Degradation kinetic study of lysine in lysine hydrochloride solutions for injection by determining its main degradation product
2015-05-15MengyingTaoMengZhuChunnuanWuZhongguiHe
Mengying Tao,Meng Zhu,Chunnuan Wu,Zhonggui He
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
Degradation kinetic study of lysine in lysine hydrochloride solutions for injection by determining its main degradation product
Mengying Tao,Meng Zhu,Chunnuan Wu,Zhonggui He*
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
ARTICLEINFO
Article history:
Received 8 April 2014
Received in revised form
17 July 2014
Accepted 21 August 2014
Available online 28 August 2014
Lysine hydrochloride
A limited number of researches have been reported to apply the Arrhenius equation to study the relationship between drugs and its degradation products so far.In the present work,the thermal degradation kinetics of lysine hydrochloride solutions for injection,the special solvent for ademetionine 1,4-butanedisulfonate(SAM)for injection,was investigated at selected temperatures and pH values.The main degradation product of lysine was separated,purif i ed,and conf i rmed as lysine lactam.A reversed-phase high performance liquid chromatographic(RP-HPLC)method without derivation was developed for the simultaneous determination of lysine and lysine lactam.The results conf i rmed that both the lysine degradation and lysine lactam generation followed zero-order reaction kinetics. The degradation and generation rate constants increased with increasing temperatures and decreasing pH values.The temperature-dependent degradation and generation reaction could be suff i ciently modeled on the Arrhenius equation with the activation energy of 80.14 and 83.22 kJ/mol,respectively.Meanwhile,a linear relationship existed between the amount of lysine degradation and lysine lactam generation since the approximate activation energy.Considering there could be other side effects,we established an upper limit of lysine lactam(500 μg/ml),as the acceptable criteria for stability to estimate the shelf life together with lysine,which made the prediction more accurate and credible.Extrapolation data demonstrated that the lysine hydrochloride solutions for injection could be stable for two years stored at room temperature.
©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
1.Introduction
Lysine,one of the essential amino acids,plays an important part in promoting human development,enhancing immune function and improving the function of the central nervous tissue[1].Meanwhile,lysine also has a positive effect on the absorption of calcium and the formation of collagen with other nutrients[2].Additionally,lysine is generally used as anutritional supplement to make up the possible def i ciencies of lysine in humans[3].Although lysine shows great promise,it is extremely unstable under normal conditions. Hence,the natural basic amino acidL-lysine generally exists in the form of crystalline salts of acid and basic bases, especiallyinthehydrochlorideandacetateforms,to improve the stability.
The determination of lysine is becoming increasingly important in clinical analysis,biochemistry and pharmaceutics.Several methods are reported for the lysine determination based on spectrophotometry,potentiometric titration, thin layer chromatography,liquid chromatography,micellar liquid chromatography and hydrophilic interaction chromatography[4-9].The determination of lysine by f l ow injection analysis techniques[10]has also been described.Even though there are so many methods available,there are still problems need to be solved,including the complex sample pretreatment process,time-consuming analysis and the uncommon instrumentations.As a zwitterionic compound,the reversed-phase chromatography of lysine with buffered mobile phase at different pH values usually presents typically poor retention[11].However,mobile phase containing ionpairing reagents[12]can improve retention of such situations,which makes the rapid and direct determination of underivatized lysine possible.
Since the poor stability of Ademetionine 1,4-butanedisulfonate(SAM)under weakly acidic and alkaline conditions,it is infeasible to directly adjust the pH of the solution to neutral during the preparation of SAM for injection. Hence,a special solvent,lysine hydrochloride solutions for injection need to be prepared as pH modif i ers to adjust the pH of the SAM solution before injection to improve the stabilityduringprocessingandstorage.However,our research found that a signif i cant degradation product had generated in solutions for injection after sterilization,and no information was available on the structure and possible generation pathways of the degradation product.To guarantee the quality of solutions for injection,the contents of both lysine and the degradation product should be used as the control parameters.Also,since few studies have investigated the kinetic stability of lysine and the degradation product to date,a thermal degradation kinetic study on the lysine hydrochloride solutions for injection should be conducted applying the Arrhenius equation to quantify the effect of temperature on the product quality and to predict the shelf life[13].
In this study,we attempted to separate and conf i rm the main degradation product in lysine hydrochloride solutions for injection,and to develop a sensitive RP-HPLC method to rapidly detect lysine and the degradation product.Our efforts focused on the kinetic investigation of the lysine degradation and the main degradation product generation in solutions for injection under various conditions.The aim of the present work was to study the thermal stability of lysine hydrochloride solutions for injection,and determine the kinetic parameters of lysine degradation and the degradation product generation,with respect to the effects of pH value and temperature;and to apply the Arrhenius equation for predicting the shelf life of solutions for injection during storage and/or thermal processing.
2.Materials and methods
2.1.Materials
Lysine hydrochloride was purchased from Kyowa Amino Acid Co.,Ltd.(Shanghai,China;Purity,100.1%).Lysine lactam standard substance was purchased from Sigma-Aldrich (Shanghai,China;Purity≥97.0%).MethanolofHPLCgradewas purchased from Concord Technology Co.,Ltd(Tianjin,China). All other materials were analytical grade quality.All solutions were prepared using distilled water throughout the study.
2.2.Preparation of lysine hydrochloride solutions for injection
To study the thermal stability,lysine hydrochloride solutions for injection with a lysine concentration of 83.5 mg/ml were prepared by the following methods.The accurately weighed lysine was dissolved in 60%of the total volume of water for injection,and 0.1%(w/v)activated carbon was added with stirring.After stirring at room temperature for 15 min,the solution was f i ltered through 0.45 μm membrane to remove the activated carbon.Next,the pH of the solution was adjusted with sodium hydroxide solution to pH 10.3,and then water for injection was added to volume.The obtained solution was f i ltered again using a 0.22 μm membrane f i lter to make the solution clear and sterile.Finally,the solution was nitrogen-f i lled sealed and autoclaved at 121°C for 12 min.
2.3.Separation,purif i cation and characterization of the main degradation productSince the rare information available on the degradation productinlysinehydrochloridesolutionsforinjection, method for separating and purifying the degradation product was designed as follows:the solvent was removed from the lysine hydrochloride solutions for injection by vacuum evaporation to form a concentrated mixture of lysine and the main degradation product.Subsequently,the mixture was dissolved in methanol and lysine was f i ltered out because of its poor solubility.The f i ltrate was collected and dried in a vacuum rotary evaporator.The resulting crude product was loaded onto a silica gel column,with n-propanol-ammonia (5:1)as eluent.The fractions were collected and sprayed to yield the degradation product-rich powders.
The structure of the main degradation product was characterized by proton nuclear magnetic resonance(1H NMR) spectra and infrared spectra,applying a Bruker AV-600 MHz NMR spectrometer and a Bruker IFS-55 infrared spectrometer, respectively.
2.4.Degradation kinetic modeling of lysine hydrochloride solutions for injection
The thermal stability of the lysine hydrochloride solutions for injection was studied at selected temperatures(60,80,90 and 100°C).The pH 10.3 lysine hydrochloride solutions for injection were placed in a thermostatic water bath(Zhengzhou Greatwall Scientif i c Industrial and Trade Co.Ltd.,China)preheated to a given temperature.At specif i ed time intervals (0,2,5,8,12,24 and 36 h for 80,90 and 100°C;while,0,48,96, 144,192,240 and 288 h for 60°C),one ampoule of each temperature was randomly removed from the water bath and promptly cooled by putting into an ice water bath[14,15].The contents of lysine and the degradation product in cooled ampoules were determined simultaneously by an HPLC method.
Since the purpose of preparing the lysine hydrochloride solutions for injection was to adjust the SAM solution to neutral before injection,the pH value of the solutions need to be controlled between 10.0 and 10.6.Therefore,the effect of pH on degradation stability was conducted at three different pH values(10.0,10.3 and 10.6)at an extreme 100°C.To carry on the study,lysine hydrochloride solutions for injection at specif i ed pH values were prepared.
Parameters of the kinetic reaction included the rate constant.The rate constants of degradation and generation during heat treatment could be determined using the following equation:
where C is the concentration of lysine or the degradation product at a heating time and temperature(mg/ml);t is the heating time(h);k is the reaction rate constant([mg/ml]1-n/h); and n is the kinetic order of the reaction.
2.5.Analytical method
The concentrations of lysine and the degradation product were determined using a RP-HPLC system by the method of Sultana H et al.[16]with some modif i cations.The mobile phase was consisted of 0.1 mol/l ammonium dihydrogen phosphate containing 0.2%(w/v)sodium heptanesulfonate:-methanol(96:4,v/v).The pH of the buffer was adjusted with ammoniumhydroxideto 6.0.Separation was achieved at 30°C using an ODS C18(4.6×250 mm,5 μm)column.The f l ow rate was set at 0.8 ml/min.Samples were detected using a UV detector at 210 nm,and the injection volume was 20 μl.
2.6.Statistical analysis
All the content determination results of lysine and the main degradation product were expressed in the form of concentration.Linear regression analysis was used to obtain the rate constants of lysine degradation and the degradation product generation.Data was analyzed using the Microsoft Off i ce Excel software.
3.Results and discussion
3.1.Structure identify of the main degradation product
The1H NMR spectra and infrared spectra of the main degradation product were shown in Fig.1.The spectral data analysis was as follows:1H NMR(600 MHz,D2O,25°C):1.41(δ,1H, m,H-5),1.72(δ,1H,m,H-3),1.77(δ,1H,m,H-4),1.87(δ,1H,m, H-5),2.02(δ,1H,m,H-3),2.08(δ,1H,m,H-4),3.28(δ,2H,m,H2-6),4.30(δ,1H,dd,J=1.9 Hz,11.5 Hz,H-2).FTIR(KBr):3440.1(ν (N-H));2936.0,2855.1(ν(-CH2-));1665.6(ν(C=O));1430-1490 (δ(-CH2-));1000-1200(ν(C-N)).In Fig.1A,the labile protons signalofthenitrogenhydrogenbonddisappearedfor exchanging with the solvent D2O,resulting only nine protons can be seen in the1H NMR spectra.
Based on the1H NMR spectra and infrared spectra,we deducedthatthemaindegradationproductwaslysinelactam. Our result was in agreement with the previous studies[17,18] that the thermal degradation of amines usually generated higher molecular polyamines at stripping temperatures,such as amine dimer,amine trimer and cyclic amines.Judging from the structure of lysine and lysine lactam as well as the enzymatic conversion,one of the major manufacture process of lysine[19],itwasmoreprobablythatlysinelactamwasderived from the dehydration cyclization of lysine.Certainly,mechanism studies of lysine degradation and lysine lactam generation in solutions for injection are required for future work.
3.2.Validation of the analytical method
The developed HPLC method was characterized by evaluation of system suitability,sensitivity,linearity,accuracy,precision and stability.
3.2.1.System suitability
The determination of system suitability was accomplished by assaying the system suitability solution(n=6).The acceptance criteria for evaluating system suitability were as follows:therelativestandarddeviation(RSD)within2%, theoretical plates greater than 2000,the tailing factor within 1.5,and the resolution greater than 1.5.The results of system suitability showed that all the parameters gained were within the typical values.Fig.2 showed the chromatogram of the system suitability solution.
3.2.2.Sensitivity
The limit of detection(LOD)and quantization(LOQ)were determined adopting the proposed method by stepwise diluting the sample solutions.The LOQs for lysine and lysine lactam corresponding to a signal/noise ratio(S/N)of 10 were 3.2 μg/ml and 0.35 μg/ml,respectively.And the LODs for lysine and lysine lactam corresponding to the S/N of 3 were 0.80 μg/ ml and 0.10 μg/ml.
3.2.3.Linearity
The linearity was conducted with the standard solutions (n=7)over the concentration range of 1-12 mg/ml and 0.01-0.07 mg/ml for lysine and lysine lactam,respectively. The regression analysis was employed to establish the linearity.Typical regression equations for lysine and lysine lactam were A=596744C+65464 and A=15096C+7351.4.The correlation coeff i cient(r)was 0.9999 and 0.9998 for lysine and lysine lactam,separately.
3.2.4.Accuracy
The accuracy for the determination of lysine and lysine lactam was conducted by assaying drug substance sample at 80, 100 and 120%of the target.The apparent recovery of lysine and lysine lactam were found to be from 98.6 to 101.7%.Theaverage percent recovery was 99.9%(RSD%0.9)and 100.5% (RSD%1.0)for lysine and lysine lactam.
3.2.5.Precision
The precision of the proposed method were assessed by assaying 6 replicate sample solutions in the standard concentrations of lysine and lysine lactam.The same sample was determined 6 times to study the instrument precision.The RSD(%)of the instrument precisions and the method precisions were all less than 1.0%for lysine and lysine lactam.
3.2.6.Stability
The stability of lysine and lysine lactam in water was evaluated at ambient temperature for 12 h.No signif i cant changes of the chromatographic responses(<2%)were observed forthe sample solutions of lysine and lysine lactam,relative to freshly prepared standards.The results indicated that the sample solutions were stable at room temperature during analysis over a period of 12 h.
3.3.Kinetic stability of lysine hydrochloride solutions for injection under different heating temperatures and pH values
The stability of lysine hydrochloride solutions for injection was studied at different temperatures and pH values.The kinetics of lysine degradation and lysine lactam generation during heat treatment were investigated using Eq.(1).Under all the selected conditions,zero-order,f i rst-order and secondorder reaction kinetics were appropriate to model the thermal degradation behavior of lysine,as suggested by the relatively highcorrelationcoeff i cient(r).But,underthesameconditions, only zero-order kinetics described the generation of lysine lactam well(data not shown).For instance,at pH 10.3 and 100°C,correlation coeff i cient of lysine degradation for zeroorder,f i rst-order and second-order kinetics were 0.9992, 0.9993 and 0.9994,respectively,whereas,for lysine lactam generation,the values were 0.9997,0.9379 and 0.7705,individually.Hence,under the given conditions,lysine degradation and lysine lactam generation over time followed a zeroorder reaction model with perfect correlation coeff i cient (r≥0.9980).Accordingly,zero-order plots for lysine degradation and lysine lactam generation at different pH values and temperatures were demonstrated in Figs.3 and 4.The rate constants of lysine degradation and lysine lactam generation, calculatedbylinearregressionbasedontheexperimentaldata in Figs.3 and 4,were presented in Tables 1 and 2.As shown in Table 1,the rate constants increased with decreasing pH, which indicated that the lysine hydrochloride solutions for injection were more stable at higher pH values.The temperature also had a signif i cant impact on the stability of solutions for injection.As demonstrated in Table 2,when the temperature increasedfrom 60°C to 100°C,the rate constantsof lysine degradation and lysine lactam generation increased from 0.0040 to 0.0739 and 0.0008-0.0197 mg/ml/h,respectively.The results all showed the sensitivity of the lysine hydrochloride solutionsforinjectiontohightemperaturesandlowpHvalues.
As it was reported,when applying the integration method to accurately estimate the reaction order,the concentration range of the experimental data should be large enough.While, our results showed that lysine was stable during heat treatment,the contentoflysinewas only3.3%lowerevenheatedat 100°C for 36 h.In order to obtain a suff i ciently large range oflysine concentration,more intense test conditions were needed.Since the tolerance of the existing instruments at high temperature for long time was not desirable,adopting more advanced equipment with better tolerance to do further research on the lysine degradation to validate its reaction order should be take into consideration.
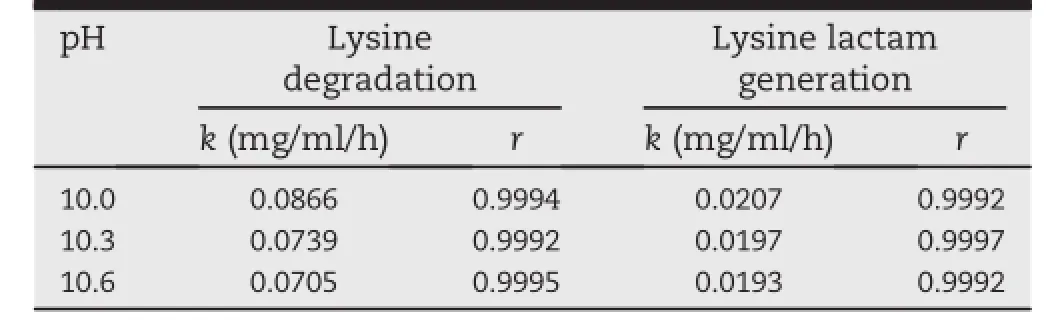
Table 1-Rate constant(k)and correlation coeff i cient(r)of lysine degradation and lysine lactam generation at 100°C and pH 10.0,10.3 and 10.6.
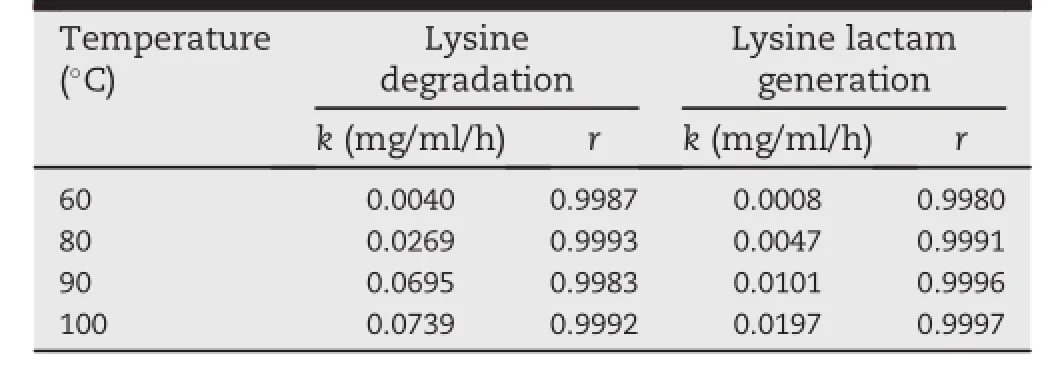
Table 2-Rate constant(k)and correlation coeff i cient(r)of lysine degradation and lysine lactam generation at pH 10.3 and temperatures of 60,80,90 and 100°C.
3.4.Application of Arrhenius equation and predication of the shelf life
Since the lysine degradation and lysine lactam generation followed the zero-order reaction kinetics,the relationship between the rate constants and temperature could be established by f i tting the experimental data to the Arrhenius equation:
where k is the reaction rate constant;A is pre-exponential factor;Eais activation energy(J/mol);T is thermodynamic temperature(K),and R is molar gas constant(8.314 J/K/mol). By moving the exponential we can have:
Activation energy and other kinetic parameters were calculated by the linear relationship between lnK and 1/T as described in Eq.(3).Fig.5 showed the Arrhenius plots for lysine degradation and lysine lactam generation at pH 10.3, and the statistical parameters were showed in Table 3.
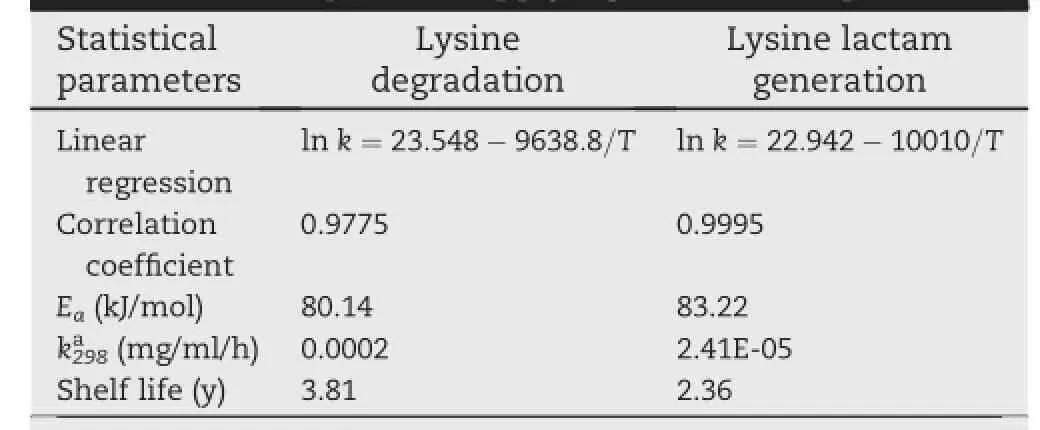
Table 3-Statistical parameters and extrapolated shelf life at room temperature applying Arrhenius equation.
The Eavalue of lysine lactam generation(83.22 kJ/mol)was approximate to that of lysine degradation(80.14 kJ/mol), indicating that the change rates of the reaction rate constants with temperature for lysine lactam generation and lysine degradation were the same.Since the approximate activation energy,we concluded that a linear relationship existed between the amount of lysine degradation and lysine lactam generation.Therefore,we could study the degradation of lysine hydrochloride solutions for injection by determining the generation amount of lysine lactam,which made the research more simple and convenient.
The Arrhenius plots of lysine degradation and lysine lactam generation were used simultaneously to estimate the shelf life of lysine hydrochloride solutions for injection at room temperature,which made the prediction more accurate and credible.It was worth mentioning that an upper and lower limit of 8%of initial lysine concentration in lysine hydrochloride solutions for injection was established as acceptancestandardforstability.Besides,consideringother possible side effects,an upper limit of lysine lactam,500 μg/ ml,was also used.By extrapolating from Arrhenius equation, the lysine degradation and lysine lactam generation rate constants at room temperature(k298)were calculated(shown in Table 3),and the shelf life were obtained,3.81 y for lysine and 2.36 y for lysine lactam.Therefore,we concluded that the lysine hydrochloride solutions for injection could be stable stored at room temperature for two years.
4.Conclusion
The thermal degradation product lysine lactam was successfully separated from lysine hydrochloride solutions for injection and was simultaneously determined with lysine using a new ion-pair RP-HPLC method without derivation.The present study provided detailed information with respect to the kinetic stability of lysine and lysine lactam at different temperatures and pH values.The study showed that the lysine degradation and lysine lactam generation were adequately fi tted to a zero-order reaction kinetic model,and the temperatureand pH levelshad a signi fi cantimpactonthe stability of lysine hydrochloride solutions for injection,which were more stable at higher pH values and lower temperatures.The dependence of degradation and generation process on temperature was described by Arrhenius equation,and the main kinetic parameters were calculated.The shelf life of lysinehydrochloride solutions for injection was estimated by lysine degradation and lysine lactam generation together,with a consequence of 2.36 years.Further studies on the mechanism of lysine degradation and lysine lactam generation during heat treatment are certainly required considering the great potential of lysine in nutritional enhancement.
REFERENCES
[1]Flodin N.The metabolic roles,pharmacology,and toxicology of lysine.J Am Coll Nutr 1997;16:7-21.
[2]Bremer H,Duran M,Kamerling J,et al.Disturbances of amino acid metabolism:clinical chemistry and diagnosis. Baltimore:Urban&Schwarzenberg;1981.p.23.
[3]Saurina J,Hernˊandez-Cassou S,Alegret S,et al. Amperometric determination of lysine using a lysine oxidase biosensor based on rigid-conducting composites.Biosens Bioelectron 1999;14:211-220.
[4]Hasani M,Yaghoubi L,Abdollahi H.A kinetic spectrophotometric method for simultaneous determination of glycine and lysine by artif i cial neural networks.Anal Biochem 2007;365:74-81.
[5]Saurina J,Hernˊandez-Cassou S,Alegret S,et al. Determination of lysine in pharmaceutical samples containing endogenous ammonium ions by using a lysine oxidase biosensor based on an all-solid-state potentiometric ammonium electrode.Biosens Bioelectron 1999;14:67-75.
[6]Nabi S,Khan M.Selective TLC separation of lysine and threonine in pharmaceutical preparations.Acta Chromatogr 2003:161-171.
[7]Fabregas J,Beneyto J.Simultaneous determination of cephalexin and lysine in their salt using high-performance liquid chromatography of derivatives.J Pharm Sci 1980;69:1378-1380.
[8]Garcı´a-Villar N,Saurina J,Hernˊandez-Cassou S.Liquid chromatographic determination of lysine by potentiometric detection with a biosensor.Anal Lett 2002;35:1313-1325.
[9]Douˇsa M,Bˇrichˊaˇc J,Gibala P,et al.Rapid hydrophilic interaction chromatography determination of lysine in pharmaceutical preparations with f l uorescence detection after postcolumn derivatization with o-phtaldialdehyde.J Pharm Biomed Anal 2011;54:972-978.
[10]Divritsioti M,Karalemas I,Georgiou C,et al.Flow injection analysis system for l-lysine estimation in foodstuffs using a biosensor based on lysine oxidase immobilization on a goldpoly(m-phenylenediamine)electrode.Anal Lett 2003;36:1939-1963.
[11]Hancock W,Bishop C,Hearn M.The analysis of nanogram levels of free amino acids by reverse-phase high-pressure liquid chromatography.Anal Biochem 1979;92:170-173.
[12]Frey J,Chamson A,Raby N.Separation of amino acids using ion-paired reversed-phase high-performance liquid chromatography with special reference to collagen hydrolysate.Amino Acids 1993;4:45-51.
[13]Ertel K,Carstensen J.Examination of a modif i ed Arrhenius relationship for pharmaceutical stability prediction.Int J Pharm 1990;61:9-14.
[14]Wu Y,Mao J,Mei L,et al.Kinetic studies of the thermal degradation of sulforaphane and its hydroxypropyl-βcyclodextrin inclusion complex.Food Res Int 2013;53:529-533.
[15]Hou Z,Qin P,Zhang Y,et al.Identif i cation of anthocyanins isolated from black rice(Oryza sativa L.)and their degradation kinetics.Food Res Int 2013;50:691-697.
[16]Sultana H,Onodera R,Or-Rashid M,et al.Convenient method for the determination of arginine and its related compounds in rumen f l uid by reversed-phase highperformance liquid chromatography.J Chromatogr B Biomed Sci Appl 2001;755:321-329.
[17]Lepaumier H,da Silva E,Einbu A,et al.Comparison of MEA degradation in pilot-scale with lab-scale experiments. Energy Procedia 2011;4:1652-1659.
[18]Davis J,Rochelle G.Thermal degradation of monoethanolamine at stripper conditions.Energy Procedia 2009;1:327-333.
[19]Tosaka O,Enei H,Hirose Y.The production of l-lysine by fermentation.Trends Biotechnol 1983;1:70-74.
*Corresponding author.Tel./fax:+86 24 23986321.
E-mail address:hezhgui_student@aliyun.com(Z.He).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.08.012
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.All rights reserved.
Lysine lactam
Degradation kinetics
Shelf life
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Delivery systems for siRNA drug development in cancer therapy
- Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs
- Evaluation of chitosan-anionic polymers based tablets for extended-release of highly watersoluble drugs
- Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modif i ed by two-layered membrane techniques
- Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets:A study in diet induced hyperlipidemic rabbits
- Targeted delivery of docetaxel to the metastatic lymph nodes:A comparison study between nanoliposomes and activated carbon nanoparticles
