Preparation and evaluation of taste masked oral suspension of arbidol hydrochloride
2015-05-15LingWangYinghuaSunChenKuangXiangrongZhang
Ling Wang,Yinghua Sun,Chen Kuang,Xiangrong Zhang
School of Pharmacy,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
Preparation and evaluation of taste masked oral suspension of arbidol hydrochloride
Ling Wang,Yinghua Sun,Chen Kuang,Xiangrong Zhang*
School of Pharmacy,Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
ARTICLEINFO
Article history:
Received 10 June 2014
Received in revised form
12 July 2014
Accepted 18 July 2014
Available online 27 August 2014
Arbidol hydrochloride
The purpose of this study was to cover the bitter taste of arbidol hydrochloride(ARB)and develop dry suspension with combination of solid dispersion and f l avors.Taste masking was successfully done by solid dispersion using octadecanol as the carrier by fusion method.Suspending agents,carriers and other excipients were selected.Differential scanning calorimetry(DSC)and Fourier transform infrared spectroscopy(FTIR)were performed to identify the physicochemical interaction between drug and carrier,DSC analysis indicated that ARB was amorphous in the solid dispersion,FTIR spectroscopy showed no interaction between drug and carrier.Taste masking was evaluated on six volunteers with a score of 4.9.The results demonstrated successful taste masking.Water was used to study the in vitro dissolution performance of the three formulations of commercial tablet,capsule and self-made suspension.The self-made suspension showed a lower and slower release, the insoluble carrier octadecanol blocked the drug dissolving from the solid dispersion.It was indicated from the primary stability study,the self-made suspensions were sensitive to high temperature,high humidity and strong light conditions,they should be stored in sealed containers away from heat,light and humidity.
©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1.Introduction
ARB(1-methyl-2-phenyl-thiomethyl-3-carboxy-4-dimethylaminomethyl-5-hydr-oxy-6-bromoindole hydrochloride monohydrate)(Fig.1)is a small indole-derivatives molecule used for the treatment and prevention of infections by inf l uenza A, B viruses,A/H1N1 is included[1].ARB was listed in Russia in 1993andinChinain2006,forthecontrolofinf l uenzaandother acute respiratory virus infection[2-4].It is proved that ARB is eff i cient to reduce the duration of illness and to prevent the development of post-inf l uenza complications[5].Moreover, despite years of clinical trials and application reveal that,ARB show high eff i cacy,minor adverse effect,and no drug resistance[6-9].A major problem in the development of an oral solid dosage form of ARB is its intensely bitter taste,leading to poor patient compliance.To overcome the above problem,we try to develop a bitterness-covered ARB for dry suspension.
The commercially available products include tablets and capsules.It is estimated that 50%of the population have problem of swallowing tablets and capsules,especially the pediatric and geriatric population[9].Dry suspension has been used to address the problem.In addition to good patient compliance,there are many advantages of dry suspension,such as safety,stability,convenience and ease absorption.
Without changing its safety and eff i cacy,a drug's taste has to be masked and techniques are being adapted to meet this need,especially for the pediatric and juveniles patients.These are as follows:taste masking with f l avors,taste masking by granulation,microencapsulation,ion exchange resins,solid dispersionmethod,bitternessinhibitor.Whensingle approach for taste masking is not very successful for highly bitter drugs,using combination of various taste masking technologies is found to be a more eff i cient strategy[9]. Amongst the strategies mentioned,we choose the combination of f l avors with solid dispersion[10].
Solid dispersion have been def i ned as dispersion of one or more active ingredients in an inert carrier or matrix at solid state prepared by melting method,hot melt extrusion,solvent evaporation method,spray drying,supercritical f l uid precipitation and so on[11,12].
In our study,melting method was chosen owing to their effective taste masking and ease of industrial production.To prepare ARB solid dispersion,octadecanol was used as the carrier,then f l avors such as sucrose,mannitol,aspartame and orange f l avor were added.
2.Materials and methods
2.1.Materials
Arbidol hydrochloride was purchased from Sichuan Baili Pharmaceutical Co.,Ltd.(China).Arbidol hydrochloride commercial tablet was purchased from Shiyao Group Pharmaceutical(Shijiazhuang)Co.,Ltd.(China).Arbidolhydrochloride commercial capsule was purchased from Shijiazhuang Siyao Co.,Ltd.(China).Xanthan,mannitol,orange f l avor concentration and aspartame were purchased from Beijing Fengli Jingqiu Commerce and Trade Co.,Ltd.(China).Sodium carboxymethylcelluloseandhydroxypropylcellulosewere kindly supplied by Anhui Sunhere Pharmaceutical Excipients Co.,Ltd.(China).Arabic gum,octadecanol,sucrose,citric acid, poloxamer 188,glyceryl monostearate,sodium dodecyl sulfate,pentane sulfonate and ammonium dihydrogen phosphate were purchased from Tianjin Bodi Chemical Co.Ltd. (China).Sunset yellow dye was purchased from Shanghai Institute Dye Co.(China).PEG6000 and phosphoric acid was purchased from Tianjin Kermel Chemical Reagent Co.,Ltd. (Tianjin,China).Methanol of chromatographic grade was purchasedfrom Concord TechnologyCo.,Ltd.(Tianjin,China).
2.2.Methods
2.2.1.Preparation of solid dispersions
Conventional melting method was used in our work.Several typical carriers with a low melting point including PEG6000, citric acid,octadecanol,poloxamer 188 and glyceryl monostearate were evaluated using organoleptic evaluation and in vitro dissolution study.
Different carriers were heated at 70°C directly to melt entirely,then the mixture of ARB and sucrose with a ratio of 1:2 was slowly added to the carriers with constantly stirring. After totally dispersed,the melted mixture was solidif i ed in an ice-bath under vigorous stirring until forming particles. After dried at room temperature for 2 h,the f i nal solid dispersion was crushed,pulverized and screened by 40#mesh;particles with diameters under the size were chosen for further use.
2.2.2.Organoleptic evaluation[13]
The objective of this study was to conduct and evaluate the palatability of different formulations of ARB dry dispersion. Taste evaluation was done on a team of 3 members.Each volunteer took dispersion equivalent to 100 mg ARB in the mouth for 15 s and then spit out.The taste score between 1 and 5 was given to evaluate the taste of formulation.Namely, the scores were set as follows:1(Distasteful,equivalent to ARB taste),2(Slightly taste,ARB taste remaining fairly),3 (Mean,ARB taste remaining to some extent),4(slightly tasty, ARB taste slightly remaining),5(Tasty,no taste of ARB).
2.2.3.In vitro dissolution study
Thein vitrodissolution testwasstudiedusinga ChP2010Type2 dissolution apparatus(paddle method),and all the tests were carried out in triplicate.The temperature of the dissolution medium(a volume of 900 ml puri fi ed water)was maintained at 37±0.5°C with stirring from a paddle at 75 rpm.A certain amount of samples equivalent to 100 mg ARB were used in all of the dissolution tests.At prescheduled time intervals(5,10, 15,20,30,45,60 min),samples aliquots were withdrawn and fi ltered(0.45 μm membrane,Millipore)immediately,then replaced with an equal volume of drug-free dissolution fl uid. The samples were suitably diluted with blank dissolution fl uid and the concentration of ARB was determined using on a UV spectrophotometer(Beijing Rayleigh Analytical Instrument Co.)at 257 nm.
2.2.4.Differential scanning calorimetry analysis
Thermal analysis of octadecanol,raw arbidol hydrochloride, powdered sucrose,physical mixture,and the solid dispersion of the three substances were performed on a DSC 1(Metter Toledo,Greifensee,Switzerland).Samples each 2-5 mg wasplaced in non-hermetical aluminum pans,with an empty aluminum pan as a reference.The temperature increased with a heating rate of 10°C/min from 30°C to 230°C under a nitrogen gas f l ow.
2.2.5.FTIR spectroscopy
A Bruker Ifs 55 FTIR spectrometer(Bruker,Switzerland) equipped with a DTGS detector was used for infrared analysis. Samples were prepared with KBr disc method(1 mg sample in 100 mg KBr)and examined in the transmission mode.The scanning range was 400-4000 cm-1and the resolution was 2 cm-1.
2.2.6.Selection of suspending agents
Suspending agent was selected by using single factor method. Arabic gum,xanthan gum,sodium alginate,sodium carboxymethyl cellulose and hydroxypropylmethyl cellulose were investigated for the ability of suspending using sedimentation ratio evaluation index.
The sedimentation ratio of the suspensions was determined by transferring samples equivalent to 100 mg ARB into a 50 ml stoppered cylinder,made up to volume with distilled water,shaken vigorously for 1 min and then stored at room temperature.It was enough to note the sedimentation at intervals of 30 min because ARB suspension was single-dose packaging.The sedimentation ratio,F(%),was then calculated using the following formula.
F=100H/H0
where H is the ultimate height of the sediment and H0is the initial height of the suspension[14].
2.2.7.Selection of f l avors and colorants
The amount of f l avors including aspartame and orange f l avor were chosen by smell and odor;and the amount of colorants agent depended on the color of the suspension.
2.2.8.Preparation of ARB dry suspension
The ARB solid dispersion using octadecanol as the carrier was prepared by the method described in Section 2.2.1.The other components including powdered mannitol,aspartame,arabic gum,orange f l avor,sodium dodecyl sulfate and sunset yellow dye were passed by 60#mesh and mixed well with the prepared solid dispersion powders.The f i nal dry suspension was packaged for further quality evaluation.
2.2.9.Determination of ARB content
Analysis of the content of ARB in the suspensions was performed by high performance liquid chromatography(HPLC) system(Hitachi,Tokyo,Japan)consisting of an isocratic pump (Model L-2130),an autosampler(Model L-2200),a UV detector (Model L-2420)(operating at 254 nm)and HPLC System Manager Hitachi Model D-2000 software.A VP-ODS C18column (5 μm,250×4.6 mm)was used with a mobile phase of methanol-aqueous phase(1.45 g pentane sulfonate and 3.45 g ammonium dihydrogen phosphate were dissolved in 900 ml of distilled water,then adjusted to pH 2.50 with phosphoric acid)(75:25,v/v)at a f l owrate of1 ml/minwith a totalinjection volume of 20 μl.
Accurate amount of dry suspension was weighed equivalent to a concentration about 0.1 mg/ml of ARB with mobile phase as the solvent.The content was calculated by the external standard method.The retention time was about 6.5 min.
2.2.10.Determination of ARB impurities
The chromatographic conditions were the same to the drug content of ARB,except that the f l ow rate of the mobile phase was 0.8 ml/min.Accurate amount of dry suspension was weighed equivalent to a concentration about 0.5 mg/ml of ARB with mobile phase as the solvent.Area normalization method was used to calculate the impurities of ARB.The retention time was about 8.5 min.
2.2.11.Preliminary stability study of the suspensions
The suspensions were exposed to high temperatures of 40°C and 60°C,relativehumidityof 75%and 92.5%,and lightof4500 l×for 10 d.Samples were taken at 0 d,5 d and 10 d separately.
3.Results and discussions
3.1.Preparation of ARB dry suspension
In the present study,a combination of f l avors with solid dispersion dry suspension was prepared to mask the bad taste of ARB.In the method,solid dispersion played an important role in taste masking,carriers which drugs were dispersed in created a physical barrier between the drug and the taste buds,then taste of active could be masked,f l avors and polymers were added to have a more pleasant taste,f l avors could reduce the sensitivity of taste buds to bitter, polymers as viscosity enhancers could complement the taste masking.
3.1.1.Effect of the carriers in solid dispersion method
In solid dispersion,one or more active ingredients are homogeneously dispersed in an inert carrier or matrix in solid state.The easiest and most affordable melting method was used for preparation of solid dispersion.Carriers with a low melting point are preferred.In the experiment,PEG6000,citric acid,poloxamer 188,octadecanol and glyceryl monostearate were evaluated.All f i ve carriers were selected according to the organoleptic evaluation with a team of 3 members.The mean score was shown in Table 1.
Both octadecanol and glyceryl monostearate could effectively cover the intense bitter mask of ARB,but glyceryl monostearate had an unpleasant odor.Octadecanol was the best choice.Sucrose was added to the solid dispersion topromote the drug dissolution,and obtained a better taste. Then drug-sucrose-carrier weight ratios of 1:2:2,1:2:4 and 1:2:6 were evaluated by organoleptic evaluation and in vitro dissolution study.The mean value of taste evaluation was shown in Table 2.

Table 1-Organoleptic evaluation of ARB solid dispersion with different carriers.
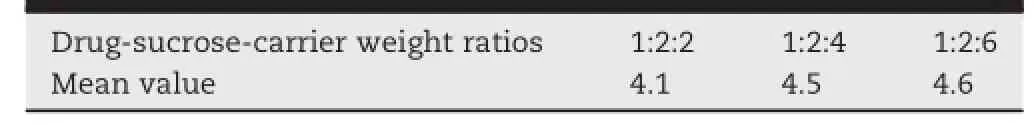
Table 2-Organoleptic evaluation of ARB solid dispersion with different ratios.
The dissolution of solid dispersion with different drugsucrose-carrier weight ratios(1:2:2,1:2:4,1:2:6)were performed in purif i ed water and the corresponding prof i les were shown in Fig.2.
With the increase of the amount of octadecanol,the tastecovered capability of the solid dispersion improved,but the dissolutionpercentagesofsoliddispersionsgradually decreased.The weight ratio(1:2:4)of drug-sucrose-carrier was a better selection.
3.1.2.Effect of different suspending agents
Most suspending agents perform two functions.Besides acting as a suspending agent they also imparts viscosity to the solution.Suspending agents form f i lm around particle and decrease interparticle attraction.Suspending agents also act as thickening agents.They increase viscosity of the solution, whichis necessary topreventsedimentationofthesuspended particles as the Stoke's law.A good suspension should have well developed thixotropy.At rest the solution is suff i cient viscous to prevent sedimentation and thus aggregation or caking of the particles.When agitation is applied the viscosity is reduced and provide good f l ow characteristic from the mouth of bottle.
In this study,arabic gum,sodium alginate,sodium carboxymethylcelluloseandhydroxypropylcellulosewere investigated for the ability of suspending.Only with arabic gum as suspending agents,the particles could disperse easily and uniformly in water.While other suspending agents were used,the particles stuck together even shaking vigorously. Arabic gumwas the best choice of suspending agents and f i nal suspending agent weight ratios of 5%,10%and 15%were evaluated using sedimentation ratio evaluation index.The sedimentation ratio was shown in Table 3.10%arabic gum was selected as the optimal suspending agents.

Table 3-The sedimentation ratio of suspensions made up of different suspending agents.
The amount of f l avoring and coloring agents were determined by organoleptic evaluation as follows:aspartame 3%, orange f l avor concentration 1%,sunset yellow dye 0.5%.Along with other excipients added,the f i nal formulation was formed in Table 4.
3.2.Differential scanning calorimetry analysis
The DSC thermograms of ARB,octadecanol,sucrose,solid dispersion and the corresponding physical mixture of ARB, sucrose and octadecanol were shown in Fig.3.The thermogram curves octadecanol exhibited the single endothermic peaks at around 60°C,and sucrose showed endothermic peaks at around 189°C.During scanning of ARB,a relatively broad endotherm ranging from 90 to 150°C was observed, indicating the loss of crystal water;the observed little peaks ataround 155°C and 180°C were ARB's melting peak,which showed that the pure drug was polymorphs.The DSC prof i les of the physical mixture of ARB,sucrose and octadecanol were the combination of three melting processes.The curve of the solid dispersion showed the absence of ARB peaks,indicating that ARB might be in amorphous state,both ARB and sucrose wereincorporatedinthesoliddispersion,octadecanol covered the bitter taste of ARB.
3.3.FTIR spectroscopy
FTIR is often used to investigate any possible chemical interactions between drugs and matrixes in solid dispersions [15].In this paper,FTIR was mainly used to study the possible interactions between ARB and octadecanol in the solid dispersion.The infrared spectra of ARB,octadecanol,sucrose, their solid dispersion and physical mixture were shown in Fig.4.No additional shifts or peaks was found on comparison with FTIR spectrum of the solid dispersion and physical mixture in the existing peaks,which means that there was no appreciable interaction between the drug and polymer.
3.4.Evaluation of dry suspension
Organoleptic properties are important considerations for development of a solid dosage form that can inf l uenceconsumer preference and compliance.In the case of bitter drugs,taste is one of the most important parameter governing patient compliance[16]and oral administration of bitter drugs with an acceptable degree of palatability is a key issue for health care providers especially for pediatric and geriatric[17].

Table 5-Evaluation ofdry suspensionspreparedby solid dispersion.
Taste evaluation is the most important index to assess the formulations prepared by combination of f l avors with solid dispersion.In addition to taste,we also investigated some other items such as appearance,sedimentation ratio,drugcontent and impurities.All the evaluation parameters were shown in Table 5.
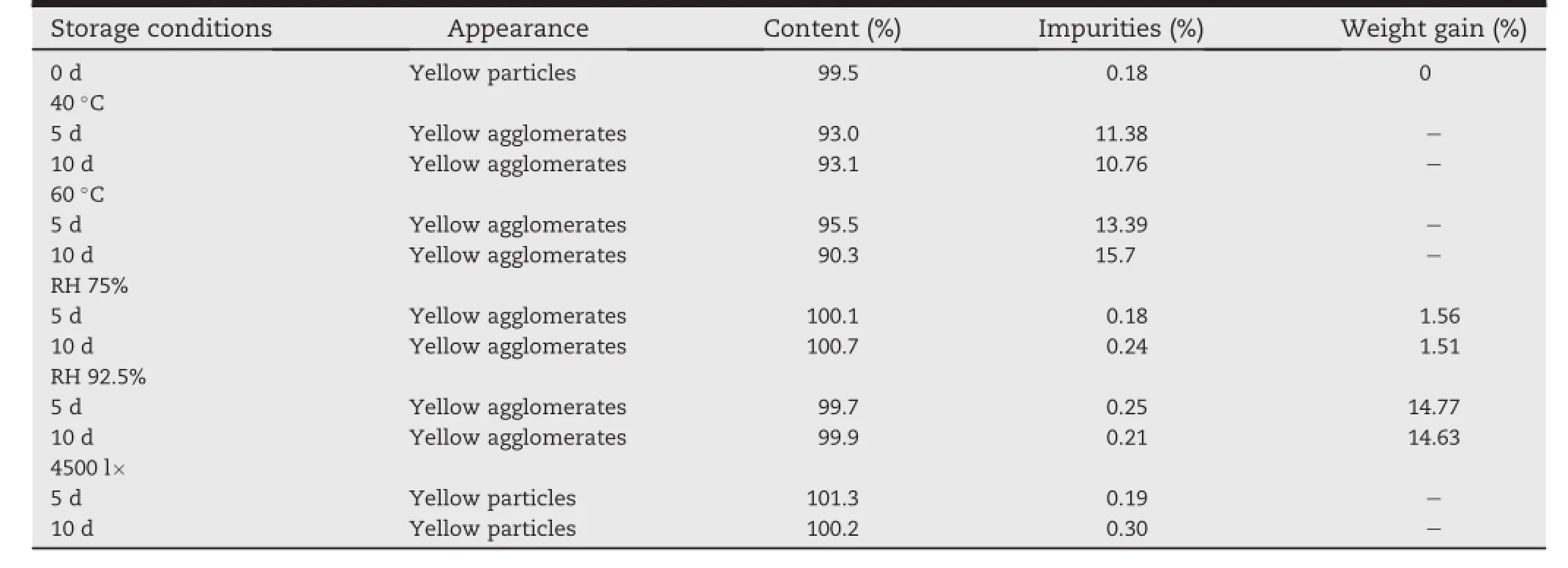
Table 6-Stability results of the suspensions.
For oral dosage form,in vitro dissolution is important.The dissolution of commercial tablet,capsule and self-made dry suspension were performed in water and the corresponding prof i les are shown in Fig.5.
With a taste evaluation score of 4.9,the self-made formulation successfully covered the bitter taste of ARB.
In comparison with the commercial tablets and capsules, the self-made formulations which showed a slower and lower release,around 90%of the drug dissolved within 60 min,it would increase the residence time of the dosage form in the gastrointestinal and achieve a prolonged therapeutic effect in some extent.Further in vivo research would investigate to compare the absorption of them.The insoluble carrier octadecanol blocked the drug dissolving from the solid dispersion.
3.5.Primary stability study of the suspensionsAppearance,drug content,impurities,weight gain were investigated and the results were shown in Table 6.
After 10 d,the formulation showed a decreased content of ARB of 93.1%and 90.3%,an increased content of impurities of 10.76%and 15.7%under the high temperature of 40°C and 60°C,respectively.Theresultsshowedthatthechoiceofthese excipients maybe not suitable for ARB,studies of active drugexcipient compatibility will be performed to support our fi ndings.And weight gained a lot under the high relative humidity of 75%and 92.5%.So the self-made suspensions should be stored in sealed containers away from heat,light and humidity to keep its properties unchanged.
4.Conclusion
In the research,we developed a method which was a combination of solid dispersion with f l avors for preparing ARB dry suspension.It was proved the approach successfully covered the bitter taste by the organoleptic evaluation. Octadecanol as carrier in the solid dispersion covered the bitter taste of ARB effectively,and a better taste producedwhile the addition of an appropriate amount of f l avors.In comparison with the commercial tablets and capsules,the self-made formulations showed a lower and slower release. The insoluble carrier octadecanol blocked the drug dissolving from the solid dispersion.The results of primary stability study showed that the self-made suspensions were very sensitive to high temperature;they should be stored in sealed containers away from heat,light and humidity.Similar solid dispersion techniques could be applied to other poor taste drugs,which could cover the bitter taste of bitter drugs by oral administration.
REFERENCES
[1]Shi L,Xiong H,He J,et al.Antiviral activity of arbidol against inf l uenza A virus,respiratory syncytial virus,rhinovirus coxsackie virus and adenovirus in vitro and in vivo[J].Arch Virol 2007;152:1447-1455.
[2]Obrosova-serova NP,Burtseva EI,Nevskii IM,et al.The protective action of arbidol during a rise in respiratory disease in 1990[J].Vopr Vinisol 1991;36:380-381.
[3]Boriskin YS,Leneva IA,Pecheur EI,et al.Arbidol:a broadspectrum antiviral compound that blocks viral fusion.Curr Med Chem 2008;15:997-1005.
[4]Brooks MJ,Sasadeusz JJ,Tannock GA.Antiviral chemotherapeutic agents against respiratory viruses:where are we now and what's in the pipeline?Curr Opin Pulm Med 2004;10:197-203.
[5]Leneva IA,Russell RJ,Boriskin YS,et al.Characteristics of arbidol-resistant mutants of inf l uenza virus:implications for the mechanism of anti-inf l uenza action of arbidol.Antivir Res 2009;81:132-140.
[6]Burtseva EI,Shevchenko ES,Leneva IA,et al.Rimantadine and arbidol sensitivity of inf l uenza viruses that caused epidemic morbidity rise in Russia in the 2004-2005 season. Vopr Virusol 2007;52(2):24-29.
[7]Wang MZ,Cai BQ,Li LY,et al.Eff i cacy and safety of arbidol in treatment of naturally acquired inf l uenza.Acta Acad Med Sin 2004;26(3):289-293.
[8]Liu HB,Qu WX,Li SQ,et al.Multicenter randomized double blind parallel clinical trial of inf l uenza arbidol hydrochloride tablet in the treatment of naturally acquired.Chin J Clin Pharmacol 2006;22(6):403-405.
[9]Sharma Deepak,Kumar Dinesh,Singh Mankaran,et al.Tste masking technologies:a novel approach for the improvement of organoleptic property of pharmaceutical active substance.Int Res J Pharm 2012;3(4):2230-8407.
[10]Dimitrios N.Solid dispersions,part II:new strategies in manufacturing methods for dissolution rate enhancement of poorly water-soluble drugs.Expert Opin Drug Deliv 2011;8:1663-1680.
[11]Parinda S,Hans de W,Henderik WF,et al.Improved dissolution behavior of lipophilic drugs by solid dispersions: the production process as starting point for formulation considerations.Expert Opin Drug Deliv 2011;8:1121-1140.
[12]Mohd A,Raisuddin A,Fahad I,et al.Solid dispersions:a strategy for poorly aqueous soluble drugs and technology updates.Expert Opin Drug Deliv 2012;9:1419-1440.
[13]Kawano Yayoi,Ito Akihiko,Sasatsu Masanaho,et al. Preparation and evaluation of taste masked orally disintegrating tablets with granules made by wet granulation method.Pharm Soc Jpn 2010;130(12):1737-1742.
[14]Mann AS,Jain NK,Khrya MD.Evaluation of suspending properties of Cassia tora mucilage on sulphadimide suspension.Asian J Exp Sci 2007;21(1):63-67.
[15]Liu C,Wu JH,Zhang YX.Chin J Clin Pharm 2005;15:332-335.
[16]Patel A,Amrit S.Formulation taste masking-from bitter to better:the latest taste masking techniques can yield more palatable drugs.Pharm Formul Qual 2009:1-2.
[17]Sohi H,Sulta Y,Khar RK.Taste Mashing Technologies in oral pharmaceuticals,recent development and approaches. Drugs Dev Ind Pharm 2004;30(5):429-448.
*Corresponding author.Tel./fax:+86 24 23986522.
E-mail address:xrzhxr@126.com(X.Zhang).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.07.001
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
Taste masking
Dry suspension
Solid dispersion
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Targeted delivery of docetaxel to the metastatic lymph nodes:A comparison study between nanoliposomes and activated carbon nanoparticles
- Degradation kinetic study of lysine in lysine hydrochloride solutions for injection by determining its main degradation product
- Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets:A study in diet induced hyperlipidemic rabbits
- Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modif i ed by two-layered membrane techniques
- Evaluation of chitosan-anionic polymers based tablets for extended-release of highly watersoluble drugs
