Anti-depression effects of electroacupuncture through up-regulating serum E2and BDNF and expression of BDNF in hippocampus in chronic depression rats
2015-05-18MuDaozhou穆道周HuangXi黄熙
Mu Dao-zhou (穆道周), Huang Xi (黄熙)
1 The First Clinical Medical College of Nanjing University of Chinese Medicine, Nanjing 210023, China
2 Basic Medical College of Nanjing University of Chinese Medicine, Nanjing 210023, China
Basic Study
Anti-depression effects of electroacupuncture through up-regulating serum E2and BDNF and expression of BDNF in hippocampus in chronic depression rats
Mu Dao-zhou (穆道周)1, Huang Xi (黄熙)2
1 The First Clinical Medical College of Nanjing University of Chinese Medicine, Nanjing 210023, China
2 Basic Medical College of Nanjing University of Chinese Medicine, Nanjing 210023, China
Objective:To investigate the effects of electroacupuncture (EA) at acupoints Baihui (GV 20), Neiguan (PC 6) and Sanyinjiao (SP 6) on the levels of estradiol (E2) and brain-derived neurotrophic factor (BDNF) in serum, as well as the expression of BDNF in hippocampus in chronic depression rat models.
Acupuncture Therapy; Electroacupuncture; Depression; Hippocampus; Brain-derived Neurotrophic Factor; Estradiol; Rats
Previous researches have shown that serum estradiol (E2) and brain-derived neurotrophic factor (BDNF) decrease in depression and increase after treatment[1-2]. In the central nervous system (CNS), there are widespread and diverse interactions between E2and BDNF, and they seem to share common targets and effects. There is no clear biological marker for depression yet. A recent study suggests that plasma BDNF might be a peripheral marker for the action mechanism of antidepressant agents in humans[3]. Increased variability of E2around the woman’s own mean level is strongly associated with new onset of depressed mood among women with no history of depression[4]. E2can ease anxiety and depressive behaviors of ovariectomized rats via estrogen receptor beta (ERβ) in the hippocampus[5]and induce synaptogenesis between mossy fibers and cornu ammonis 3 (CA3) neurons by enhancing BDNF release from dentate gyrus (DG) granule cells in a nuclear estrogen receptor (NER)-independent and protein kinase A (PKA)-dependent manner[6]. The antidepression effects of electroacupuncture (EA) have been proven both clinically and in laboratory[7-8]. However, the mechanism is still not clear. Whether serum E2, BDNF and the expression of BDNF in hippocampus contribute to the anti-depression effects of EA hasn’t confirmed yet. In this study, the effects of EA at Baihui (GV 20), Neiguan (PC 6) and Sanyinjiao (SP 6) on serum E2and BDNF as well as the expression of BDNF in hippocampus in chronic depression rats were investigated.
1 Materials and Methods
1.1 Animals
Thirty adult female Wistar rats (weighing 180-240 g, offered by Experimental Animal Care Center of Nanjing Medical University) were allowed to acclimatize in cages for 7 d prior to experiments. All the experiments were conducted in accordance with all relevant guidelines and legislations to minimize pain and suffering of the animals. A 12-hour light/dark cycle was used (lights on: 7:00-19:00; lights off: 19:00-7:00). The temperature was 21-25 ℃ and humidity was (55±2)%.
Rats with the score of horizontal plus vertical movement >120 or <30 were excluded from further experiments. Thirty eligible rats were enrolled and randomly divided into three groups according to their weight. The three groups were normal control (NC) group, model group, and EA group, 10 rats in each group. The depression model was established by chronic unpredicted mild stimulus (CUMS) combined with individual caging for 21 d.
1.2 Depression model and EA treatment
Rats in the model group and EA group were caged individually. Stress was applied consecutively for 21 d using different mild stressors. There were altogether 9 kinds of stressors, including tail clamping for 1 min, cold water swim (14 ℃, 5 min), high-temperature stress in oven (45 ℃, 5 min), water deprivation for 24 h, electric shock to foot (voltage 30 V, 1 min), shaking the cage horizontally (160 times/min, 25 min), moist bedding for 24 h, food deprivation for 24 h and reversed 12-hour light/dark cycle (lights off: 7:00-19:00; lights on: 19:00-7:00) for 24 h. Each stimulation was applied 2-3 times. The rats in the model group and EA group were randomly exposed to one of the nine stressors each day and caged individually. The EA group was then treated with EA at Baihui (GV 20), Neiguan (PC 6) and Sanyinjiao (SP 6) once daily for 14 d, 25 min each time. Sparsedense wave was adopted for EA treatment, and bilateral Neiguan (PC 6) and Sanyinjiao (SP 6) were connected to the EA apparatus alternately. Rats in the model group were bounded in the same way as those in the EA group but not treated with EA. The NC rats were kept without any stress but behavior test during the experiment, with free access to water and food.
1.3 Behavior observation
1.3.1 Sucrose preference test
Sucrose preference test was performed according to Willner P[9]. The sucrose preference test was conducted over a 24-hour period of time using a two-bottle test, one with 1% sucrose solution and the other with water. The positions of the bottles were randomly chosen. The sucrose solution and water consumptions were determined by measuring the volume of the fluid. Sucrose preference was calculated as the percentage of ingested sucrose solution relative to the total liquid consumption.
1.3.2 Open-field (OF) test
The OF test was used to observe the exploratory behavior and emotional responses[10]. The OF box was a 100 cm × 100 cm × 50 cm carton. The bottom area was divided into 20 cm × 20 cm squares. The squares along the side were taken as peripheral squares and the rest as central squares. A rat was placed in the center of the OF box and observed for 5 min. The following indices were observed and recorded by a person who did not know the test objectives: number of squares crossed as the horizontal movement score (defined by numbers of time when four paws crossing the nearby squares, a square as one score), vertical movement score (indexed as both forelimbs raising at least 1 cm above the ground, one time as one score). Then the horizontal score and vertical score were put together as the OF score.
1.4 Serum E2and BDNF
Serum E2was measured by radioimmunoassay and BDNF by enzyme-linked immunosorbent assay (ELISA) test. Briefly, rats were anesthetized with 3.6% chloral hydrate at the end of the experiment. The thorax wall was then opened and the heart was exposed. Blood samples were drawn from the right ventricular. Approximately 3 mL blood was collected from each rat. All blood samples were immediately placed on ice for 30 min and then centrifuged at 3 000 rpm for 10 min. Serum was separated and stored at -70 ℃ until it was thawed for assay. Serum E2was measured by radioimmunoassay and BDNF was assessed using a BDNF ELISA kit (Shanghai HORA Biological Technology Co., Ltd., Shanghai, Batch No. 201008247) according to the manufacturer’s instruction.
1.5 BDNF in hippocampus
The animals were anesthetized with 3.6% chloral hydrate (1 mL/100 g) by intraperitoneal injection. The thorax wall was opened and the heart was exposed. Aortic cannulation was performed and the right auricle was cut open. A total of 100 mL normal saline (4 ℃) was used to wash out blood, followed by 4% paraformaldehyde (4 ℃, pH adjusted to 7.4) 350 mL perfusion to fix the tissues for 30 min. The skull was then opened and the brain was removed. The brain was fixed again in 4 % paraformaldehyde for one week. The brain was further sliced into 4 μm sections to perform hematoxylin-eosin (HE) staining and BDNF immunehistochemistry assays. Mean optical density (MOD) was used to analyze the picture.
1.6 Statistical analysis
Measurement data of normal distribution were shown as mean ± standard deviationand evaluated by one-way analysis of variance (ANOVA), then if appropriate, by individual comparisons with the control using the post-hoc SNK and LSD test. As for the sucrose preference, because rats in the NC group were raised at 5 per cage, and the five rats in a cage were considered as a whole when sucrose preference rate was measured, there are only two numbers in the NC group, and the data were shown as. One sample t-test was used and the mean value of NC group was taken as test value to compare with those of the EA group and model group, while two independent samples t-test was used to compare the EA group with the model group. Tests were two tailed. P<0.05 indicates a statistical significant difference.
2 Results
2.1 Behavioral response
2.1.1 Sucrose preference test
Rats undergoing stress showed a decreased sucrose preference rate following the stress period compared with the NC group, and a rat in the model group died in the process of stress protocol. This decrease was statistically significant (model group: t=11.61, P<0.01; EA group: t=15.24, P<0.01). But in the EA group, after 14-day treatments with EA, sucrose preference rate increased significantly compared with that in the model group (t=11.27, P<0.01), (Table 1).
2.1.2 OF test
There was no significant difference among the three groups before experiment in OF test (P>0.05). At the end of test, differences among the three groups were statistically significant (F=86.43, P<0.01). Compared with the NC group, the model group and EA group had significantly lower scores after 21-day stress (P<0.01). Compared with the model group, score of the EA group had significantly increased after treatment (P<0.01), (Table 2).
2.2 Serum E2and BDNF
Differences of serum E2and BDNF among the three groups were statistically significant (F=9.62, P<0.01; F=52.89, P<0.01). Compared with the NC group, the model group had significantly lower serum BDNF concentration (P<0.01). But a significant higher serum BDNF concentration occurred in the rats of EA group after EA treatment (P<0.05), (Table 3).
2.3 Expression of BDNF in the hippocampus
Differences in BDNF expression in hippocampus among the three groups were statistically significant (F=3.662, P<0.05). Rats in the model group showed significantly lower expression than rats in the NC group (P<0.05), while the expression of BDNF increased remarkably in the EA group compared with that in the model group (P<0.05), (Table 4, Figure 1 and Figure 2).
Table 1. Effects of EA on sucrose preference rate

Table 1. Effects of EA on sucrose preference rate
Note: Compared with the NC group, 1) P<0.01; compared with the model group, 2) P<0.01
Group n Pre-test After 21-day stress Post-treatment Model 9 88.16±2.76 52.94±9.101) 52.81±8.611)EA 10 86.67±3.35 52.72±7.351) 86.78±2.912)NC 10 88.59 88.15 88.55
Table 2. Effects of EA on OF test scores (horizontal score plus vertical score
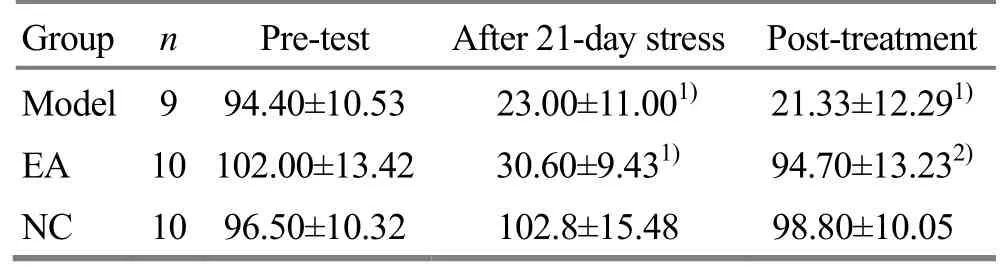
Table 2. Effects of EA on OF test scores (horizontal score plus vertical score
Note: Compared with the NC group, 1) P<0.01; compared with the model, 2) P<0.01
Group n Pre-test After 21-day stress Post-treatment Model 9 94.40±10.53 23.00±11.001) 21.33±12.291)EA 10 102.00±13.42 30.60±9.431) 94.70±13.232)NC 10 96.50±10.32 102.8±15.48 98.80±10.05
Table 3. Effects of EA on serum E2and BDNF levels

Table 3. Effects of EA on serum E2and BDNF levels
Note: Compared with the NC group, 1) P<0.01; compared with the model group, 2) P<0.05
Group n E2(pg/mL) BDNF (pg/mL) Model 9 68.24±4.941) 74.20±6.321)EA 10 72.19±5.41 84.75±8.182)NC 10 75.12±5.14 115.38±11.80
Table 4. Effects of EA on the expression of BDNF
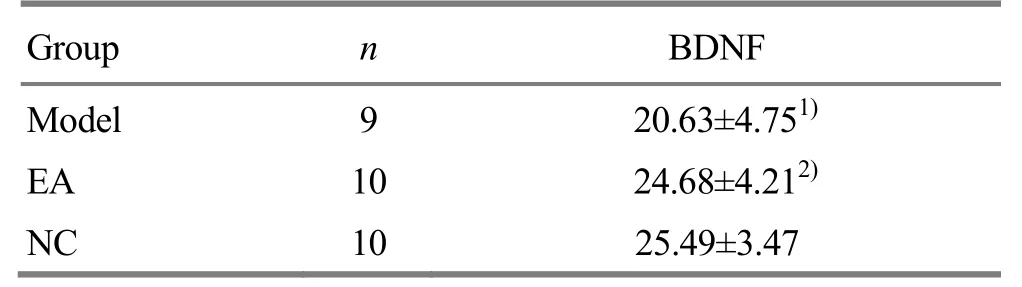
Table 4. Effects of EA on the expression of BDNF
Note: Compared with the NC group, 1) P<0.01; compared with the model group, 2) P<0.05
Group n BDNF Model 9 20.63±4.751)EA 10 24.68±4.212)NC 10 25.49±3.47
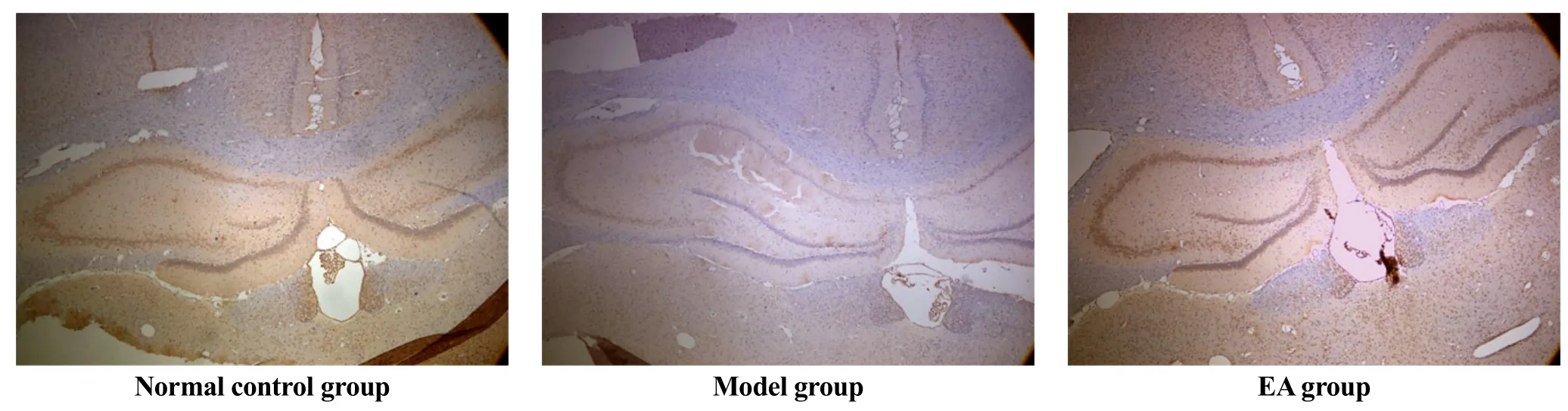
Figure 1. Effects of EA on expression of BDNF in rats’ hippocampus (immunohistochemistry, ×40)
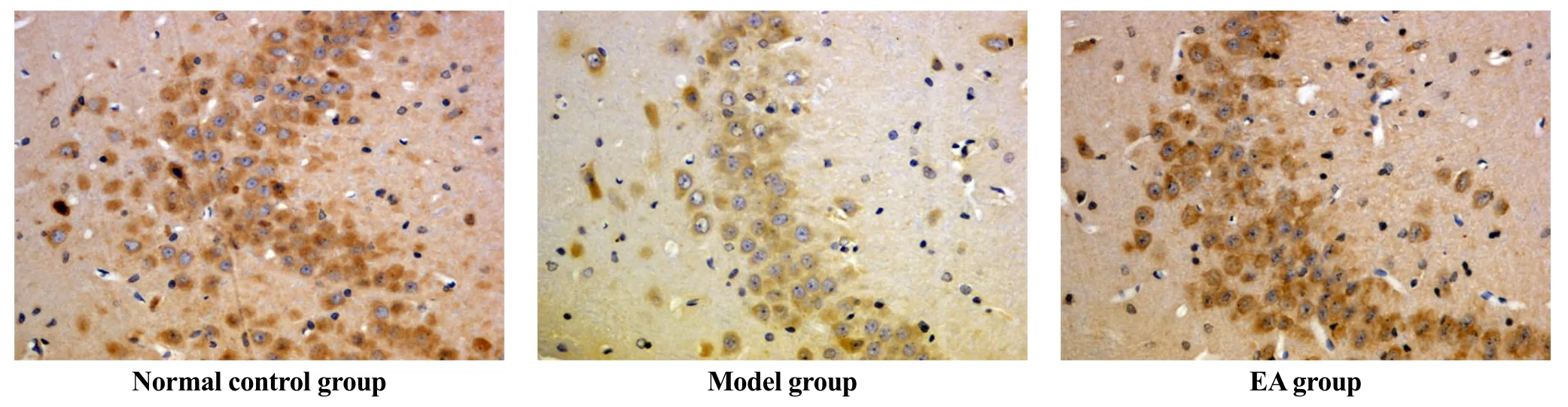
Figure 2. Effects of EA on expression of BDNF in rats’ hippocampus (immunohistochemistry, ×400)
3 Discussion
Psychosocial stress is one of the factors that may impact multiple neurobiological systems relevant to major depressive disorder[11]. CUMS model for depression has been proven to be valid and can be used to study problems that are extremely difficult to address by other means[12]. Previous research showed that chronic stress depression model is superior to other models[13]and unpredictable chronic stress can decrease BDNF expression in the hippocampus of rats[14]. In this study, CUMS combined with individual caging was used to establish depression model to study the effect of EA on depression. Consistent with Zheng H, et al[15]and Duan DM, et al[8], we found that sucrose preference rates and OF scores decreased significantly in the model group and EA group after stress period, while they increased remarkably in the EA group after EA treatment, suggesting that depression model is successfully established, and EA treatment reduced depression-like behavior. In addition, E2and BDNF in serum, as well as expression of BDNF in hippocampus were remarkably lower in the model group. Furthermore, EA treatment significantly increased BDNF expression in serum and hippocampus. We hypothesize that EA can produce anti-depression effect by promoting BDNF expression.
It has been implied that BDNF and E2that have similar effects in this region of brain, they are the two factors associated with depression. and there are complex interactions between them in hippocampus. Tri-molecular cascade, estrogen-BDNF-NPY, may be important in understanding the hormonal regulation of hippocampal function[16]. Oral corticosterone (CORT) exposure can lead to anhedonic-like behavior in mice but direct hippocampal BDNF microinfusion can mimick chronic antidepressant treatment (ADT) by reversing this CORT-induced anhedonic-like behavior[17]. Expression of BDNF and BDNF serum level are related[18]. The above studies indicate that BDNF and E2are two factors which contribute to depression and can exert anti-depression effects. This research shows that serumE2level and the expression of BDNF both in serum and hippocampus were remarkably lower in the model group than in the control group, while rats in EA group had significantly increased serum BDNF and expression of BDNF in hippocampus as well as improved behavioral response. Serum E2level rose in the subjects in EA group after EA treatment but not significantly, which may be because that the 14-day EA duration is not long enough. These results have indicated that both BDNF and E2contribute to depression and they have similar changes in depression pathology and rehabilitation and are consistent with researches in the similar field. They also prove that EA may produce an anti-depression effect through regulating serum BDNF and expression of BDNF in hippocampus of rats, while serum E2may also relate to the anti-depression effect of EA.
As limited by the experimental conditions, we didn’t analyze the relationship among serum E2, BDNF and expression of BDNF in hippocampus during stress period and EA treatment. To offer experimental evidence that whether serum BDNF can be a peripheral marker for depression and the action mechanism of antidepressants, further investigation on how serum BDNF is related to the expression of BDNF in hippocampus and its dynamic changes in depression pathology and rehabilitation in both animal and human should be conducted. Relationship between serum E2and BDNF in depression will also be further studied.
Conflict of Interest
There was no conflict of interest in this article.
Acknowledgments
This work was supported by Natural Science Key Research Project of Education Department of Anhui Province (安徽省教育厅自然科学重点科研项目,No. KJ2008A42ZC).
Statement of Informed Consent
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 25 October 2014/Accepted: 28 November 2014
[1] Sen S, Duman R, Sanacora G. Serum BDNF, depression and anti-depressant medications: meta-analyses and implications. Biol Psychiatry, 2008, 64(6): 527-532.
[2] Luo Y, Zheng LZ, Zhou JW. Research on relationship between post partum depression and plasma estrogen, monoamine concentration. Zhonghua Fuchanke Zazhi, 2007, 42(11): 745-748.
[3] Lee HY, Kim YK. Plasma brain-derived neurotrophic factor as a peripheral marker for the action mechanism of antidepressants. Neuropsychobiology, 2008, 57(4):194-199.
[4] Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry, 2006, 63(4): 375-382.
[5] Walf AA, Frye CA. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol Biochem Behav, 2007, 86(2): 407-414.
[6] Sato K, Akaishi T, Matsuki N, Ohno Y, Nakazawa K. Beta-estradiol induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells. Brain Res, 2007, 1150: 108-120.
[7] Kim H, Park HJ, Han SM, Ohno Y, Nakazawa K. The effects of acupuncture stimulation at PC 6 (Neiguan) on chronic mild stress-induced biochemical and behavioral responses. Neurosci Lett, 2009, 460(1): 56-60.
[8] Duan DM, Tu Y, Chen LP. Efficacy evaluation for depression with somatic symptoms treated by electroacupuncture combined with Fluoxetine. Zhongyiyao Zazhi, 2009, 29(3): 167-173.
[9] Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl), 1997, 134 (4): 319-329.
[10] Sarkisova KY, Kulikov MA. Prophylactic actions of the antioxidant agent AEKOL on behavioral (psychoemotional) disturbances induced by chronic stress in rats. Neurosci Behav Physiol, 2001, 31(5): 503-508.
[11] Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009, 180(3): 305-313.
[12] Murua VS, Gomez RA, Andrea ME, Molina VA. Shuttle-box deficits induced by chronic variable stress: reversal by imipramine administration. Pharmacol Biochem Behav, 1991, 38(1): 125-130.
[13] Jia BH, Li ZG, Lu J. Research on model choice for analyzing the effect of electroacupuncture in antidepression. Zhen Ci Yan Jiu, 2005, 30(1): 23-25.
[14] Li Y, Ji YJ, Jiang H. Effects of unpredictable chronic stress on behavior and brain-derived neurotrophic factor expression in CA3 subfield and dentate gyrus of the hippocampus in different aged rats. Chin Med J, 2009, 122(13): 1564-1569.
[15]Zheng H, Liu YY, Li W, Yang B, Chen D, Wang X, Jiang Z, Wang H, Wang Z, Cornelisson G, Halberg F. Beneficial effects of exercise and its molecular mechanisms on depression in rats. Behav Brain Res, 2006, 168(1): 47-55.
[16] Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol, 2006, 27(4): 415-435.
[17] Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal BDNF restores motivational and forced swim performance after corticosterone. Biol Psychiatry, 2008, 64(10): 884-890.
[18] Karege F, Schwald M, Cisse M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci Lett, 2002, 328(3): 261-264.
电针上调慢性抑郁大鼠血清E2和BDNF及影响海马区BDNF表达的抗抑郁效果
目的:探讨电针百会、内关和三阴交穴对慢性抑郁模型大鼠血清雌二醇(estradiol, E2)和脑源性神经营养因子(brain-derived neurotrophic factor, BDNF)水平及海马区BDNF表达的影响。方法:将 30只雌性Wistar大鼠随机分为三组, 包括正常对照组, 模型组和电针组, 每组 10只。采用慢性中等程度不可预见性刺激(chronic unpredicted mild stress, CUMS, 如冷水游泳、夹尾、电击足底等)结合孤养21 d造模。模型组和电针组大鼠每天随机应用9种不同的刺激结合孤养。造模结束后, 电针组给予电针百会、内关、三阴交治疗, 每天一次, 共14 d;模型组给予同等程度束缚。血清 E2用放免法检测, 血清 BDNF用酶联免疫吸附试验法(enzyme-linked immunosorbent assay, ELISA)检测,海马BDNF的表达用免疫组化法。结果:应激结束后, 与正常对照组比较, 模型组和电针组糖水偏爱率显著下降(P<0.01),强迫游泳不动时间显著增加(P<0.01)。此外,电针显著减少了大鼠的抑郁样行为(P<0.01)。与正常对照组比较,模型组血清E2、BDNF水平及海马区BDNF的表达显著下降 (P<0.01,P<0.01,P<0.05)。与模型组比较, 电针组血清BDNF和海马区BDNF表达显著增加(P<0.05),血清E2水平增加但差异无显著性 (P>0.05)。结论:E2和BDNF可能与慢性轻度不可预见性应激大鼠抑郁样行为有关。电针可能通过增加血清和海马BDNF的表达发挥抗抑郁作用。
R2-03 【
】A
】针刺疗法; 电针; 抑郁; 海马; 脑源性神经营养因子; 雌二醇; 大鼠
Author: Mu Dao-zhou, doctoral candidate, nurse-in-charge
Huang Xi, M.D., professor, chief physician.
E-mail: tcmhuangx59@163.com
Methods:Thirty female Wistar rats were randomly divided into three groups, including a normal control (NC) group, a model group and an EA group, 10 rats in each group. The depression model was established by using chronic unpredicted mild stress (CUMS), such as cold-water swimming, tail clamping, electric shock to foot, etc., combined with individual caging for 21 d. Rats in the model group and EA group were randomly exposed to one of the 9 stressors each day and caged individually. After modeling, rats in the EA group were then treated with EA at Baihui (GV 20), Neiguan (PC 6) and Sanyinjiao (SP 6) once daily for 14 d, and the model group was not treated with EA but bounded in the same way as the EA group. Serum E2was measured by radioimmunoassay and BDNF was assessed by an enzyme-linked immunosorbent assay (ELISA) and the expression of BDNF in hippocampus was detected by using immunohistochemistry.
Results:After the stress stimulation, compared with the NC group, the model group and EA group showed a significant reduction of sucrose preference rate (P<0.01) and remarkable increase of forced-swimming immobility time (P<0.01). In addition, EA significantly reduced the depression-like behavior of rats in the EA group (P<0.01). The expressions of E2and BDNF in serum as well as the expression of BDNF in hippocampus were remarkably lower in the model rats than those in the NC group (P<0.01,P<0.01,P<0.05). The expression of BDNF in rats’ serum and hippocampus in the EA group was significantly higher than that in the model group (P<0.05), while serum E2increased but insignificantly (P>0.05).
Conclusion:E2and BDNF may contribute to the depression-like behaviors of the rats during CUMS period. EA may exert its anti-depression effects through promoting BDNF expression in serum and hippocampus.
【
猜你喜欢
杂志排行
Journal of Acupuncture and Tuina Science的其它文章
- Effects of wrist-ankle acupuncture on associated factors in uterus tissue and serum in rats with primary dysmenorrhea
- Clinical study on acupuncture combined with low-frequency electric stimulation for scissor gait in children with spastic cerebral palsy
- Observation on therapeutic effect of half puncture plus transcutaneous acupoint electric stimulation for infantile facial paralysis
- Tuina combined with needling distal points for pseudo-myopia in adolescents
- Clinical observation on regulating Conception Vessel and unblocking Governor Vessel by acupuncture combined with tuina for cerebral infarction
- Therapeutic efficacy observation on electroacupuncture for Alzheimer’s disease
