在LiCl-KCl共晶熔体中电化学制备钬镍金属间化合物
2015-06-01孙婷婷王珊珊
李 梅 孙婷婷 韩 伟 王珊珊 张 密 林 颜 永 得 张 萌
(哈尔滨工程大学材料科学与化学工程学院,教育部超轻材料与表面技术重点实验室,哈尔滨150001)
在LiCl-KCl共晶熔体中电化学制备钬镍金属间化合物
李 梅 孙婷婷 韩 伟*王珊珊 张 密 林 颜 永 得 张 萌
(哈尔滨工程大学材料科学与化学工程学院,教育部超轻材料与表面技术重点实验室,哈尔滨150001)
采用循环伏安、方波伏安和开路计时电位等方法研究了Ho离子在LiCl-KCl共晶熔体中的电化学行为及Ho-Ni合金化机理。在惰性W电极上,Ho离子在-2.06 V(vs Ag/AgCl)发生电化学还原,该还原过程为3个电子转移的一步反应。与惰性W电极上的循环伏安相比,Ho离子在活性Ni电极的循环伏安曲线上还出现了3对氧化还原峰,是Ho与Ni形成了金属间化合物,导致了Ho离子在活性Ni电极发生了欠电位沉积。在不同的电位进行恒电位电解制备的3个不同的Ho-Ni合金,采用X-射线衍射(XRD)和扫描电子显微镜-能谱仪(SEM-EDS)等测试手段进行表征,结果表明:制备的3种合金分别是Ho2Ni17,HoNi5和HoNi23种合金化合物。
电化学行为;钬-镍合金化合物;恒电位电解;镍电极
Asstructuralandfunctionalmaterials, intermetallic compounds have attracted considerable attention due to their high potential forvarious applications[1-3].Therareearth-Niintermetallic compounds are essential for high-temperature structural materials,magneticmaterials,catalytic,hydrogen storage alloys,etc[4-5].As a new preparation method of the rare earth-transition metal intermetallic compounds, electrochemical synthesis using molten salts is an effective method because composition and thickness ofthealloyscanbecontrolledbyelectrochemical parameters[6].Kobayashi et al.[7-8]and Yasuda et al.[9-10]have investigated the electrochemical formation of Dy-Ni and Nd-Ni alloys in a molten LiF-CaF2-LnF3and NaCl-KCl-LnCl3(Ln=Dy and Nd)melts,respectively. Chamelot and co-worker[11]have obtained NdNi2,NdNi3, GdNi2,GdNi,Ni2SmandNi3Smintermetallic compounds on a reactive nickel electrode by the electroreduction of the lanthanides(Nd,Gd and Sm)in LiF-CaF2-LnF3molten salts.Nohira et al.[12]have studied the electrochemical preparation of Pr-Ni alloys in a molten LiCl-KCl-PrCl3(0.50 mol%)system.Iida and co-worker[13-14]have explored the formation of Sm-Ni and Yb-Ni alloys in a molten LiCl-KCl-LnCl3(Ln=Sm and Yb)melts.Yang and co-worker[15]have explored the electroreduction of Ho3+on nickel cathode in molten KCl-HoCl3.The free energies of formation for the intermetallic compounds between Ho and Ni,the diffusion coefficients and diffusion activation energy of Ho atom in the alloy phase were determined.
Sincetheoperationconditionssignificantly influencesthefeasibilityofpyrometallurgical reprocessing,it is of crucial importance to have the knowledge of the electrochemical behavior of Ln in different melts for the understanding of the process.The electrochemical reduction of Hoon a Ni electrode has only been studied in the KCl system[15],whereas the LiCl-KCl eutectic melts has not been used for the electrochemical synthesis of Ho-Ni alloys.Thus,the electrochemical behavior of Howas studied on an inert W electrode and a reactive Ni electrode in LiCl-KCI-HoCl3melts by cyclic voltammetry,square wave voltammetry and open circuit chronopotentiometry.The preparation of Ho-Ni intermetallic compounds was carried out by potentiostatic electrolysis at different potentials,and the samples were characterized by XRD and SEM-EDS.
1 Experimental
1.1 Preparation and purification of the melt
A mixture of LiCl-KCl eutectic melts(58.5∶41.5,n/ n)(analytical grade)was first dried under vacuum for more than 48 h at 573 K to remove excess water and then melted in an alumina crucible located in an electric furnace.The temperature of the melts was measured with a nickel chromium-nickel aluminium thermocouple sheathed with an alumina tube.Metal ion impurities in the melts were removed by pre-electrolysis at-2.10 V(vs Ag/AgCl)for 4 h.Anhydrous HoCl3(99.9wt%;High Purity Chemical Co.Ltd.)was added directly to the melts as a Hoion source.
1.2 Electrochemical apparatus and electrodes
All electrochemical measurements were performed using an Autolab PGSTAT 302N(Metrohm,Ltd.)with NOVA1.8softwarewheretechniquesofcyclic voltammetry(CV),square wave voltammetry(SWV)and open circuit chronopotentiometry(OCP)were employed. The working electrodes were W wire(d=1 mm;99.99%) and Ni plate(2 mm×11 mm;99.99%),which were polished thoroughly using SiC paper(2000 grit),and then cleaned ultrasonically with dilute hydrochloric acid and ethanol prior to use.The Ag/AgCl electrode (Ag:d=1 mm;99.99%)used as reference electrode(RE) was encased in a Pyrex tube,in which the LiCl-KCl eutectic melts contain 1.0wt%AgCl(99.998%).All potentials are referred to the Ag/AgCl couple.
1.3 Characterization of deposits
The deposits were prepared by potentiostatic electrolysisunderdifferent conditions.After electrolysis, the alloy samples were washed in hexane(99.8%)in an ultrasonic bath to remove salts and stored in a glove box for analysis.These deposits were analyzed by X-ray diffraction(XRD,X′Pert Pro;Philips Co.,Ltd.)using Cu Kαradiation(λ=0.154 06 nm)at 40 kV and 150 mA with a 2θ-θ scan mode in the 2θ range of 20°~90°,and the step size was 0.02°.The microstructure and micro-zone chemical analysis were measured by scanning electron microscopy and energy dispersive spectrometer(SEMEDS,JSM-6480A,JEOL Co.,Ltd.)under conditions of accelerating voltage of 20.0 kV,the valid time of 30.0 s andtheincidentangleof90.0°.
2 Results and discussion
2.1 Electrochemical behavior of Hoions in
LiCl-KCl eutectic melts on a W electrode
2.1.1 Cyclic Voltammetry
Fig.1 presents the typical cyclic voltammograms (CVs)obtained in the LiCl-KCl melts before and after the addition of HoCl3at 1 023 K.On the dotted curve, peaks A and A′correspond to the deposition and dissolution of Li metal,since no alloys or intermetallic compounds exist in the W-Li phase diagram[16]at 1 023 K.The solid curve represents the CVs after the addition of HoCl3in the LiCl-KCl melts.Except for the cathodic/ anodic peaks A/A′,the peaks B/B′observed at-2.06~1.89 V(vs Ag/AgCl)are ascribed to the deposition and subsequent reoxidation of holmium.
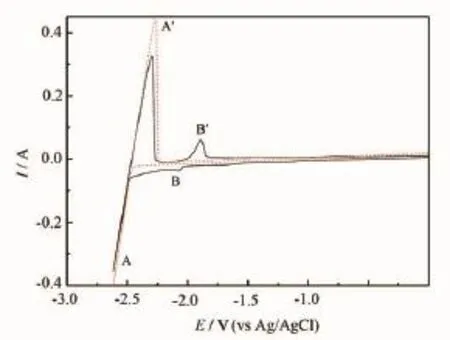
Fig.1 Typical CVs of the LiCl-KCl melts before(dotted line)and after(solid line)the addition of HoCl3(2.50×10-4mol·cm-3)on a W electrode(S=0.322 cm2)at 1 023 K.Scan rate:0.1 V·s-1
2.1.2 Square wave voltammetry
The square wave voltammetry technique was used to determine the number of electrons involved in the electrochemical step.For a simple reversible reaction, the width of the half-peak,W1/2,depends on the number of electrons exchanged and on the temperature as follows[17]:

where R is the universal gas constant,T is the absolute temperature(K),n is the number of exchanged electrons,and F is the Faradays constant(96 485 C· mol-1).
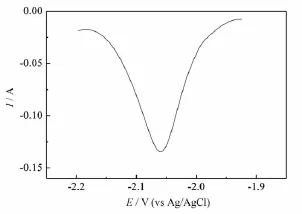
Fig.2 Square wave voltammogram of the LiCl-KCl-HoCl3(2.50×10-4mol·cm-3)melts obtained on a W electrode(S=0.322 cm2)at 1 023 K.Pulse height:25 mV;potential step:1 mV; frequency,30 Hz
Fig.2 shows a square wave voltammogram obtained in the LiCl-KCl-HoCl3melts on a W electrode.The peak shown in Fig.2 exhibits an asymmetrical Gaussian signal attributed to the nucleation overpotential,the rise of the current is delayed by the overpotential caused by the solid phase formation.The relationship(1)can be utilized to calculate the number of electrons involved in the reduction reactions of Ho/Ho(0)when we apply this technique at low frequencies and small step potentials[17-18].Using this methodology,the value of n is 2.86,close to three electrons,which indicates that the electroreduction of Hoproceeds in a one-step process involving three electrons.The result is consistent with the ones obtained from the cyclic voltammograms.
2.2 Electrochemical behavior of Hoions on a nickel electrode and formationofHo-Ni intermetallic compounds
2.2.1 Electrochemical behavior of Hoions in LiCl-KCl eutectic melts on a Ni electrode
In order to investigate the formation of Ho-Ni intermetalliccompounds,cyclicvoltammetrywas conducted on a Ni electrode in the LiCl-KCl-HoCl3melts.Fig.3 shows the typical CVs of the LiCl-KCl melts before(dotted line)and after(solid line)the addition of HoCl3(2.50×10-4mol·cm-3)on a Ni electrode at 1 023 K.On the dotted curve in Fig.3, comparison to the CVs of the LiCl-KCl melts on a W electrode,the peaks A/A′correspond to the deposition and re-oxidation of Li metal.The sharp increase signals F/F′can be ascribed to the deposition and dissolution of Ni metal.
The shape of the solid curve in Fig.3 obtained on a Ni electrode is very different from the one obtained on a W electrode after the addition of HoCl3in the LiCl-KCl melts(solid curve in Fig.1).Except for the peaks A/A′and B/B′at-2.42 V/-2.33 V and-2.06 V/-1.89 V(vs Ag/AgCl),corresponding to the reduction/oxidation of lithium and holmium,respectively,additional peaks appear prior to the reduction of Hoto give pure metal on a W electrode.Of evidence,these peaks,C/C′,D/D′and E′,are attributed to the formation and re-oxidation of Ho-Ni intermetallic compounds.

Fig.3 Typical CVs of the LiCl-KCl melts before(dotted line)and after(solid line)the addition of HoCl3on a Ni electrode(S=0.322 cm2)at 1 023 K.Scan rate:0.1 V·s-1
Inaddition,squarewavevoltammetrywas employed to further investigation of the electrochemical behavior of Hoon a Ni electrode,since square wave voltammetry is more sensitive and has a higher resolution than cyclic voltammetry[19].Fig.4 shows a square wave voltammogram obtained at a step potential of 1 mV and frequency of 30 Hz in the LiCl-KCl-HoCl3(2.50×10-4mol·cm-3)melts on a Ni electrode at 1 023 K.It exhibits four peaks B,C,D,and E,corresponding to the reactions of Ho/Ho(0),the formation of three different intermetallic compounds,respectively,which is consistent with the results obtained from cyclic voltammogram on a Ni electrode.
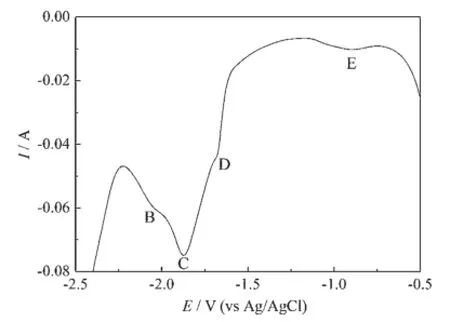
Fig.4 Square wave voltammogram of the LiCl-KCl-HoCl3melts obtained on a Ni electrode(S=0.322 cm2)at 1 023 K;Pulse height:25 mV;potential step: 1 mV;frequency:30 Hz
Open circuit chronopotentiometry was carried out tofurtherinvestigatetheformationofHo-Ni intermetallic compounds.Since the deposited Ho metal reacts with Ni and diffuses into the Ni electrode,the electrode potential gradually shifts to more positive values.During this process,a potential plateau is observed when the composition of the electrode surface is within a range of a two-phase coexisting state[13,20].Fig. 5 shows an open circuit chronopotentiogram after potentiostatic electrolysis at-2.60 V(vs Ag/AgCl)for 35 s on a Ni electrode.Five potential plateaus are observed at-2.28,-2.01,-1.82,-1.61 and-1.10 V(vs Ag/AgCl),respectively.The first potential plateau A is thought to be due to the presence of the codeposited Li metal on the electrode.The second plateau B is considered to be ascribed to the Ho/Ho(0)potential. After that,three plateaus C,D and E correspond to the coexistingstatesofdifferentHo-Niintermetallic compounds.
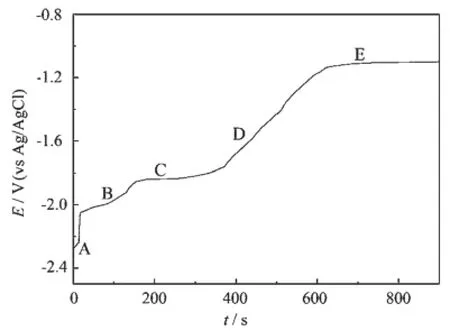
Fig.5 Open circuit potentiogram of LiCl-KCl-HoCl3(2.50×10-4mol·cm-3)melts on a Ni electrode (S=0.322 cm2)at-2.6 V vs Ag/AgCl for 35 s at 1 023 K
2.2.2 Potentiostatic electrolysis and characterization of deposits
Based on the results of cyclic voltammogram, squarewavevoltammogramandopencircuit potentiogram,theHo-Nialloycompoundswere prepared by potentiostatic electrolysis on a Ni electrode(S=2.88 cm2)in the LiCl-KCl-HoCl3(2.50×10-4mol cm-3)melts.
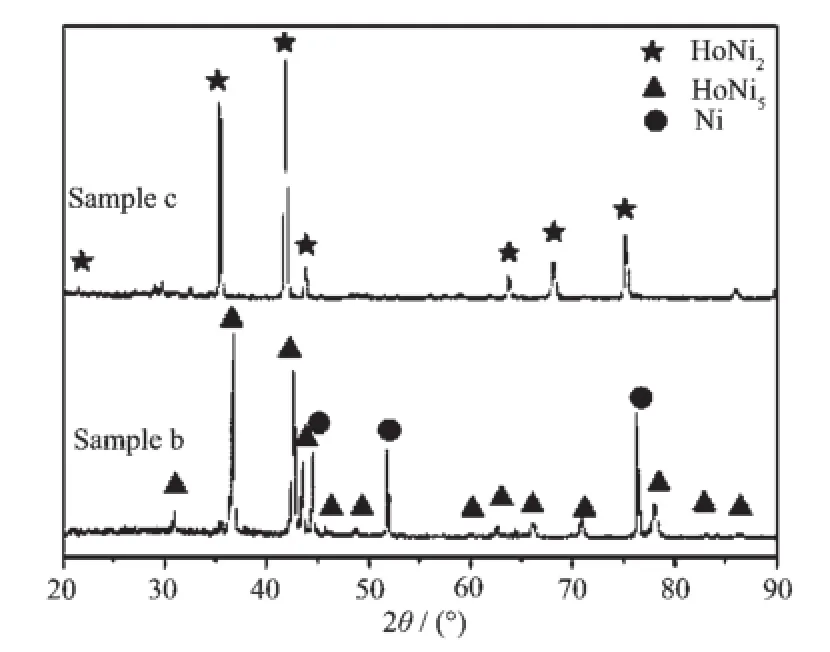
Fig.6 XRD patterns of the deposits obtained by potentiostatic electrolysis in the LiCl-KCl-HoCl3melts on a Ni electrode at 1 023 K; sample b:at-1.8 V for 10 h;sample c at -2.2 V for 10 h
Fig.6 shows the XRD patterns of Ho-Ni alloy samples obtained by potentiostatic electrolysis in the LiCl-KCl-HoCl3melts at-1.8 V(1 023 K,10 h,sample (b))and-2.2 V(1 023 K,10 h,sample(c)), respectively.XRD patterns indicate that the Ho-Ni alloy samples are comprised of HoNi5and HoNi2, respectively.
At-1.8 V(vs Ag/AgCl),the sample(a)was obtained by potentiostatic electrolysis at-1.8 V for 5 h. The cross-sectional SEM image of the sample(a)in Fig. 7a shows the thickness of the alloy layer is around 20 μm.The EDS point analysis of labeled A in the Fig. 7a shows that the atomic ratio of Ni to Ho is about 17∶2 (Fig.7b).Because the alloy layer is very thin,there is no corresponding signal can be detected by XRD.Nohira et al.[6]pointed out that one phase forms preferentially and another phase starts to form upon the completion of the preferentially phase formation.According to the Ho-Ni phase diagram[21]and the analysis of EDS,we think the observed alloy layer in the sample(a)is Ho2Ni17layer with 20 μm thickness.

Fig.7 SEM and EDS analysis of the Ho-Ni alloys obtained by potentiostatic electrolysis in the LiCl-KCl-HoCl3melts;SEM image(a)and EDS point analysis(b)at-1.8 V for 5 h;SEM image(c)and EDS point analysis(d)at-1.8 V for 10 h; SEM image(e)and EDS point analysis(f)at-2.2 V for 10 h
Fig.7 c shows the SEM image of sample(b)obtained at-1.8 V(vs Ag/AgCl)for 10 h at 1 023 K.The thickness of alloy layer is close to 60 μm.The EDS of labeled A in Fig.7c indicates that the atomic ratio of Ni to Ho is close to 5∶1(Fig.7d).The XRD pattern confirms the presence of HoNi5in the sample(b).At the same potential,the Ho-Ni intermetallic compound obtained is different at different electrolysis time.The longer the electrolysis time,the richer Ho intermetallic compound is obtained by potentiostatic electrolysis at the same potential.
At-2.2 V,only one intermetallic compound HoNi2is characterized by XRD in Fig.6.The cross-sectional SEM image of the sample(c)is presented in Fig.7e.The result shows that the thickness of deposited alloy layer is about 130 μm and the atomic ratio of Ni to Ho isclose to 2∶1(Fig.7f).Combined with the XRD pattern of the sample(c)in Fig.6,we think that the alloy layer is a uniform HoNi2layer.
3 Conclusions
The electrochemical behavior of Hoin the eutectic LiCl-KCl melts on W and Ni electrodes were investigated by a series of electrochemical techniques. The results show that the reduction mechanism of Hoproceeds in a one-step process exchanging three electrons on a W electrode.Theunder-potential deposition of Hoon a Ni electrode is due to the formation of different Ho-Ni intermetallic compounds. According to the study of cyclic voltammograms,square wavevoltammogramandopen-circuit chronopotentiograms,theHo-Niintermetalic compounds were obtained by potentiostatic electrolysis under different potentials.The formation of Ho-Ni alloys with different ratios of the two metals,i.e. Ho2Ni17,HoNi5and HoNi2,is confirmed by XRD, SEM-EDS.
[1]Yaropolov Y L,Andreenko A S,Nikitin S A,et al.J.Alloys Compd.,2011,509:S830-834
[2]Haraguchi T,Kogachi M.Mater.Sci.Eng.A,2002,329-331: 402-407
[3]GUO Xin(郭欣),LI Shu-Cun(李书存),WANG Li(王丽)et al. Chinese J.Inorg.Chem.(无机化学学报),2014,30(9):2019-2024
[4]Domnguez-Crespo M A,Torres-Huerta A M,Brachetti-Sibaja B,et al.Int.J.Hydrogen Energy,2011,36:135-151
[5]Li P,Li Q Q,Jin T,et al.Mater.Sci.Eng.A,2014,603:84-92
[6]Konishi H,Nohira T,Ito Y.Electrochim.Acta,2003,48:563-568
[7]Kobayashi S,Nohira T,Kobayashi K,et al.J.Electrochem. Soc.,2012,159(12):E193-197
[8]Kobayashi S,Kobayashi K,Nohira T,et al.J.Electrochem. Soc.,2011,158(12):E142-146
[9]Yasuda K,Kobayashi S,Nohira T,et al.Electrochim.Acta, 2013,106:293-300
[10]Yasuda K,Kobayashi S,Nohira T,et al.Electrochim.Acta, 2013,92:349-355
[11]Chamelot P,Massot L,Hamel C,et al.J.Nucl.Mater., 2007,360:64-74
[12]Nohira T,Kambara H,Amezawa K,et al.J.Electrochem. Soc.,2005,152(4):C183-189
[13]Iida T,Nohira T,Ito Y,et al.Electrochim.Acta,2001,46: 2537-2544
[14]Iida T,Nohira T,Ito Y,et al.Electrochim.Acta,2003,48: 1531-1536
[15]SU Yu-Zhi(苏育志),YANG Qi-Qin(杨绮琴),LIU Guan-Kun (刘冠昆).J.Rare Earths,2000,18(1):34-38
[16]Sangster J,Pelton A D.J.Phase Equilib.,1991,12:203
[17]BardAJ,FaulknerLR.ElectrochemicalMethods: Fundamental and Applications.New York:John Wiley& Sons,Inc,2001:291
[18]Castrillejo Y,Fernández P,Bermejo M R,et al.Electrochim. Acta,2009,54:6212-6222
[19]Strycker J D,Westbroek P,Temmerman E.Electrochem. Commun.,2002,4:41-46
[20]Konishi H,Nishikiori T,Nohira T.Electrochim.Acta, 2003,48:1403-1408
[21]Zhou H Y,Ou X L,Zhong X P.J.Alloys Compd.,1991,117 (1):102-106
Electrochemical Preparation of Ho-Ni Intermetallic Compounds in LiCl-KCl Eutectic Melts
LI MeiSUN Ting-TingHAN Wei*WANG Shan-ShanZHANG Mi-LinYAN Yong-DeZHANG Meng
(Key Laboratory of Superlight Materials and Surface Technology,Ministry of Education,College of Material Science and Chemical Engineering,Harbin Engineering University,Harbin 150001,China)
The electrochemical behavior of Hoin LiCl-KCl eutectic melts and the alloying mechanism of Ho-Ni alloys were investigated by cyclic voltammetry,square wave voltammetry and open circuit chronopotentiometry. On an inert W electrode,the electroreduction of Hoproceeds in a one-step process involving three electrons at -2.06 V(vs Ag/AgCl).Compared with the cyclic voltammograms on an inert W electrode,three reduction peaks are observed which indicates the under-potential deposition of Hoon the reactive Ni electrode due to the formation of Ho-Ni intermetallic compounds.Three alloy samples were produced by potentiostatic electrolysis at various potentials and characterized by X-ray diffraction(XRD),scanning electron microscopyand energy dispersive spectrometer(SEM-EDS),respectively.The results confirm the three alloy samples of Ho2Ni17,HoNi5and HoNi2intermetallic compounds,respectively.
electrochemical behavior;Ho-Ni intermetallic compounds;potentionstatic electrolysis;LiCl-KCl melts
0614.111;0614,22;TB323
A
1001-4861(2015)01-0177-06
10.11862/CJIC.2015.006
2014-06-30。收修改稿日期:2014-10-08。
国家自然科学基金(No.21271054,21173060,51104050),国家自然基金重大研究计划(No.91326113,91226201)以及中央高校研究基金(HEUCF201403001)资助项目。
*通讯联系人。E-mail:weihan@hrbeu.edu.cn,Tel:0451-82569890;会员登记号:S06N3035M1005。
