浸渍/共沉淀法对BaO改性单Pd催化剂净化甲醇汽油车尾气性能的影响
2015-06-01张雪乔田浩杞叶芝祥陈耀强
张雪乔 田浩杞 叶芝祥 陈耀强
(1成都信息工程学院资源环境学院,成都610225)
(2四川大学催化材料科学研究所,成都610064)
浸渍/共沉淀法对BaO改性单Pd催化剂净化甲醇汽油车尾气性能的影响
张雪乔1,2田浩杞1叶芝祥1陈耀强*,2
(1成都信息工程学院资源环境学院,成都610225)
(2四川大学催化材料科学研究所,成都610064)
采用浸渍和共沉淀两种方法分别制备了BaO改性的Pd/CeO2-ZrO2-La2O3-Al2O3催化剂。运用N2吸附-脱附,X射线衍射(XRD),H2程序升温还原(H2-TPR),NH3程序升温脱附(NH3-TPD),透射电子显微镜(TEM)和X射线光电子能谱(XPS)对催化剂进行表征,并考察其对甲醇,CO,C3H8和NO的催化性能。活性测试结果表明,BaO的引入可明显改善Pd催化剂对甲醇,CO,C3H8和NO的催化活性,且浸渍法最佳,起燃温度(T50)分别降低了43,31,45和35℃。XRD,H2-TPR及XPS结果表明,浸渍法引入BaO主要通过表面改性方式,强化Pd-Ce界面间的相互作用,改善催化剂的还原性能,进而提高催化剂的低温活性;而共沉淀法则是通过结构改性方式增加CeO2晶格缺陷,加速活性氧物种的流动,Ce3+浓度的增加是促使CO氧化活性显著提高的主要原因。
氧化钡;钯;铈;甲醇;催化转化
Alternative fuels become important research interests owing to an increased concern on environmental protection and the need to reduce the dependency on petroleumoil[1].Methanol(orits mixture with gasoline)has been suggested as an alternative fuel due to its larger octane number and less air pollution[2].Although vehicles operating on the gasoline-methanol fuel produce an exhaust with a lower carbon monoxide(CO),hydrocarbons(HCs)and nitrogen oxides(NOx),etc,it emits various partial oxidationproductssuchasformaldehydeand unburned methanol vapor[3],which are harmful to the environment.Therefore,it is crucial to develop an efficient catalyst for purification of methanol,CO,HCs and NOx,simultaneously.
Pd-based catalysts have attracted much attention for its lower price,more facilities and higher activity for the oxidation of HCs and CO compared with Rh,Ptbased catalysts.Ceria species widely used in three-way catalysts(TWC)exhibit a multiple effects on catalytic performance,such as increasing in oxygen storage capacity(OSC)of TWC[4],improving CO and NO conversion[5],promotinglow temperaturewater-gas shift[6],stabilizing noble metal dispersion[7]and minimizing the thermally induced sintering ofsupports[8]. Hence,increasing attentions are focused on TWC with Pd species as catalytic active sites and ceria as the support[9-10].Barium oxide(BaO)is regarded an effective promoter for improving OSC of ceria-zirconia[11],NOxstorage capacity[12],and thermal stability of ceria-based catalysts,etc[13].Moreover,BaO can also improve catalytic activity for the conversion of CO,HC,NOxin vehicle exhaust[14].So far,there have been available many kinds of introduction methods,including microemulsion[15],sol-gel[16],impregnation[17]and co-precipitation methods[18].These preparation methods play an important role in promoting the performance of the catalyst.However,BaO-modifiedPdcatalystsby different methods to purify methanol exhaust have not yet been reported,to the best of our knowledge.
In the present work, Pd-BaO catalysts modified by impregnation/co-precipitation method were preparedand applied in purification of gasoline-methanol exhaust.The effect of preparation methods on textural, structural,redox,acidity,electronpropertiesand catalytic performance was investigated.
1 Experimental
1.1 Catalyst preparation
Ce0.45Zr0.45La0.1O1.95-Al2O3(CZLA)solid solution was preparedbyco-precipitationmethodfromthe corresponding raw materials:Ce(NO3)3·6H2O,ZrOCO3· H2O,La(NO3)3·6H2O and Al(NO3)3·9H2O.The precipitates were filtered, washed, dried at 105 ℃ overnight, and then calcined at 600 ℃ in air for 3 h to obtain CZLA support. The theoretical mass percentage of Al2O3in the oxides is 50.0%.
To obtain the Pd/CZLA-Ba mixed oxides prepared by co-precipitation method(co-catBa),the BaO-doped Ce0.45Zr0.45La0.1O1.95-Al2O3(CZLA-Ba)was prepared according to the same process for CZLA; then Pd(NO3)2aqueous solution was deposited on CZLA-Basuppor tmaterials by in cipient wetness method.The obtained sample was dried and calcined at 550℃for 3 h.The resulting powders were milled with desired deionized water to obtain slurry,then the resulting slurry was washcoated onto a honeycomb cordierite(2.5 cm3,the weight was 1.1 g,Corning, America).The loading of washcoat was kept about 140 g·L-1.The washcoated catalysts were dried at 120℃for 2 h and calcined at 550℃for 3 h.Another type ofBaO-loadedPd-Ba/CZLAmixedoxideswas obtained by impregnation method(im-catBa).The asprepared CZLA powders were impregnated in the aqueoussolutionofBa(NO3)2andPd(NO3)2..The successive process followed was the same as the process for Pd/CZLA-Ba.The loadings of Pd and BaO relativetosupportwere2.0wt%and5.0wt%, respectively.Inordertocompare,thePd/CZLA sample without BaO(cat0)was prepared.
1.2 Catalysts characterization
Textural properties were evaluated by low temperaturenitrogenadsorption-desorptionona QUADRASORB SI Automated Surface Area&Pore Size Analysizer(U.S.).The samples were evacuated at 300℃for 3 h.
X-ray diffraction(XRD)patterns of catalysts were recorded on a DX-1000 X-ray diffractometer operated at 30 kV and 20 mA,using Cu Kα radiation(λ=0.154 18 nm).The crystalline phases were identified according to PDF(The Powder Diffraction File)reference data from International Centre for Diffraction Data(ICDD) of Joint Committee on Powder Diffraction Standards (JCPDS).
Hydrogen temperature-programmed reduction (H2-TPR)experiments were performed in a selfassembledexperimental setup with a thermal conductivity detector. All samples(100 mg)were pretreated in a quartz tubular micro-reactor in a flow of pure N2at 500℃for 1 h,and then cooled down to room temperature.The reduction was carried out in a flow of 5%H2-95%N2from 200 to 1 000℃with a heating rate of 8℃·min-1.
Temperature-programmed desorption of NH3NH3(NH3-TPD)experiments were carried out in a fixedbed quartz reactor.A typical sample mass of 80 mg and a gas flow rate of 30 mL·min-1were used during the experiments.The experiment included four stages: (1)degasification of the sample in Ar at 400℃for 1 h to clear surface,(2)adsorbed 2%NH3at room temperature for 1 h,(3)isothermal desorption in Ar at room temperature until no NH3was detected and(4) temperature programmed desorption in Ar at 10℃· min-1up to 550℃.The detector was a thermal conductivity detector.
The size of the precipitates was observed with transmission electron microscopy(TEM)using a Tecnai G2 F20 S-TWIN apparatus operated at 200 kV.
X-ray photoelectron spectroscopy(XPS) measurements were performed on a spectrometer(XSAM-800,KRATOS Co)using Mg Kα radiation (hν=1 486.6 eV)under ultra-high vacuum condition. The binding energy was determined by reference to the C1s binding energy of 284.8 eV.
1.3 Activity evaluation
The catalytic purification for methanol exhaust was evaluated in a continuous flow fixed-bed reactor by passing mixed gases similar to the gasohol exhaust,and the gases were regulated using mass-flow controllers.The simulated exhaust gas was a mixture of 0.5%~0.6%CO,0.07%~0.08%C3H8,0.08%~0.09%NO,1.9%~2.2%O2,0.02%~0.03%methanol, and N2as the balance gas.The gas space velocity was 30 000 h-1.The organic reaction products were analyzed by gas chromatography(GC-2000,China)equipped with FID detector and a Porapak-Q column.The concentrations of CO,HC,NO,O2and CO2were analyzed online by a five-component FGA-4001.
2 Results and discussion
2.1 XRD
The XRD patterns of Ba-modified Pd/CZLA are shown in Fig.1.

Fig.1 XRD patterns of Pd/CeO2-ZrO2-La2O3-Al2O3(Pd/CZLA)doped by BaO
As seen from Fig.1,all of the diffractograms show the main reflections typical of a cubicfluoritestructured material,with fcc unit cells at 29.0°,33.6°, 48.2°,and 58.6°,corresponding to the(111),(200), (220)and(311)planes[14,19].Forco-catBa,no diffraction peaks for BaO are detected,indicating that Ba2+ions are doped into CeO2-ZrO2framework forming homogeneous CeO2-ZrO2solid solutions.Based on BraggslawandScherrerformula,thelattice parameters and crystallite sizes of all samples are calculated and the results are listed in Table 1.It can be observed that the lattice parameter of co-catBa is larger than that of cat0.The ionic radius of Ba2+(0.134 nm)is larger than that of Ce4+/Ce3+(0.097 nm/ 0.114 nm)or Zr4+(0.084 nm).Therefore,the addition of Ba2+into the CeO2-ZrO2lattice will result in lattice expansion.However,the lattice parameter of im-catBa is smaller than that of cat0.The diffraction peak reveals the phase segregation for the mixed oxide, with the presence of characteristic Ba2AlLaO5phase peaks.It may be resulted from the fact that due to bigger radius of La3+(0.106 nm)than that of Ce4+,a portion of La3+ions has been extracted from the mixed oxides and combined with Ba2+forming the Ba2AlLaO5, which leads to the significant lattice shrinkage of imcatBa.The similar result has been reported by other groups[20].The XPS results in the following discussion will give more details on this point.So,it can be considered that co-precipitation method will lead to most Ba2+ions into the CeO2-ZrO2lattice,while the impregnation method can lead to the most Ba2+ions remaining on the surface of samples.Besides,no visible PdO or metallic Pd is present in the XRD patterns for all the catalysts,indicating that the content of Pd is too low to be detected or that the Pd particles are well dispersed on the supports.

Table1 Textural and structural properties of Pd/CZLA mixed oxides doped by BaO
2.2 Nitrogen adsorption-desorption
The textural properties of Pd catalysts modified by BaO with different methods are summarized in Table 1.As shown in Table 1,BET specific surface area of the im-catBa and co-catBa catalyst is 192 m2· g-1and 147 m2·g-1,smaller than that of cat0(276 m2· g-1).But the average pore diameters are obviously affected and even increased from 5.1 nm to 7.9 nm (im-catBa)and 7.4 nm(co-catBa),respectively.This reveals that the introduction of BaO by two methods does not increase the surface area of samples but can broaden the average pore diameter.The cumulative pore volume of im-catBa is 0.33 mL·g-1,higher than that of co-catBa(0.22 mL·g-1).This phenomenon may be resulted from the blocking of small pores or the formation of larger ones.It is confirmed by the increase of the average pore diameter.The same conclusion is alsoreportedinref.[21].Moreover, previous studies consider that this result can be explained by the formation of new phases[22].So the increased pore volume may be resulted from the presence of Ba2AlLaO5as detected by XRD.The bigger pore volume and average pore diameter are beneficial to the adsorption/desorption of reaction species,leading to an improvement of the catalytic activity.This is further confirmed by the result of the catalytic performance of the mterial.In summary,the textural properties of Pd catalyst are enhanced by two different preparation methods and the impregnation method is superior to co-precipitation method.
2.3 H2-TPR
The TPR profiles of catalysts are shown in Fig.2. The TPR profile of cat0 shows two peaks β and γ at 150℃and 300℃,which are associated with the reduction of PdO species and surface oxygen of CeO2[23].In the case of BaO modified catalysts,the in tensity of the peak soverlow-temperatureis in creased with asimul taneous decrease of the intensityofthepeakγ.Andthereduction temperatures of peaks also shift to lower temperatures. There is a direct correlation between the peak area and the amount of reductive species.The reduction peak areas of co-catBa and im-catBa are larger than that of cat0.These demonstrate that the addition of Ba2+is beneficial for the reduction of PdO species and also can increase the amount of reductive species on the surface of Pd-based catalysts.

Fig.2 H2-TPR profiles of Pd/CZLA doped by BaO with impregnation/co-precipitation
Compared with cat0,the im-catBa catalyst shows two peaks α and β at 80℃and 110℃,which is attributed to the reduction of PdO species highly dispersed on the surface of the support and the interactionbetweenPdOandthesupport[24], respectively.But,unlike the im-catBa catalyst,cocatBa exhibits only one peak α below 200℃,and the intensity of α peak is obviously higher than that of im-catBa.It indicates that the introduction of BaO by co-precipitation clearly promotes the high dispersion of PdO species on the surface of the support and accelerates the reduction rate of PdO.However,the easierreductionofim-catBaandco-catBahas differentreasons.Fortheco-catBasample,the intensity of reduction peak of PdO specie dispersed on the surface of the support increases obviously.We mayattributethisphenomenontothestructural modification in the CeO2-ZrO2lattice when some Ce4+cations are substituted by Ba2+leading to the formation of more homogeneous solid solution.This is confirmed by XRD.As reported in refs[25-26],the introduction of Ba2+in CeO2-ZrO2lattice can induce structuredisorderandcreateadditionalanion vacancies,thus increasing the oxygen mobility in the bulk of solid solution,causing partial O2-anion diffusion from bulk to surface,and then increasing the reducibility of Pd catalyst.For the im-catBa sample, two reduction peaks α and β have been improved,and the shoulder peak β area is distinctly larger than that of peak α.This fact is mainly associated with the surface modification.The enrichment of Ba2+dispersed on the surface of samples prepared by impregnation method will promote the high dispersion of PdO species on the surface of the support,especially strengthentheinteractionbetweenPdOandthe support.So,except for the highly dispersed PdO,the Pd-CeinteractionspeciesformedinthePd-Ce interfaceisanothermainspecies.Inaddition, reference[27]reports that the hydrogen spillover may occur during the PdO reduction and promotes the reduction of CeO2-support.The presence of BaO strongly modifies the dispersion of PdO,facilitating diffusion of hydrogen between in PdO particles or PdO particles and the support,which favors the reduction of PdO and the ceria surface.
Based on the above analyses,co-catBa prepared by co-precipitation has a better redox property than im-catBa prepared by impregnation.Different from the co-catBa samples with structural modification,there are two kinds of activity species in the im-catBa sample,such as highly dispersed PdO and the Pd-Ce species formed in the Pd-Ce interface.
2.4 NH3-TPD
Fig.3 shows the NH3-TPD of the samples doped by BaO with different methods.The desorption peak of the cat0 sample not only has the most wide temperature range from 100℃to 700℃,but also has the largest peak area.In addition,the NH3-TPD profileofun-dopedsampleexhibitsthree distinguished desorption peaks α,β and γ at 180,250and 500℃,respectively,which can be ascribed to the NH3desorption of weak acid sites,middle strong acid sites and strong acid sites on the surface of aluminumcontaining samples.

Fig.3 NH3-TPD profiles of Pd/CZLA doped by BaO with impregnation/co-precipitation
As for im-catBa and co-catBa,both the peak temperature and the peak area decrease,and the middle strong acid sites and strong acid sites nearly fade away. This is because that BaO is a basic alkaline compound. The addition of alkaline earth metal oxides will first neutralize some strong and middle strong acid sites on the surface,and then neutralize the weak acid sites, resulting in a decline in the surface acidity.Moreover, the peak area of co-catBa is smaller than that of imcatBa.This may be due to the following reasons:(1)the surface acid sites of catalysts are mainly determined by the number of hydroxyl groups on the surface of aluminum and the aluminum atoms,the more hydroxyl groups and aluminum atoms,the more H+and empty electron orbital[28].Combined with the results of XRD and H2-TPR,it can be inferred that co-precipitation method,leading to almost all of Ba2+ions into the CZ lattice,forming homogeneous solid solution,will result maximumneutralizationforallacidsites.The impregnation method only modifies the surface of the catalyst by promoting high dispersion of the PdO,which must neutralize part of the surface acid sites;(2) Furthermore,the amount of surface acidity vary in the order of cat0>im-catBa>co-catBa.This result corresponds well with the trend of BET specific surface area.According to the principle of NH3adsorption/ desorption,the higher surface area for a catalyst,the more surface acid sites.So,the decrease in surface area of im-catBa and co-catBa catalysts is a crucial factor leading to the decline of surface acid sites.The cocatBa has lesser amount of surface acidity than imcatBa.
2.5 TEM
TEM images of the catalyst after doping BaO by impregnation/co-precipitation method are shown in Fig. 4.From Fig.4(a)and(b),the average sizes of Pd particle for co-catBa and im-catBa are about 2 and 4 nm, respectively.The dispersion of Pd particles of co-catBa is seemingly higher than that of im-catBa.This may be due to the formation of homogeneous solid solutions as implied by XRD result,which leads to a more compact bond between Pd and the support.So the more highly dispersed Pd particles,the higher reducibility as proved by Wang et al[24].This conclusion is in good agreement with the result of H2-TPR in this work.Therefore,it can be concluded that doping with BaO by co-precipitation is more conductive to the dispersion of Pd particles than by impregnation.

Fig.4 TEM images of Pd/CZLA doped by BaO with impregnation/co-precipitation
2.6 XPS
Fig.5 (a)and(b)show the XPS spectra of Pd3d and Ce3d after treatment by XPSPEAK,respectively. Table 2 summarizes the binding energy(BE)values and the surface atomic ratios calculated from XPS.

Fig.5 Pd3d(a)and Ce3d(b)XPS spectra of catalysts

Table2 Binding energy and surface composition results of three catalysts
As shown in Table 2 and Fig.5(a),the binding energies of the Pd3d5/2electrons of all catalysts fall in the range of 336.4~337.0 eV.Recent XPS reference values for the binding energies of PdO and metallic Pd0are 336.8 and 335.2 eV,respectively[29].The bindingenergyofcat0Pd3d5/2is336.4eV, significantly higher than that of Pd0,but lower than that of PdO.It indicates that Pd exists in a partly oxidized state.Compared with the cat0,the Pd3d peaks of im-catBa and co-catBa shift to higher BE by 0.5~0.6 eV.As reported in Ref.[30],the addition of BaO could increase the electron density around PdO as an electron donor,resulting in a decrease in the binding energy value,which is in contrast to our results.In this study,the surface atomic ratios of Pd on the cat0,im-catBa and co-catBa are 0.22%,0.41% and 0.79%,respectively.According to the results of H2-TPR and TEM,it can be speculated that the binding energy shift is related with the increase of the Pd species dispersion.Furthermore,the Ce3d5/2BE values of im-catBa and co-catBa are all enhanced from881.8eVto882.5eVand882.2eV, respectively.Usually,thisphenomenoncanbe understood as the strong metal-support interaction (SMSI)effect[31].Unlike co-catBa,the im-catBa sample has more obvious increase for the Ce3d5/2BE values by 0.7 eV.This result implies that impregnation method is more beneficial to promote the Pd-Ce interaction than co-precipitation method.SMSI could change the surface chemical surrounding of PdO and CeO2forming chemical bonding such as Pd-O-Ce in the interface of palladium particles and the support. This is consisted with the result of H2-TPR.
Fig.5 (b)shows the Ce3d XPS spectra of three samples.The peaks for Ce3d are complex,and they aresplitintotheCe3d5/2andCe3d3/2spin-orbit component of cerium ion.The peaks are assigned as V,V″and V″′for Ce3d5/2,while the corresponding Ce3d3/2peaks are labeled as U,U″and U″′[32]. According to the literatures[32-33],the peaks at V′,U″represent the presence of Ce3+,while characteristic peaks of Ce4+present at V,V″,V″′,U,U″,U″′.As seen from(b),all samples display characteristic peaks of Ce3+and Ce4+,which indicates that combination of Ce3+and Ce4+for cerium species coexists in the samples.The concentrations of surface Ce3+in the samples,obtainedbycalculatingtherelative integrated areas under the curve of each deconvoluted peaks,are shown in Table 2.From Table 2,the concentration of surface Ce3+over co-catBa is 26.57%, higherthanthatofim-catBa(25.84%).Theconcentrations of surface Ce3+vary in the order of cocatBa>im-catBa>cat0.The higher the Ce3+concentration is,the more Ce3+/Ce4+redox couples are. Therefore,the materials with higher Ce3+concentration will possess better redox property.This observation agrees well with the H2-TPR results.
In addition,the quantitative XPS analysis(Table 2)shows a higher concentration of Ce,Zr,especially Al and La ions on the surface of im-catBa than that of cat0andco-catBa.Itfurthersubstantiatesthe formation of Ba2AlLaO5compound oxides.Moreover, the higher concentration of Ce and Zr ions on the surface of co-catBa may be related to the substitution Ba2+for Ce4+and Zr4+ions corresponding to the lattice expansion.These results have been confirmed by XRD.
2.7 Catalytic performance of catalysts
The catalytic activities of cat0,im-catBa and cocatBa catalysts for conversion of methanol,CO,C3H8and NO in the simulated exhaust gas are shown in Fig.6(a),(b),(c),(d).As seen from Fig.6,the conversion of methanol,CO,C3H8and NO over all catalysts increases continuously with the raising of temperature.Compared(a)and(b),it can be seen that the catalytic activity of co-catBa is lower for methanol conversion,but much higher for CO conversion than that of im-catBa.The data of light-off temperature(T50) and complete-conversion temperature(T90)obtained from Fig.6 are listed in Table 3.(T50and T90are used to evaluate the performances of catalysts.The T50and T90are the temperature at which a given pollutant conversion reaches 50%and 90%,respectively.)Compared with cat0,the T50of CO over co-catBa is 140℃, while over im-catBa is 160℃.These phenomena probably relate to the adsorption competition between CO and CH3OH on the surface of the catalyst.The interaction between the molecular CO and Pd atom can result in strongly adsorbed CO on the surface of PdcatalystandtheformationofthePd-CO complexes.The strong adsorption of CO on Pd catalyst isunfavorableformethanoloxidationsincea dominating adsorption of CO is achieved during the competition adsorption process[34].From H2-TPR,TEM and XPS characterizations,the co-catBa catalyst hasthe best reducibility,more highly dispersion,maximum surface Ce3+concentration and the most surface atomic ratio of Pd.So,the catalyst co-catBa has the best catalytic activity for CO conversion[35].Differently,the best catalytic activity for CH3OH conversion over the im-catBa catalyst may be related to the chemical bond Pd-O-Ce formed in the Pd-CZ interface.The doping of CZ into Pd catalyst will generate plenty of oxygen adspecies on the Pd/CeO2interface[36],which can be desorbed and participate in the methanol oxidation at lower temperature.Moreover,Arosio et al.[37]demonstrated that the interaction of Pd-Ce bond could contribute to the catalytic activity of Pd/CeO2for the methane oxidation.So it can be inferred that the higher activity for methanol conversion over the imcatBacatalystisrelatedwiththestrongPd-Ce interaction.
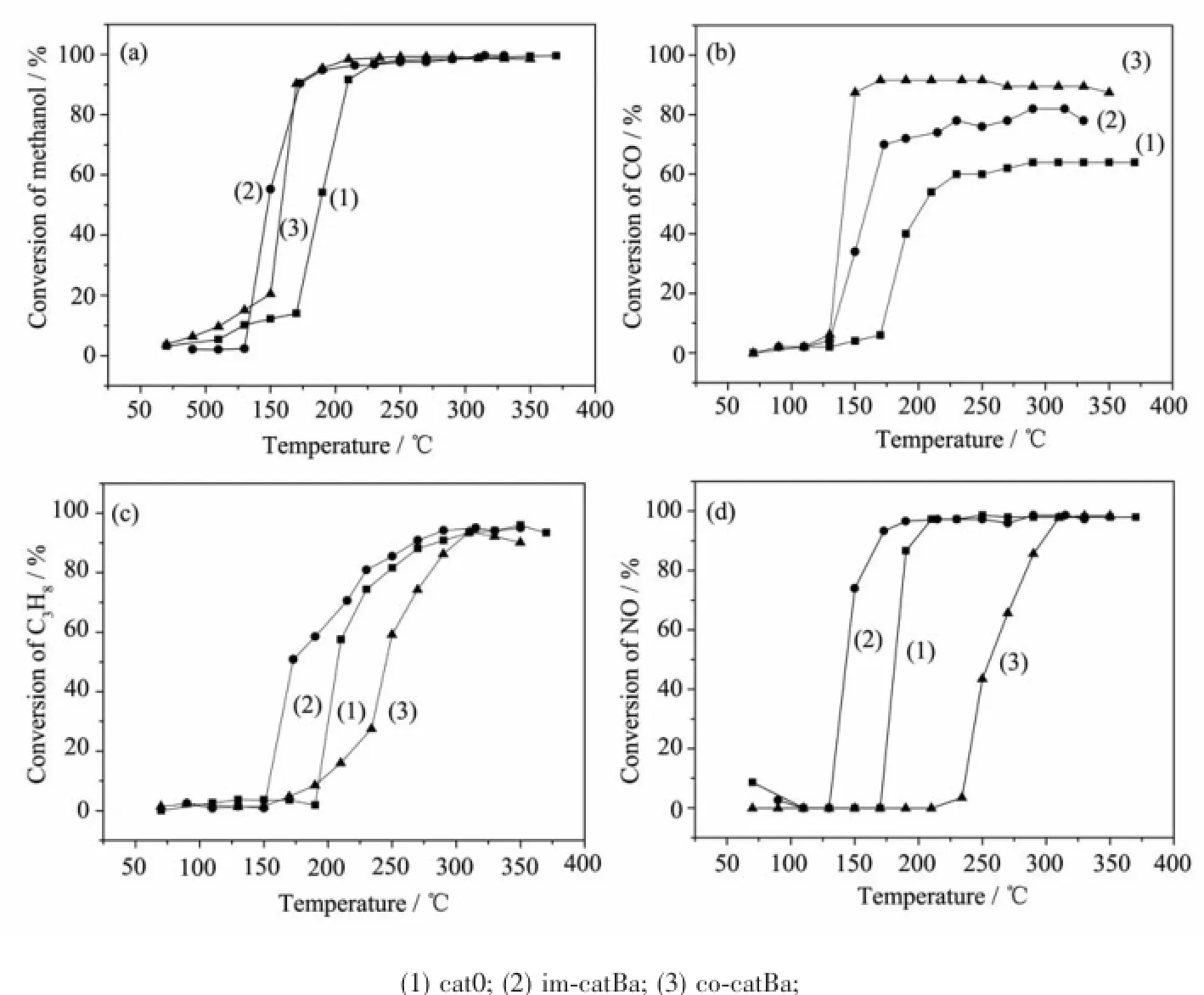
Fig.6 Conversion of methanol(a),CO(b),C3H8(C),NO(d)as a function of reaction temperature under stoichiometric CH3OH+CO+C3H8+NO+O2
Furthermore,we focus on studying C3H8and NO, which are the most difficult to convert in exhaust gases from gasoline-methanol vehicles.As seen from Fig.6(c),(d)and Table 3,the trend of catalytic activity for C3H8conversion is consistent with NO conversion. The conversion of C3H8and NO varies in the order of im-catBa>cat0>co-catBa.This may be ascribed to the propane-assisted decomposition of NO.Compared with the catalyst cat0,the T50of C3H8and NO over the catalyst im-catBa decreases by 31,35℃,but the T50increases by 35,75℃over the catalyst co-catBa.It is obvious that the addition of BaO with the impregnation method can be more effective to improve the catalytic activity,and with the co-precipitation method isnegative.TheactivityforNOreductionis dependent upon the amount of acidity,not just its strength,when saturated hydrocarbons are used as the reducing agent[38].Similarly the surface acidity of catalysts decreases due to the doping of alkaline earth metals,whichcausesadecreaseinpropane conversion[28].Soitisspeculatedthatthecoprecipitation method resulting the lowest acidity is the important reason leading to the decrease of the C3H8and NO conversion.
However,im-catBa has less total acidity,but has better catalytic activity for C3H8and NO conversion. This may be resulted from following reasons:1)it is generally accepted that the NOx storage takes place on multiple types of barium sites which have different activities toward NOx storage reduction[39].BaO on the Pd-Ba-OSC/Al2O3catalyst surface can increases the amount of active sites for NOreactions at low temperature as suggested by Tanja et al[40].So it can be considered that the formation of Ba2AlLaO5phases may be beneficial to the conversion of NO;2)Pd ions areactivesitesforNO,C3H8adsorptionand activation.So the more surface enrichment of Pd species,the higher catalytic activity.On the other hand,the Pd-CZ interface where exists additional sites for oxidant(NO)activation has a direct effect on the NO de-oxidation and C3H8oxidation[41-42].From H2-TPR and XPS characterizations,the catalyst im-catBa has more surface enriched Pd species and the Pd-O-Ce species on the Pd-CZ interface due to the surface modification.These may be the crucial factors leading to the excellent catalytic activity towards NO and C3H8.
Based on the above analyses,the redox property and highly dispersed PdO species have an important impactonthecatalyticperformanceforCO conversion.ThePd-CeinteractioninthePd-Ce interface may be the primary factor leading to the excellent catalytic activity towards methanol,NO and C3H8conversion.

Table3 Light-off(T50)and complete-conversion temperature(T90)of methanol,CO,C3H8and NO over catalysts
3 Conclusions
The addition of BaO to Pd-based catalyst by impregnation/co-precipitation method greatly improves thetextural,redoxpropertiesandeffectively strengthens the metal-support interaction.The Pd-Ba catalysts exhibit better catalytic performance,and imcatBa is superior to co-catBa.Different synthesis methods modify Pd-based catalyst in different ways. Co-precipitation method is mainly based on the lattice modification when some Ce4+cations are substituted by Ba2+,causing structure disorder and additional anionvacancies.So,co-precipitationmethodwill cause the formation of more Ce3+,accompanied by the creation of more Ce3+/Ce4+redox couples,which leads to a better redox property.The redox property of the catalysthelpstheCOconversion.However, impregnation method is mainly based on the surface modification.The enrichment of dispersed Ba2+on the surface of the catalyst will promote high dispersion of PdO species on the surface of the support,especially willstrengthenthePd-CeinteractioninPd-Ce interfaceofPd-O-Cespecies.ThestrongPd-Ce interaction may be beneficial to the conversion of methanol,C3H8and NO.
[1]Kowalewicz A,Wojtyniak M.Proc.Inst.Mech.Eng.J.Autom. Eng.,2005,219:103-125
[2]Cenk S,Kadir U,Mustafa C.Renew.Energy,2008,33:1314 -1323
[3]McCabe R W,Mitchell P J.Appl.Catal.,1986,27:83-98
[4]Mondelli C,Santo D V,Trovarelli A,et al.Catal.Today, 2006,113:81-86
[5]Monte D R,Kaspar J,Fornasiero P,et al.Inorg.Chim.Acta, 2002,334:318-326
[6]Kenevey K,Valdivieso F,Soustelle M,et al.Appl.Catal.B: Environ.,2001,29:93-101
[7]Liotta L F,Longoa A,Macaluso A,et al.Appl.Catal.B: Environ.,2004,48:133-149
[8]Magdalena K,Elbieta T,Bogusaw M,et al.Appl.Catal.A: General,2012,445-446:280-286
[9]Hungría A B,Browning N D,Erni R P,et al.J.Catal., 2005,235:251-61
[10]Osorio G P,Moyado S F,Petranovskii V,et al.Catal.Lett., 2006,1/2:110-116
[11]Vidmar P,Fornasiero P,KaŠpar J,et al.J.Catal.,1997,171: 160-168
[12]Tanja K,Ulla L,Katariina R T,et al.Appl.Catal.A: General,2006,298:65-72
[13]Groppi G,Cristiani C,Lietti L,et al.Catal.Today,1999,50: 399-412
[14]AtribakI,BuenoLA,GarcíaGA.J.Catal.,2008,259:123-132
[15]Martínez A A,Fernández G M,Hungría A B,et al.Catal. Today,2007,126:90-105
[16]Thammachart M,Meeyoo V,Risksomboon T,et al.Catal. Today,2001,68:53-60
[17]Damyanova S,Bueno J M C.Appl.Catal.A:General,2003, 253:135-141
[18]Laurent S,Forge D,Port M,et al.Chem.Rev.,2008,108: 2064-2067
[19]Corbos E C,Courtois X,Bion N,et al.Appl.Catal.B: Environ.,2008,80:62-71
[20]Li G F,Wang Q Y,Zhao B,et al.J.Mol.Catal.A:Chem., 2010,326:69-74
[21]Corbos E C,Courtois X,Bion N,et al.Appl.Catal.B: Environ.,2007,76:357-367
[22]Piacentini M,Maciejewski M,Baiker A.Appl.Catal.B: Environ.,2006,66:126-136
[23]Sun K P,Lu W W,Wang M,et al.Appl.Catal.A:General, 2004,268:107-113
[24]Wang Q Y,Li G F,Zhao B,et al.J.Hazard.Mater., 2011,189:150-157
[25]Yamazaki S,Matsui T,Ohashi T,et al.Solid State Ionics, 2000,136-137:913-919
[26]Mikulova J,Rossignol S,Gerard F,et al.J.Solid State Chem.,2006,179:2511-2519
[27]Feio L S F,Hori C E,Damyanova S,et al.Appl.Catal.A: General,2007,316:107-116
[28]HE Shen-Nan(何胜楠),SHI Zhong-Hua(史忠华),CHEN Yao-Qiang(陈耀强),et al.Acta Phys.-Chim.Sin.(物理化学学报),2011,27(5):1157-1162
[29]Voogt E H,Mens A J M,Gijzeman O L J,et al.Surf.Sci., 1996,350:21-31
[30]YAO Yan-Ling(姚艳玲),FANG Rui-Mei(方瑞梅), SHI Zhong-Hua(史忠华),et al.Chin.J.Catal.(催化学报), 2011,32:589-594
[31]Zhao M,Li X,Zhang L H,et al.Catal.Today,2011,175: 430-434
[32]Larachi F,Pierre J,Adnot A,et al.Appl.Surf.Sci.,2002,195:236-245
[33]Hungría A B,Fernández G M,Anderson J A,et al.J.Catal., 2005,235:262-271
[34]Wang J A,Aguilar R G,Wang R.Appl.Surf.Sci.,1999, 147:44-51
[35]Corbos E C,Courtois X,Bion N,et al.Appl.Catal.B: Environ.,2007,76:357-367
[36]Luo Y J,Xiao Y H,Cai G H,et al.Fuel,2012,93:533-538
[37]Arosio F,Colussi S,Trovarelli A,et al.Appl.Catal.B: Environ.,2008,80:335-342
[38]Armor J N.Catal.Today,1996,31:191-198
[39]Yang M,Li Y P,Wang J,et al.J.Catal.,2010,271:228-238
[40]Tanja K,Ulla L,Katariina R T,et al.Appl.Catal.A: General,2006,298:65-72
[41]Peng N,Zhou J F,Chen S H,et al.J.Rare Earths,2012,30: 342-349
[42]Fernández G M,Martnez A A,Iglesias J A,et al.Appl. Catal.B:Environ.,2001,31:39-50
BaO Modified Pd-Based Catalysts:Synthesis by Impregnation/Co-Precipitation and Application in Gasoline-Methanol Exhaust Purification
ZHANG Xue-Qiao1,2TIAN Hao-Qi1YE Zhi-Xiang1CHEN Yao-Qiang*,2
(1College of Resources and Environment,Chengdu University of Information Technology,Chengdu 610225,China)
(2Institute of Catalytic Material Science,Sichuan University,Chengdu 610064,China)
Barium oxide was developed to modify palladium catalysts supported on CeO2-ZrO2-La2O3-Al2O3(CZLA) compound oxides by impregnation/co-precipitation methods.Low temperature N2adsorption-desorption,X-ray diffraction(XRD),H2-temperature-programmed reduction(H2-TPR),NH3-temperature programmed desorption(NH3-TPD),transmission electron microscopy(TEM)and X-ray photoelectron spectroscopy(XPS)were employed to characterize the influence of the preparation method on physicochemical properties of the catalyst.Catalytic activity performance for methanol,CO,C3H8and NO conversion was evaluated.Catalytic activity results show that the addition of BaO has a positive effect on the conversion of all pollutants,and the best results are achieved by the impregnation method.The light-off temperature decreases by 43,31,45 and 35℃,respectively.The XRD,H2-TPR and XPS results confirm that the impregnation method is mainly based on the surface modification.The enrichment of Ba2+strengthens the Pd-Ce interaction in Pd-Ce interface,promoting the reductive ability,thus increasing the catalytic activity at low temperature.The co-precipitation method results in structure disorder and additional anion vacancies accompanied by the formation of more Ce3+,which may be beneficial to the conversion of CO.
barium oxide;palladium;ceria;methanol;catalytic activity removal
O643.36+1;O643.31
A
1001-4861(2015)01-0166-11
10.11862/CJIC.2015.002
2014-04-24。收修改稿日期:2014-09-08。
国家自然科学基金(No.51408076,11405113),四川省教育厅重点科研基金(No.14ZA0163),成都信息工程学院科研人才基金(No.J201416)资助项目。
*通讯联系人。E-mail:nic7501@scu.edu.cn,Tel:+86 28 85418451;会员登记号:S06N4556M1006。
