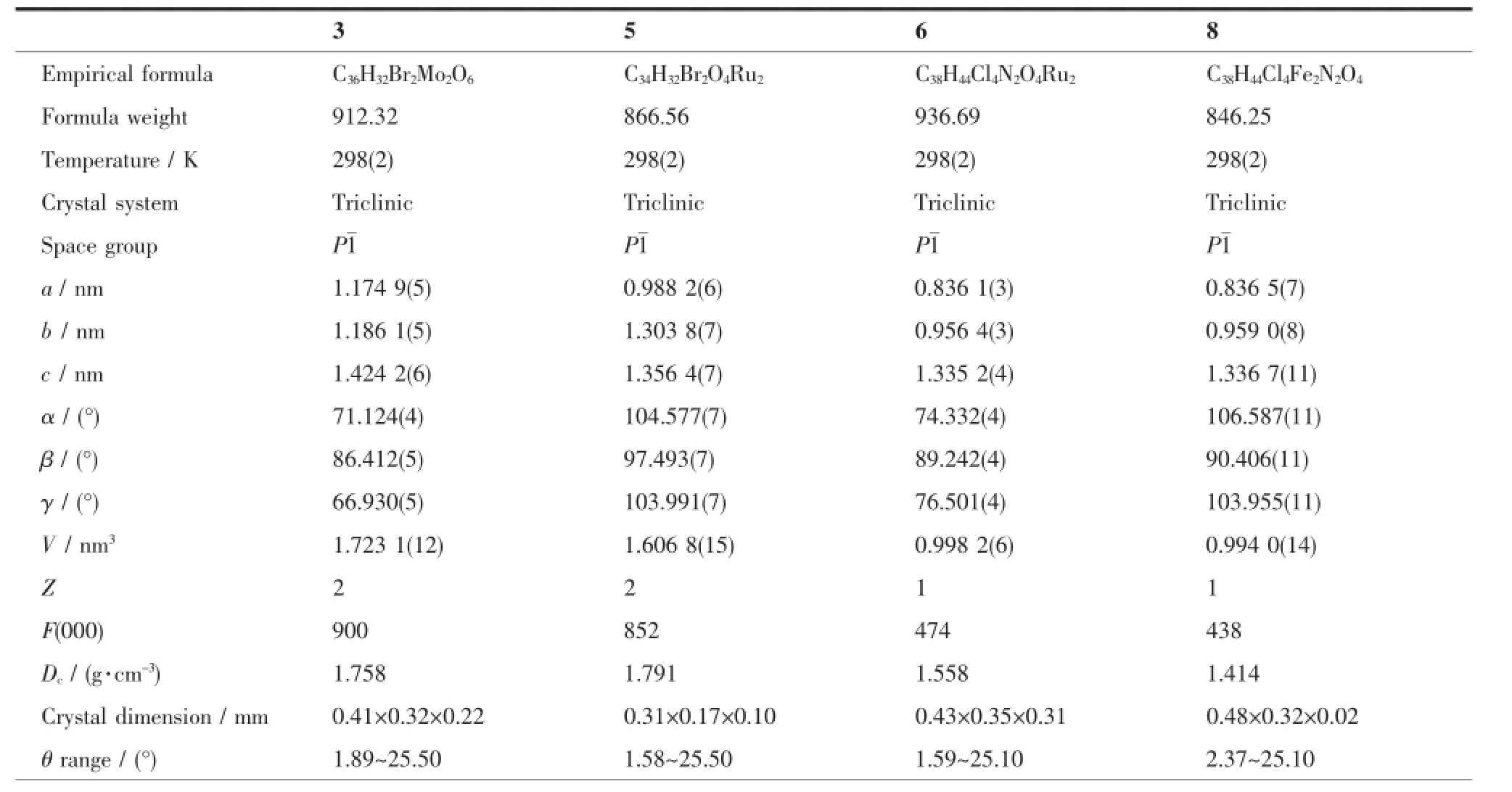
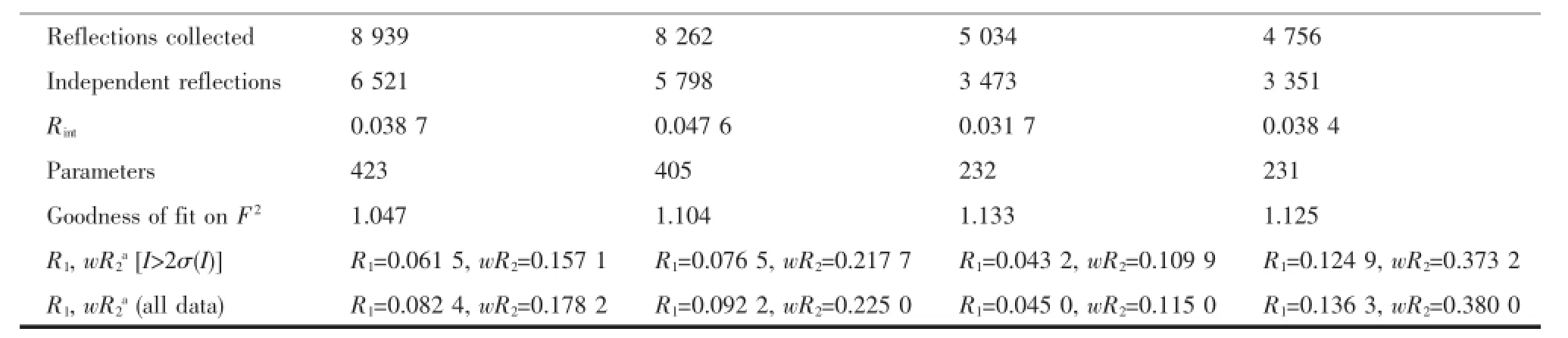
Table1 Crystal data and structure refinement parameters for the complexes 3,5,6 and 8
1.8 Crystal structure determination
Crystals of the complexes 3,5,6 and 8 suitable for X-ray diffraction were isolated from the slow evaporation of hexane-dichloromethane solution.Data collection were performed on a Bruker SMART APEX-CCD detector with graphite monochromated Mo Kα (λ=0.071 073 nm)radiationusingtheφ-ωscan technique.Thestructuresweresolvedbydirect methodsandrefinedbyfull-matrixleast-squares procedures based on F2using the SHELX-97 program system.Hydrogen atoms were included in calculated positions riding on the parent atoms and refined with fixed thermal parameters.Crystallographic data and experimental details of the structure determinations are given in Table 1.
CCDC:934733,3;944267,5;926404,6;926405, 8.
Continued Table 1
2 Results and discussion
2.1 Reactions of ligand precursors C5Me4HR(R= 4-BrPh(1),(MeC5H3N)CH2(2))with Mo(CO)6Reactions of ligand precursors C5Me4HR(R=4-BrPh(1),(MeC5H3N)CH2(2))with Mo(CO)6in refluxing xylene afforded the corresponding products 3(53.5%) and 4(43.6%)respectively(Scheme 1).
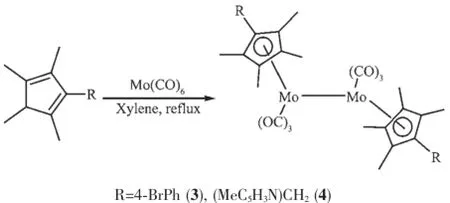
Scheme 1Synthesis of the complexes 3 and 4
Based on their1H NMR and IR spectra,3 and 4 were assigned as the normal Mo-Mo single bonded dinuclear complexes.The IR spectra of 3 and 4 all exhibited only terminal carbonyl bands(3:1 944, 1 926,1 898 cm-1;4:1 932,1 892,1 871 cm-1).The1H NMR spectra of 3 and 4 all displayed two groups of singlets for the four methyl protons,in addition,3 displayed two doublets for the phenyl protons and 4 displayed one singlet for the methylene protons and three groups of peaks for pyridyl protons,indicating the symmetrical structures in solution.
Single crystals of 3 suitable for X-ray diffraction was obtained from the slow evaporation of exane-CH2Cl2solution.Single crystals of 4 can be isolated in the same way but it is easily weathered.The singlecrystal X-ray determination of 3 is presented in Fig.1. It consists of two(C5Me4PhBr-4)Mo(CO)3units,and each of the molybdenum atoms is coordinated with a Cp(where Cp=cyclopentadienyl ligand)in an η5mode and three terminal carbonyl ligands.It has trans conformation and linked by a Mo-Mo bond,lying on a crystallographicinversion.Twoindependentbut chemically equivalent molecules appear in the unit cell.The fifth coordination position is occupied by a cyclopentadienyl ring that is essentially planar.The structural parameters of 3 are similar to those in[η5-C5Me4RMo(CO)3]2,and Mo-Mo bond distances for 3 are 0.328 9 nm(Mo1-Mo1i)and 0.329 4 nm(Mo2-Mo2ii),which are comparable to other metal-metal bond distances found for analogous derivatives of the type[η5-C5Me4RMo(CO)3]2(R=Ph-Me,(0.328 3 nm); R=Ph-OMe(0.330 7 nm)[15];R=n-butyl(0.328 6 nm)[16]). However,Mo-Mo bond distances of 3 are longer than that of trans-[η5-C5H5Mo(CO)3]2(0.323 5(1)nm)[17]and [(η5-C5H4iPr)Mo(CO)3]2(0.322 2(5)nm)[18],due to the bulky steric effect of the phenyl and four methyl groups.
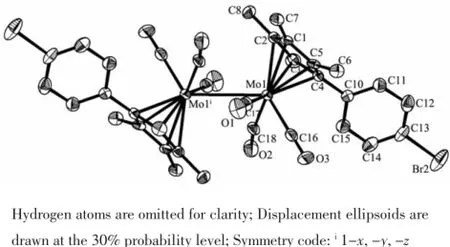
Fig.1 Molecular structure of complex 3
2.2 Reactions of ligands[C5Me4HR][R=4-BrPh (1),(MeC5H3N)CH2(2)]with Ru3(CO)12and Fe(CO)5
To develop a wider generality of the reactions oftype,we further introduced Ru3(CO)12and Fe(CO)5, carriedout by similar reaction conditions in the refluxing xylene and products 5~8 were obtained(Scheme 2).
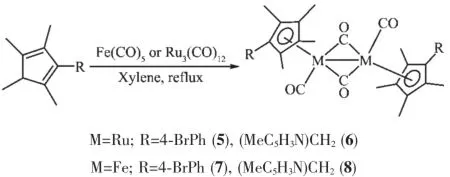
Scheme 2Synthesis of the complexes 5~8
Based on their1H NMR and IR spectra,5~8 were assigned as the normal M-M single bonded dinuclear complexes.The IR spectra of complexes 5~8 all exhibited a strong terminal carbonyl absorption at 1 921~1 930 cm-1and a strong bridging carbonyl absorption at 1 747~1 768 cm-1,which is comparable to other metal-metal bond spectra found in other substitutedcyclopentadienylrutheniumoriron carbonyl dimers.In their1H NMR spectra,5~8 all displayed two groups of singlets for the four methyl protons,in addition,both complexes 5 and 7 displayed two doublets for the phenyl protons and complexes 6 and 8 displayed one singlet for the methylene protons andthreegroupsofpeaksforpyridylprotons, indicating the symmetrical structures in solution.
Our initial idea is to introduce the pyridyl group to Cp ring as a functionalized side and then study the reactions of the side-chain-functionalized cyclopentadiene with metal carbonyls.For the pyridyl sidechain-functionalizedcyclopentadienylligand,the nitrogen atom can act as a good two-electron donor site and can coordinate to a variety of metals,for example,thermal treatment of the pyridyl side-chainfunctionalized cyclopentadiene withRu3(CO)12and Fe(CO)5could give different intramolecular C-H activated products besides the normal dinuclear metal complexes[22].Although we used different solvents,the pyridyl group only acted as substituent and did not coordinate with the Ru and Fe atoms,and the preconceivedC-Hactivatedproductswerenot obtained yet.
Selected bond lengths and angles for complexes 3,5,6 and 8 are given in Table 2.The single-crystal X-ray determination of complexes 5,6 and 8 are illustrated in Fig.2~4.
The crystal structure of 5 is shown in Fig.2,in an unit cell there are two different environmental molecules Ru(1)and Ru(2),which have the samestructure basically,just small differences in some bond lengths and angles.Similar to the cyclopentadienyl analogue trans-[η5-CpRu(CO)(μ-CO)]2,both the structures are trans form and have Cisymmetry. Two carbonyls are bridged and two carbonyls are terminal.The two cyclopentadienyl ring planes are parallel.The Ru-Ru distances are 0.275 3(2)nm(Ru1 -Ru1i)and 0.276 9(2)nm(Ru2-Ru2ii),respectively. Although there are two kinds of different environmental molecules in the unit cell,the1H NMR spectrum of 5 shows the existence of only one.This indicates that they may exist as one form in solution;however,the fact that a rapid fluxional process exist cannot be excluded[19-20].Disorder on the location of C31 and C32 has been shown in Fig.2.The disorder share of C31 is 0.50 and the disorder share of C32 is 0.50.
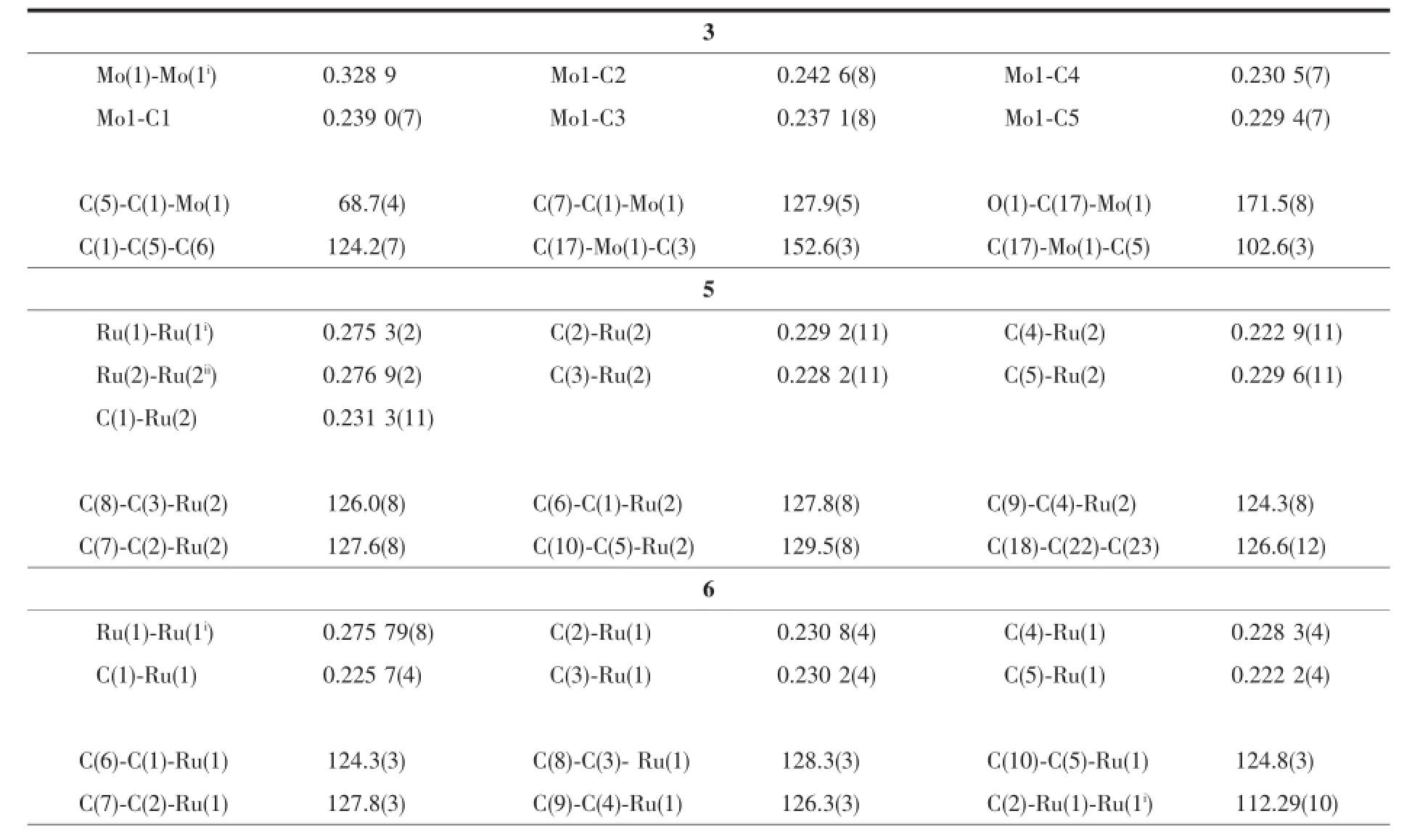
Table2 Selected bond lengths(nm)and angles(°)for complexes 3,5,6 and 8
Continued Table 1
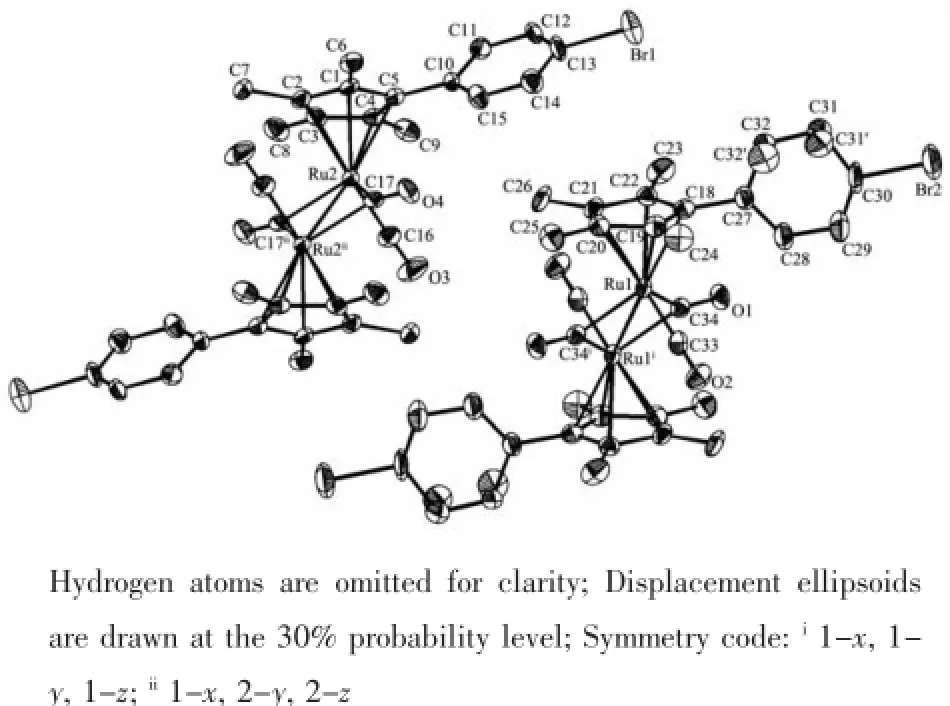
Fig.2 Molecular structure of complex 5
Ru-Ru bond distance of 5 is slightly longer than that in trans-[η5-CpRu(CO)(μ-CO)]2(0.273 5(2)nm)[21], this may be attributed to the bulky steric effect of the phenyl and four methyl groups;but slightly shorter than trans-{[η5-C5Me4Ph]Ru(CO)(μ-CO)}2(0.277 69(4) nm)andtrans-{[η5-C5Me4(4-OCH3)C6H4]Ru(CO)(μ-CO)}2(0.277 01(6)nm),which may be attributed to their different electrical effects of-Br,-OCH3and-H at the 4-position of the phenyl.
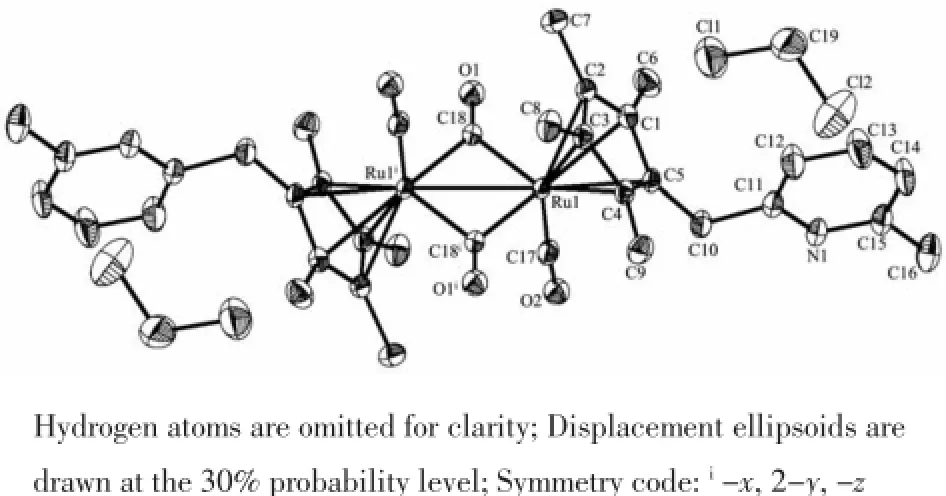
Fig.3 Molecular structure of complex 6
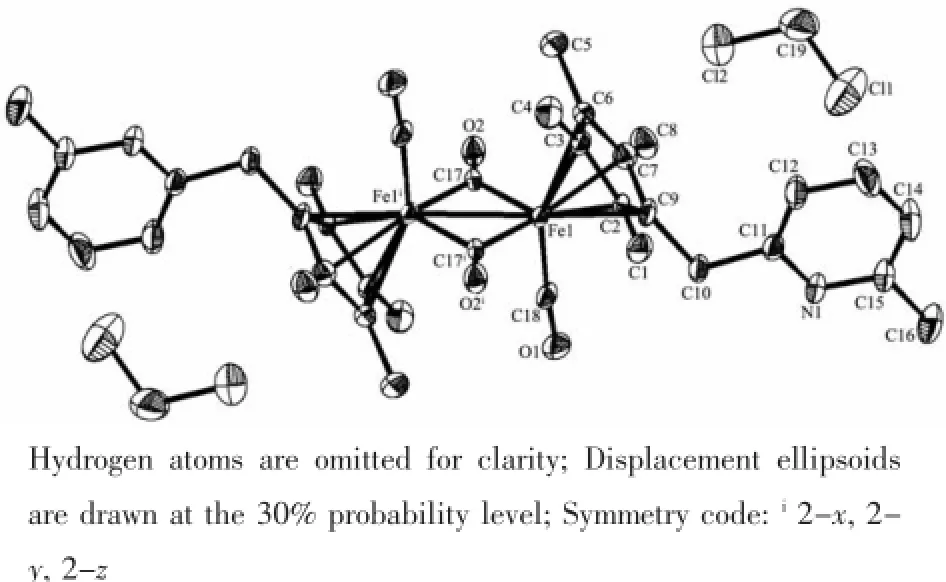
Fig.4 Molecular structure of complex 8
The crystal structures of 6 and 8 are shown in Fig.3~4.The structures of 6 and 8 are similar,and both have two types of carbonyl ligands,namely, terminal and bridging,in their molecular structures. Both structures are the symmetrical(trans)isomers. The two cyclopentadienyl ring planes and pyridyl ring planes are parallel,respectively.There are two solvent molecules in their unit cells.For complex 6,the Ru-Ru bond distance(0.275 79(8)nm)is slightly longer than those of analogous complexes trans-[C5H4Ru(CO) (μ-CO)]2(0.273 5(2)nm)[21]and[(C5H3NCH3)CH2Me2C (C5H4)Ru(CO)]2(μ-CO)2(0.273 69(8)nm)[22],and veryclosetotrans-[(η5-C5Me4Bz)Ru(CO)(μ-CO)]2(0.27537(4) nm)[23],but slightly shorter than that in[(η5-C5Me4Ph) Ru(CO)(μ-CO)]2(0.277 69(4)nm),[(η5-C5Me4PhOMe) Ru(CO)(μ-CO)]2(0.277 01(6)nm)[15]and[(η5-C5H3Ph2) Ru(CO)(μ-CO)]2(0.276 36(3)nm)[24].Ru-CEN(CEN means centroid of the cyclopentadienyl ring)distance (0.19187nm)isslightlylongerthan trans-{[(η5-C5Me4Bz) Ru(CO)(μ-CO)]2}(0.191 61 nm)[23],and very close to trans-{[η5-C5Me4(4-OCH3)C6H4]Ru(CO)(μ-CO)}2(0.1918 nm),but slightly shorter than trans-{[η5-C5Me4Ph]Ru (CO)(μ-CO)}2(0.192 9 nm)[15].These distances are comparabletothosefoundinothersubstituted cyclopentadienylrutheniumcarbonyldimers.For complex 8,the Fe-Fe bond distance is 0.258 1(3) nm,which is slightly longer than those in the unbridged analogs,trans-[(η5-C5Me4Ph)Fe(CO)(μ-CO)]2(0.256 35(6)nm),trans-[(η5-C5Me4PhOMe)Fe(CO)(μ-CO)]2(0.256 30(8)nm)[15]andtrans-[(η5-C5Me4Bz) Fe(CO)(μ-CO)]2(0.255 70(5)nm)[9],and even longer than those in the singly bridged analogs,(Me2C)[(η5-C5H4)Fe(CO)2]2(0.248 36(6)nm)and(Et2C)[(η5-C5H4) Fe(CO)2]2(0.246 71(6)nm)[18].To the best of our knowledge,the Fe-Fe bond distance is the longest distance in the unbridged analogs so far.
3 Conclusions
Reactionsofsubstitutedtetramethylcyclopentadienes C5Me4HR(R=4-BrPh(1),(MeC5H3N)CH2(2)) with Mo(CO)6,Ru3(CO)12and Fe(CO)5in the refluxing xylene were studied,respectively.Six new metal carbonyl complexes were obtained and four of them were determined by single-crystal X-ray diffraction. The results clearly revealed the coordination mode of these cyclopentadienyl metal complexes is η5and the N atom of pyridine did not coordinate to the metal atoms.These substituted cyclopentadienyl ligands are trans in the dimeric structures in the solid state. Substituent group variations display some influence on the M-M bond length of dinuclear tetramethylcyclopentadienyl metal carbonyl complexes.
[1]Borah M,Bhattacharyya P K,Das P.Appl.Organometal. Chem.,2012,26:130-134
[2]Stanowski S,Nicholas K M,Srivastava R S.Organometallics, 2012,31:515-518
[3]Do Y,Han J,Rhee Y H,et al.Adv.Synth.Catal.,2011,353: 3363-3366
[4]Kuninobu Y,Uesugi T,Kawata A,et al.Angew.Chem.Int. Ed.,2011,50:10406-10408
[5]Butenschn H.Chem.Rev.,2000,100:1527-1564
[6]Siemeling U.Chem.Rev.,2000,100:1495-1526
[7]Lin J,Zhao M X,Ma Z H,et al.J.Chem.Crystallogr.,2009, 39:642-645
[8]Lin J,Ma Z H,Li F,et al.Transition Met.Chem.,2009,34: 855-859
[9]Ma Z H,Zhao M X,Li F,et al.Transition Met.Chem.,2010, 35:387-391
[10]Tian L J,Ma Z H,Han Z G,et al.Transition Met.Chem., 2011,36:151-156
[11]Ma Z H,Tian L J,Li S Z,et al.Transition Met.Chem., 2012,37:135-140
[12]Ma Z H,Wang N,Guo K M,et al.Inorg.Chim.Acta,2013, 399:126-130
[13]Bensley D M,Mintz E A Jr,Sussangkarn S J.J.Org.Chem., 1988,53:4417-4419
[14]Enders M,Ludwig G,Pritzkow H.Organometallics,2001,20: 827-833
[15]Lin J,Gao P,Li B,et al.Inorg.Chim.Acta,2006,359:4503-4510
[16]MA Zhi-Hong(马志宏),ZHAO Ming-Xia(赵明霞),LIN Li-Zhi(林丽枝),et al.Chinese J.Inorg.Chem.(无机化学学报), 2010,26:1908-1911
[17]Adams R D,Collins D M,Cotton F A.Inorg.Chem.,1974, 13:1086-1090
[18]CHEN Shou-Shan(陈寿山),WANG Jia-Xi(王家喜),WANG Xu-Kun(王序昆),et al.Chinese J.Struct.Chem.(结构化学),1993,12:229-232
[19]Williams N A,Uchimaru Y,Tanaka M J.J.Chem.Soc.Chem. Commun.,1995,2010:1129-1130
[20]Alphonse F A,Yudin A K.J.Am.Chem.Soc.,2006,128: 11754-11755
[21]Mills O S,Nice N P.J.Organomet.Chem.,1967,9:339-344
[22]Chen D F,Xu S S,Song H B,et al.Eur.J.Inorg.Chem., 2008:1854-1864
[23]MA Zhi-Hong(马志宏),ZHAO Ming-Xia(赵明霞),LIN Li-Zhi(林丽枝),et al.Chinese J.Inorg.Chem.(无机化学学报), 2010,26:1121-1124
[24]Schumann H,Stenz S,Girgsdies F,et al.Z.Naturforsch., 2002,57b:1017-1026
Syntheses and Structures of Metal Carbonyl Complexes Based on Two Substituted Tetramethylcyclopentadienyl Ligands
FAN Dong1MA Zhi-Hong2LI Su-Zhen3HAN Zhan-Gang1ZHENG Xue-Zhong1LIN Jin*,1
(1The College of Chemistry&Material Science,Hebei Normal University,Shijiazhuang 050024,China)
(2College of Basic Medicine,Hebei Medical University,Shijiazhuang 050017,China)
(3Hebei College of Industry and Technology,Shijiazhuang 050091,China)
Via thermal reactions of the substituted tetramethylcyclopentadienes[C5Me4HR][R=4-BrPh(1), (MeC5H3N)CH2(2)]with Mo(CO)6,Ru3(CO)12and Fe(CO)5,respectively in refluxing xylene,the responding dinuclear metal carbonyl complexes trans-[η5-C5Me4R]2Mo2(CO)6(3,4),trans-[(η5-C5Me4R)Ru(CO)(μ-CO)]2(5,6),and trans-[η5-(C5Me4R)Fe(CO)(μ-CO)]2(7,8)have been obtained.These complexes have been characterized by elemental analysis,IR,and1H NMR spectra.The crystal structures of 3,5,6 and 8 have been determined by X-ray crystallography.CCDC:934733,3;944267,5;926404,6;926405,8.
cyclopentadiene;metal carbonyl complex;X-ray diffraction;structure
O614.61+2;O614.82+1;O614.81+1
A
1001-4861(2015)01-0198-07
10.11862/CJIC.2015.038
2014-07-14。收修改稿日期:2014-11-10。
国家自然科学基金(No.21372061),河北省自然科学基金(No.B2013205025,B2014205018),河北师范大学重点基金(No.L2012Z02)资助项目。*
。E-mail:linjin64@126.com;会员登记号:S06N0210M1305。
