Development and evaluation of vinpocetine inclusion complex for brain targeting
2015-05-16JiaojiaoDing,JinfengLi,ShiruiMao
Development and evaluation of vinpocetine inclusion complex for brain targeting
Jiaojiao Ding,Jinfeng Li,Shirui Mao*
School of Pharmacy,Shenyang Pharmaceutical University,Shenyang 110016,China
ARTICLEINFO
Article history:
Received 2 May 2014
Received in revised form
15 July 2014
Accepted 21 August 2014
Available online 27 August 2014
Vinpocetine
A
The objective of this paper is to prepare vinpocetine(VIN)inclusion complex and evaluate its brain targeting effect after intranasal administration.In the present study,VIN inclusion complex was prepared in order to increase its solubility.Stability constant(Kc)was used for host selection.Factors in fl uencing properties of the inclusion complex was investigated.Formation of the inclusion complex was identi fi ed by solubility study and DSC analysis.The brain targeting effect of the complex after intranasal administration was studied in rats.It was demonstrated that properties of the inclusion complex was mainly in fl uenced by cyclodextrin type,organic acids type,system pH and host/guest molar ratio. Multiple component complexes can be formed by the addition of citric acid,with solubility improved for more than 23 times.Furthermore,In vivo study revealed that after intranasal administration,the absolute bioavailability of vinpocetine inclusion complex was 88%. Compared with intravenous injection,signi fi cant brain targeting effect was achieved after intranasal delivery,with brain targeting index 1.67.In conclusion,by intranasal administration of VIN inclusion complex,a fast onset of action and good brain targeting effect can be achieved.Intranasal route is a promising approach for the treatment of CNS diseases.
©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1. Introduction
Along with the acceleration of population aging,how to treat cerebrovascular diseases effectively is a great challenge. The blood brain barrier(BBB)represents an insurmountable obstacleforalargenumberofdrugsandisthemajorbottleneck in drug delivery to the brain[1,2].So far,lots of attempts have beenmadetoovercometheBBB,includingtheusageofcarriermediated transporters(CMT),receptor-mediated transporters (RMT),and nano-sized systems such as nanosuspension, nanoparticles and micelles with different administration routes[3].Among them,intranasal drug delivery is one of the focused delivery options for brain targeting.As a kind of noninvasive route,intranasal drug delivery has the advantages of rapid onset of action,good patient compliance,and avoiding hepatic fi rst pass effect with high bioavailability[4].Moreover, the brain and nose compartments are connected to each otherviatheolfactoryreceptorcells,theyaretheonlysurfaceneural cells of the body,so the olfactory mucosa could be considered asa‘windowtothebrain’[5],thustheBBBcanbepassedbyand a wide variety of therapeutic agents,including both small molecules and macromolecules,can be successfully delivered to the CNS by intranasal route[6].
Vinpocetine(VIN)is a vasoactive vinca alkaloid and a synthetic derivative of apovincamine,it is commonly used in clinical practice for the treatment of disorders arising from cerebrovascular and cerebral degenerative diseases[7,8]. However,due to its poor solubility in water[9],short elimination half-life(1-2 h)and extensive metabolism in liver(~75%) [10,11],it has low oral bioavailability(6.7%)in human beings [12]andthereforelowdrugconcentrationinthebrain,limiting its application in the clinic.Therefore,it is highly desirable to designappropriatedrugdeliverysystemforVINwithimproved drug solubility and enhanced brain targeting effect.
Different methods can be used to increase the solubility of poorly soluble drugs.Among them,inclusion complex formation is an effective one.Cyclodextrin(CD)and its derivatives,which can form“inclusion complexes”in aqueous solutions,havebeenwidelyusedin pharmaceuticsto increase the solubility,stability and bioavailability of poorly soluble drugs,with reduced irritation and side effects[13].
Thus,the objective of this study is to improve the solubility of VIN by inclusion complex formation and increase its brain targeting effect by intranasal administration.Solubility phase diagram was used to guide the preparation of the inclusion complex.The complexes formation was con fi rmed by DSC and solubility test.In fl uence of different factors on the properties of the complex was investigated.Brain targeting effect of VIN inclusion complex was evaluated after intranasal administration in rats.
2. Materials and methods
2.1. Materials
Vinpocetine(VIN)was purchased from Haide Corporation (Benxi,Liaoning,China).β-cyclodextrin(β-CD)was from Tianjin Chemical Reagent Company(Tianjin,China),hydroxypropyl-β-cyclodextrin(HP-β-CD)was from Deli Biological ChemicalCorporation (Xi'an,Shanxi,China),randomly methyl-β-cyclodextrin(RM-β-CD)was from Xinda Chemical Corporation(Jinan,Shandong,China),citric acid was from Zhengxin InstituteDepartmentofreagents(Shenyang, Liaoning,China),tartaric acid was from Bodi Chemical Company(Tianjin,CA),Methanol(HPLC grade)was supplied by Yuwang(Jinan,Shandong,China).All other reagents and buffer components were of analytical grade.
2.2. Solubility test
The solubility of VIN underdifferentconditionswas measured using shake- fl ask method at 37°C[14].Brie fl y,an excess amount of VIN was added to 5 ml of speci fi c solvents and the samples were placed in a water bath and stirred at 100 r/min for 48 h.Thereafter,the resulting suspensions were fi ltered through a 0.45 μm membrane fi lter and concentration of VIN in the fi ltrate was analyzed using high performance liquid chromatography(HPLC)method after dilution.The HPLC instrument consists of Agilent C18 column(4.6 mm*150 mm, 5 μm,USA)and UV detector set at 273 nm.Mobile phase was a mixture of methanol:ammonium acetate(15.4 g/l)(80:20,v/v), fi ltered through 0.45 μm membrane fi lter,the fl ow rate was 1.0 ml/min,injection volume 20 μl,oven temperature 35°C.
2.3. Phase solubility studies
The phase solubility study of VIN with β-CD(0-12.5 mM),HP-β-CD(0-90.0 mM)and RM-β-CD(0-77.0 mM)was performed at 37°C in distilled water(pH=6.3).The stability constant(Kc) of the complex was calculated according to the following equation[15]:
Kc=slope/intercept(1-slope)
The higher the Kc value,the better the stability.
2.4. Preparation of inclusion complex
The inclusion complex was prepared by dissolving followed freeze-drying method.Brie fl y,1400 mg of HP-β-CD was dissolved in 10 ml of distilled water at room temperature and a solution of 10 ml 2%(w/v)citric acid or tartaric acid,aqueous solution containing appropriate amount of vinpocetine(the molar ratio of VIN and HP-β-CD was 1:1,1:2,1:3)was added under stirring,ultrasounded for 15 min to dissolve the drug and HP-β-CD.The resulting solution was stirred at 50°Cfor 1-2 h.After equilibrium to room temperature,pH of the solution was adjust to approximately 5 unless specially indicated,and fi ltered through 0.45 μm membrane fi lter,the clear solution was frozen at-20°C and subsequently freeze-dried (FD-1 freeze-dryer apparatus,Beijing Medicine and Health Technology Co,Beijing,China)for 48 h.The inclusion rate is calculated according to the following equation:
2.5. Characterization of inclusion complexes
Formation of inclusion complexes was identi fi ed by solubility study as described in 2.2 and DSC analysis.The DSC curves were determined with a DSC instrument(DSC-1,METTLER, Switzerland)underthe following conditions:samples (2-3mg)werehermeticallysealedin a fl at-bottomed aluminum pan and heated,with an empty pan sealed as reference,over a temperature range of 20-250°C with the heating rate of 10°C under nitrogen gas.
2.6. In vivo analytical method of VIN
VIN concentration in plasma was analyzed by HPLC after solvent extraction[17].Brie fl y,200 μl of the plasma sample was mixed with 40 μL of internal standard(8 μg/ml progesterone)and 50 μL of 0.5 M NaOH solution for 30 s by vortexing in a glass tube.Then hexane(3 ml)was added for VIN extraction.The mixture was centrifugalized and the supernatant was transferredinto another glass tube and the solventwas evaporated in a 40°C water bath under nitrogen fl ow.The residue was redissolved in 100 μl of methanol by vortexing, followed by centrifugation at 6650 g/min for 10 min.The supernatant was analyzed by HPLC at wavelength 228 nm to increase the sensitivity;the other analytical conditions were the same as described in Section 2.2.For the brain homogenate,0.5 ml of homogenate was mixed with 40 μl of internal standard and 50 μl of 0.5 M NaOH solution by vortexing for 30 s in a glass tube,hexane(6 ml)was added for extraction,and then follow the procedures described above.
The HPLC method described above for VIN assay in the plasmaandinthebraintissuewasspeci fi candef fi cient.There was no interference from endogenous components observed at the retention time of the analytes in the chromatogram. Good linear relationship between drug concentration and peak area was established,the concentration range was 20-1000 ng/ml for plasma sample and 20-800 ng/ml for brain samples.The mean extraction recoveries were 90.18%and 85.39%for plasma and brain samples,respectively.The RSDs of intra-and inter-day precision were both below 10.0%.
2.7. Pharmacokinetic studies in rats
In vivo absorption of VIN inclusion complex after intravenous and intranasal administration was studied in rats.All animal studies were approved by the University Ethics Committee and were carried out in accordance with the Principle of Laboratory Animal Care.The Sprague Dawley rats(male, 180-200 g)were supplied by the Lab Animal Center of Shenyang Pharmaceutical University and randomly divided into two groups,15 rats in each group.In group I the complex was given by intravenous injection via tail vein,in group II the complex was administrated intranasally according to the method reported previously[16],both with a single dose of 1 mg/kg.Blood samples(approximately 0.5 ml)were withdrawn from retro-orbital plexus at 0,3,5,10,20,30 min,1,2, 3 h post-dosing(n=3)and centrifuged at 6650 g/min for 10 min.The supernatant plasma samples were stored at -20°C until analysis.
For the biodistribution study,the rats were sacri fi ced at 10 min,30 min,1 h,2 h after dosing(n=3)and the brain was removed after cardiac perfusion with 0.9%NaCl solution,and drug concentration in the brain was determined by homogenizing the organs in 2-fold ice-cold 0.9%NaCl solution before analysis.
2.8. Statistical analysis
Pharmacokinetic parameters,the maximum plasma concentration of the drug(Cmax),the time to reach Cmax(Tmax)and AUC were calculated from the plasma concentration-time pro fi les.The absolute bioavailability(F)of the intranasally administrated inclusion complex was calculated by comparing their AUC with that of intravenous injection using DAS 2.1.1 software.Signi fi cance of difference was evaluated using one-way ANOVA at the probability level of 0.05.Drug targeting index(DTI)was calculated by the following formula, DTI>1 was considered as brain-targeting distribution[18].
3. Results and discussion
3.1. In fl uence of pH on the solubility of VINTo optimize the condition for inclusion complex preparation, in fl uence of pH on the solubility of VIN was studied fi rstly.The pH range was selected based on the physiological condition of human body fl uid and nasal mucus[19].Buffer solutions with pH 2.0,4.0,5.0,6.0,6.8,7.4 were used in this study.
As shown in Table 1,with the increase of pH,the solubility of VIN decreased signi fi cantly.The solubility of VIN was 5.43 mg/ml at pH 2,and decreased to 2.44 μg/ml at pH 6.8.As VIN is a kind of weak basic drug(pKa 7.31),its solubility was greatly affected by pH,therefore it is absolutely essential to monitor pH during the experiment.
3.2. In fl uence of cyclodextrin type on the stability of the inclusion complex
To screen which kind of cyclodextrin can form stable complex with VIN and explore the complexation ratio,phase solubility diagrams of VIN with three types of cyclodextrin,including β-CD,and two water soluble cyclodextrin derivatives,2-hydroxypropyl derivatives of β-cyclodextrin(HP-β-CD)and randomly methyl-β-cyclodextrin(RM-β-CD)were studied in distilled water(pH 6.3).As shown in Fig.1,irrespective of cyclodextrin type,a typical ALtype diagram was observed and the solubility of VIN increased almost linearly with the increase of CD concentration,implying that soluble VIN and CD inclusion complexes were formed at molar ratio 1:1.
Meanwhile,the apparent stability constant(KC)of different complexes,which represents strength of the interaction and stability of the complex,were calculated,it was in the order: HP-β-CD(77.77 M-1)>RM-β-CD(74.76 M-1)>β-CD(66.92 M-1). No signi fi cant difference in bonding force between VIN and HP-β-CD,RM-β-CD were found,both are higher than β-CD based complex.Considering the fact that HP-β-CD has better safety pro fi le for intranasal administration than that of RM-β-CD[20],and the poor aqueous solubility of β-CD(1.85%at 25°C),HP-β-CD was selected for further investigation.

However,even when the concentration of HP-β-CD was as high as 15%,the solubility of VIN was just 50-60 μg/ml,far less than the dose required for intranasal delivery.It is reported thatmulticomponentcomplexationtechnologyisquite effective in increasing drug concentration in the cavity of cyclodextrin,the perquisite for using this technology is that a tight fi t between the complexed molecule and the CD must occur(Kc at least 103M-1)[21],apparently,the interaction between VIN and cyclodextrin was far below this value. Fortunately,it was found that citric acid(CA)can increase the solubility ofVIN signi fi cantly [22],and the solubility enhancement of CA overweighed the in fl uence of solution pH. For example,the solubility of VIN in 2%CA was 15 mg/ml(the pH of different concentrated CA solution was typically in the range of pH 3-4),and in comparison,its solubility in pH 3.0 medium was only 1.87 mg/ml,implying free water soluble complexes were formed between VIN and CA[22].Therefore, adding CA might be an effective way to improve VIN concentration in the HP-β-CD by forming multicomponent complex.It has been reported that simultaneous complexation with these acids signi fi cantly increased the solubilizing power,reducing the amount of cyclodextrin necessary for making the targeted formulation[21].
?
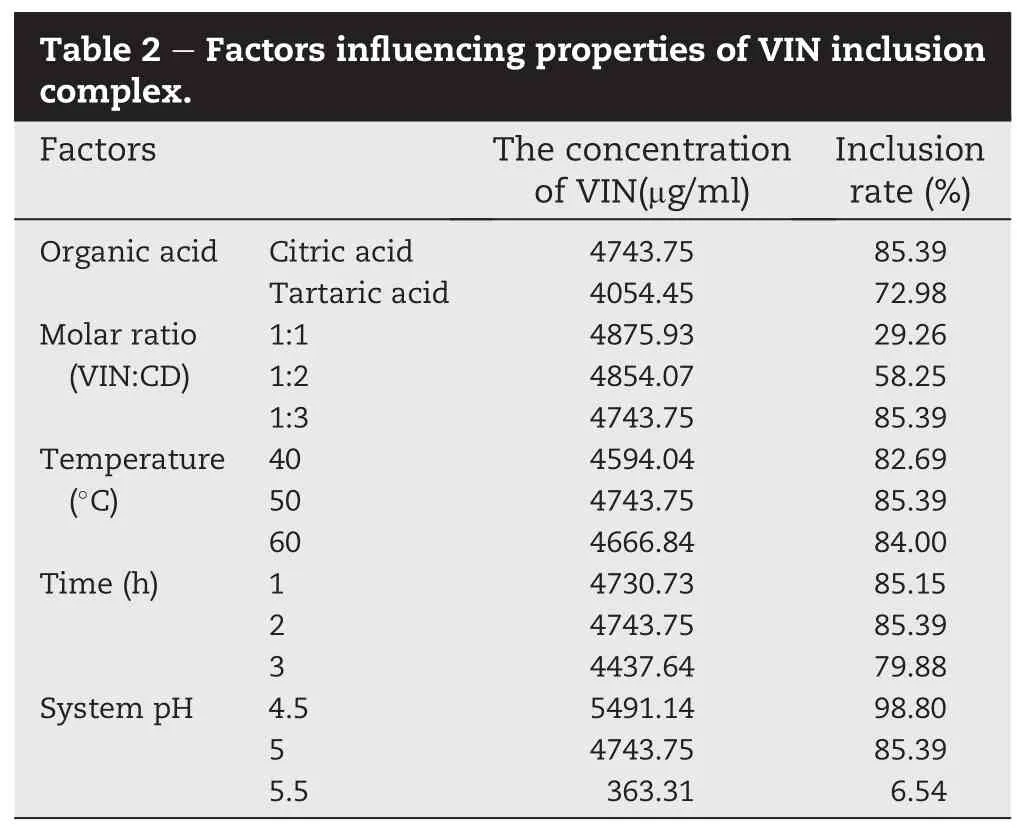
3.3. Factors in fl uencing properties of VIN inclusion complex
To optimize properties of VIN inclusion complex,in fl uence of VIN and HP-β-CD molar ratio(1:1,1:2,1:3),organic acids type (citric acid and tartaric acid),inclusion complex preparation temperature(40,50,60°C),inclusionincubation time(1,2,3 h), and the system pH(4.5,5,5.5)on achieved drug concentration and the inclusion rate were investigated.The results are presented in Table 2.
First of all,by keeping solution pH at 5,in fl uence of different parameters was investigated.Taking both drugconcentrationand inclusion rateintoconsideration,citricacid was more effective than that of tartaric acid and was selected for the followed study.No signi fi cant difference in drug concentration was found(P>0.05)when the molar ratio of VIN and HP-β-CD was 1:1,1:2,1:3,but the inclusion rate increased with the increase of molar ratio,so VIN and HP-β-CD molar ratio 1:3 was selected.In fl uence of temperature on properties of the inclusion complex was not signi fi cant in the range of 40-60°C,50°C was selected.When the incubation time was 1 h or 2 h,there was no signi fi cant difference in drug concentration or inclusion rate,however,when the reaction time was prolonged to 3 h,the drug concentration decreased slightly,therefore reaction time 1-2 h was selected.As to the in fl uenceofsystem pH,drugconcentration decreased approximately 14%when the pH was changed from 4.5 to 5, however,a sharp decrease in drug concentration was found when the pH was adjusted to 5.5,probably the organic acid was neutralized by the added base and destroyed the multiclathratecomplex[21].SincepH4.5-6.5iscommonly accepted for intranasal administration[23],pH 4.5 was selected for the followed study.
Therefore,the fi nal complex was prepared by using the following conditions:VINandHP-β-CD molarratio 1:3,organic acid types:citric acid,inclusion temperature 50°C,incubation time 1-2 h,the system pH 4.5.
3.4. Identi fi cation of inclusion complex formation
Formation of inclusion complexes was identi fi ed by both DSC and solubility study.The DSC pro fi les of the pure drug,the ternary system of the multicomponent complex and physical mixture are shown in Fig.2.The thermal curve of pure VIN was typical of a crystalline anhydrous substance with a sharp endothermic peak at 151.98°C corresponding to its melting point[24].The melting peak was also observed in the physical mixture.Concerning the ternary system of the multicomponent complex,the thermal characteristic peak of VIN was shifted to a lower temperature at around 148.0°C and its intensity decreased signi fi cantly,indicating that most of drug substance existed in amorphous state and/or being included in the complex[24].These results suggest that VIN/HP-β-CD/ CA inclusion complexes were formed.
Inclusion complex formation was further con fi rmed by solubility test.The drug solubility of the inclusion complex lyophilized powder was 4.74 mg/ml(pH=4.5),and it was 0.2 mg/ml for the free drug measured at exactly the same condition.This study indicated that the solubility of VIN in the inclusion complex was about 23-fold higher than that of the free drug in water.
3.5. Pharmacokinetic and biodistribution studies
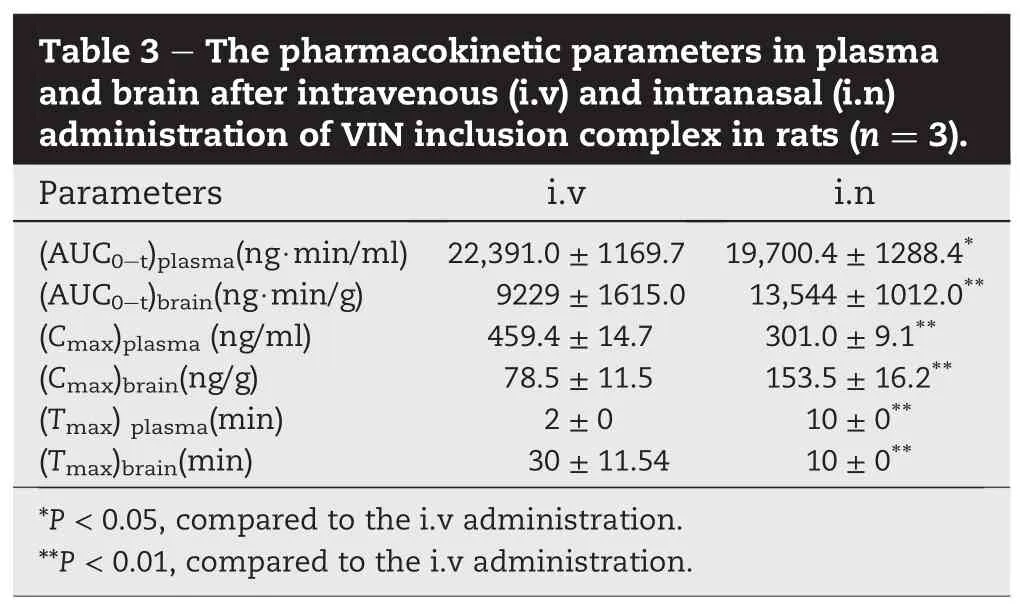
The concentration-time pro fi les of VIN in the plasma and in the brain after i.v and intranasal administration of VIN inclusion complex in rats are shown in Fig.3.The pharmacokinetic parameters are summarized in Table 3.The plasma AUC value of VIN after intranasal administration was slightly lower than that after i.v.administration,and the absolute bioavailability was 88%,with Tmax10 min,implying that VIN can be quickly absorbed into systemic circulation comparable with that of intravenous injection.
As for brain targeting,shorter Tmaxand higher drug concentration in the brain was found after intranasal administration of VIN complex.The mean Cmaxvalues in the brain (153.5 ng/g)afteri.n administrationwerefoundtobemarkedly higher than those obtained after i.v administration(78.5 ng/g). The peak concentration in the brain occurred at 10 min after i.n administration,and it was faster than that of i.v(30 min) route.Moreover,the brain AUC0-180minvalue was signi ficantly higher after intranasal administration compared to i.v. administration,with DTI value 1.67,indicating signi fi cant brain-targeting effect was achieved.This is quite reasonable and can be explained by the fact that part of the drug could reach the brain directly via olfactory mucosa in the nose whereas after i.v administration,the drug will be fi rstly absorbed into systemic circulation then redistributed in thebrain slowly[25].This is in good agreement with the higher drug concentration in plasma than that in brain after i.v. injection.
4. Conclusions
In the present study,VIN inclusion complex was prepared in order to increase its solubility.Factors in fl uencing properties of the inclusion complex was investigated.Formation of the inclusion complex was identi fi ed by solubility study and DSC analysis.In vivo study in rats revealed that after intranasal administration of vinpocetine inclusion complex,the absolute bioavailability was 88%.Signi fi cant brain targeting effect was found with intranasal administration of VIN compared with that of intravenous injection,with brain targeting index 1.67. In conclusion,by intranasal administration of VIN inclusion complex,a fast onset of action and good brain targeting effect can be achieved.
REFERENCES
[1]Wohlfart S,Gelperina S,Kreuter J.Transport of drugs across the blood-brain barrier by nanoparticles.J Control Release 2012;161:264-273.
[2]Torchilin VP.Passive and active drug targeting:drug delivery to tumors as an example.Drug Deliv 2010;197:3-53.
[3]Chen Y,Liu L.Modern methods for delivery of drugs across the blood-brain barrier.Adv Drug Deliv Rev 2012;64:640-665.
[4]Costantino HR,Illum L,Brandt G,et al.Intranasal delivery: physicochemical and therapeutic aspects.Int J Pharm 2007;337:1-24.
[5]Perry C,Mackay-Sim A,Feron F,et al.Olfactory neural cells: an untapped diagnostic and therapeutic resource. Laryngoscope 2002;112:603-607.
[6]Vyas TK,Shahiwala A,Marathe S,et al.Intranasal drug delivery for brain targeting.Curr Drug Deliv 2005;2:165-175.
[7]Singh A,Sharma V,Pandey B.Comparative effectiveness study of vinpocetine vs.nimodipine on functional recovery in patients of head injury.Int J Basic Clin Pharm 2013;2:18-25.
[8]Patyar S,Prakash A,Modi M,et al.Role of vinpocetine in cerebrovascular diseases.Pharmacol Rep 2011;63:618-628.
[9]Ribeiro L,Loftsson T,Ferreira D,et al.Investigation and physicochemical characterization of vinpocetine-sulfobutyl ether beta-cyclodextrin binary and ternary complexes.Chem Pharm Bull 2003;51:914-922.
[10]Grassi G,Hasa D,Voinovich D,et al.Simultaneous release and ADME processes of poorly water-soluble drugs: mathematical modeling.Mol Pharmacol 2010;7:1488-1497.
[11]Szak′acs T,Veres Z,Vereczkey L.In vitro-in vivo correlation of the pharmacokinetics of vinpocetine.Pol J Pharmacol 2001;53:623-628.
[12]Grandt R,Beitinger H,Schaltenbrand R,et al.Vinpocetine pharmacokinetics in elderly subjects.Arzneimittelforschung 1989;39:1599-1602.
[13]Sebesty′en Z,Buv′ari-Barcza′A,Rohonczy J.pH-dependent complex formation of amino acids with β-cyclodextrin and quaternary ammonium β-cyclodextrin.J Incl Phenom Macro 2012;73:199-210.
[14]Higuchiand T,Connors KA.Phase-solubility techniques.Adv Anal Chem Instrum 1965;4:117-212.
[15]Ribeiro LSS,Falc~ao AC,Patrı´cio JAB,et al.Cyclodextrin multicomponent complexation and controlled release delivery strategies to optimize the oral bioavailability of vinpocetine.J Pharm Sci 2007;96:2018-2028.
[16]Mei D,Mao S,Sun W,et al.Effect of chitosan structure properties and molecular weight on the intranasal absorption of tetramethylpyrazine phosphate in rats.Eur J Pharm Biopharm 2008;70:874-881.
[17]Ning M,Zhou Y,Chen G,et al.Preparation and in vitro/in vivo evaluation of vinpocetine elementary osmotic pump system. Adv Pharm Sci 2011;2011:385469.
[18]Cai Z,Hou S,Li Y,et al.Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target 2008;16:178-184.
[19]Gupta MR,Gupta BP,Nagariya AK,et al.An ornamental mucoadhesive particulate drug delivery system for nasal route:a review.J Pharm Res 2010;3:8.
[20]Irie T,Uekama K.Pharmaceutical applications of cyclodextrins.III.Toxicological issues and safety evaluation. J Pharm Sci 1997;86:147-162.
[21]Redenti E,Szente L,Szejtli J.Drug/cyclodextrin/hydroxy acid multicomponent systems.Properties and pharmaceutical applications.J Pharm Sci 2000;89:1-8.
[22]Nie S,Fan X,Peng Y,et al.In vitro and in vivo studies on the complexes of vinpocetine with hydroxypropylβ-cyclodextrin.Arch Pharm Res 2007;30:991-1001.
[23]Na L,Mao S,Wang J,et al.Comparison of different absorption enhancers on the intranasal absorption of isosorbide dinitrate in rats.Int J Pharm 2010;397:59-66.
[24]Ribeiro LSS,Ferreira DC,Veiga FJB.Physicochemical investigation of the effects of water-soluble polymers on vinpocetine complexation with β-cyclodextrin and its sulfobutyl ether derivative in solution and solid state.Eur J Pharm Sci 2003;20:253-266.
[25]Westin UE,Bostr¨om E,Gra˚sj¨o J,et al.Direct nose-to-brain transfer of morphine after nasal administration to rats. Pharm Res 2006;23:565-572.
*Corresponding author.School of Pharmacy,Shenyang Pharmaceutical University,103 Wenhua Road,110016 Shenyang,China.Tel./fax: +86 24 23986358.
E-mail addresses:maoshirui@syphu.edu.cn,maoshirui@vip.sina.com(S.Mao).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.08.008
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
Hydroxypropyl-β-cyclodextrin Citric acid
Inclusion complex
Brain targeting
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- A review on phospholipids and their main applications in drug delivery systems
- Studies on thermoresponsive polymers:Phase behaviour,drug delivery and biomedical applications
- Design and evaluation of nicorandil extended-release tablet
- A new self-emulsifying formulation of mefenamic acid with enhanced drug dissolution
- Can semipermeable membranes coating materials in fl uence in vivo performance for paliperidone tri-layer ascending release osmotic pump tablet: In vitro evaluation and in vivo pharmacokinetics study
- Mycosynthesis,characterization and antibacterial properties of AgNPs against multidrug resistant (MDR)bacterial pathogens of female infertility cases
