Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs
2015-05-15VrpornBurphcheepJunyprsertBoontidMorkul
Vrporn Burphcheep Junyprsert,Boontid Morkul
aDepartment of Pharmacy,Faculty of Pharmacy,Mahidol University,Thailand
bCenter of Excellence in Innovative Drug Delivery and Nanomedicine,Faculty of Pharmacy,Mahidol University, Thailand
Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs
Varaporn Buraphacheep Junyapraserta,b,*,Boontida Morakula
aDepartment of Pharmacy,Faculty of Pharmacy,Mahidol University,Thailand
bCenter of Excellence in Innovative Drug Delivery and Nanomedicine,Faculty of Pharmacy,Mahidol University, Thailand
ARTICLEINFO
Article history:
Received 16 June 2014
Received in revised form
15 July 2014
Accepted 17 August 2014
Available online 27 August 2014
Nanocrystals
Nanocrystals,a carrier-free colloidal delivery system in nano-sized range,is an interesting approach for poorly soluble drugs.Nanocrystals provide special features including enhancement of saturation solubility,dissolution velocity and adhesiveness to surface/cell membranes.Several strategies are applied for nanocrystals production including precipitation,milling,high pressure homogenization and combination methods such as Nano-Edge™,SmartCrystal and Precipitation-lyophilization-homogenization(PLH)technology. For oral administration,many publications reported useful advantages of nanocrystals to improve in vivo performances i.e.pharmacokinetics,pharmacodynamics,safety and targeted delivery which were discussed in this review.Additionally,transformation of nanocrystals to f i nal formulations and future trends of nanocrystals were also described. ©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
The poor solubility of drug is a major problem which limits the development of highly potent pharmaceutics.The drugs with low solubility lead to low oral bioavailability and erratic absorptionwhichis particularly pertinentto drugswithinclassII of the Biopharmaceutical Classif i cation System(BCS).Generally,the rate-limiting step for absorption of the drugs in this class is the dissolution velocity arising from low solubility. Although the drugs are high permeability,the poor solubility results in a low concentration gradient between gut and blood vessel consequent to a limitation of drug transport and oral absorption.Nowadays,there are a large percentage of drug compounds in drug development represents as poor aqueous solubility.Therefore,one ofthe mostchallenging tasksin drug development is to improve the drug solubility in order to enhance the bioavailability of these drugs.Several strategies have been employed to overcome these limitations.The approaches to increase the solubility and the available surface area for dissolution are classif i ed as physical and chemical modif i cations.For the physical modif i cation,the techniques includedecreasingparticlesize(micronization,nanonization), formation of polymorphs/pseudopolymorphs(including solvates),complexation/solubilization(by means of using surfactants or cyclodextrins,conjugation to dendrimers,and anadditionof co-solvents)and preparation of drug dispersions in carriers(eutectic mixtures,non-molecular solid dispersions, solid solutions).For the chemical modif i cation,the used technique is the synthesis of soluble prodrugs and salts[1-5].
Particle size reduction has been a much smarter approach that can be applied to nonspecif i c formulation for many years. The micronization of drug leads to an increase in their surface area which proportionally increases in rate of dissolution and rate of diffusion(absorption).However,for very low solubility compounds the micronization fails to improve the saturation solubility and increase the bioavailability of the drug.Therefore,the further step to reduce the particle dimension to nanometer size range has been invented.Recently,particle diminution to the sub-micron range has emerged to be a powerful formulation approach that can increase the dissolutionrateandthesaturationsolubility,subsequently improve the bioavailability of poorly water-soluble drugs and may also decrease systemic side effects.Over the last decade, drug nanocrystals are considered as a novel approach to improve the solubility of hydrophobic drugs since the technique is simple and effective which can quickly launch product to the market.The nanocrystals were invented at the beginning of the 1990s and the f i rst products appeared very fast on the market from the year 2000 onwards.Additionally, drug nanocrystals are a universal approach generally applied to all poorly soluble drugs for the reason that all drugs can be disintegrated into nanometer-sized particles[6].
Drug nanocrystals are nanoscopic crystals of parent compounds with the dimension of less than 1 μm.They are composed of 100%drug without carriers and typically stabilized with surfactants or polymeric steric stabilizers.A dispersion of drug nanocrystals in an outer liquid medium and stabilized by surface active agents is so-called nanosuspensions.The dispersion medium can be water,aqueous or nonaqueous media e.g.liquid polyethylene glycol(PEG)and oils.The nanosuspensions can be used to formulate compounds that are insoluble in both water and oil and to reformulate existing drugs to remove toxicologically less favorable excipients.Additionally,the poorly soluble drugs enable to be formulated as nanosuspensions alone,or with a combination of pharmaceutical excipients[2,4,7].
2.Special features of nanocrystals to enhance oral bioavailability
Poorly soluble drugs encounter biopharmaceutical delivery problems such as low bioavailability after oral administration, low penetration of the drug into the skin,large injection volume for intravenous(i.v.)administration and undesired side effectsafter i.v.injection whenusing traditional formulations. Drug nanocrystals possess outstanding features enabling to overcome the solubility problems including an increase in saturation solubility,an increase in dissolution velocity,and an increased adhesiveness to surface/cell membranes[6].
These features are resulted from transferring of particle size from macroparticle to nanodimension that changes their physicochemical properties on the basis of nanotechnology.A detailed description of the physical background of these effects is shown below.
2.1.An increase in saturation solubility(Cs)
In general,saturation solubility is a compound-specif i c constant,which is depending on physicochemical properties of the compound,dissolution medium and temperature.However,this def i nition is only valid for drug particles with a minimum particle size in the micrometer range.Furthermore, the saturation solubility is also a function of the crystalline structure(i.e.latticeenergy)andparticlesize.Thepolymorphic modif i cation with highest energy and lowest melting point leads to the best solubility.Occasionally,homogenization process generates amorphous fraction with high inner energy thatcontributestoanincreasedsolubilityofthesubstance.For the particle size aspect,the saturation solubility is also a function of particle size when a critical size is below 1-2 μm. The saturation solubility increases with decreasing particle sizebelow1000nm.Thisphenomenoncanbeexplainedbythe Kelvin and the Ostwald-Freundlich equations.
TheKelvinequation(Eq.(1))isoriginallyusedtodescribethe vapor pressure over a curved surface of a liquid droplet in gas (aerosol).A decrease in the particle size of liquid droplet contributes to an increase in curvature of the surface and the increasing vapor pressure.The situation of a transfer of moleculesfromaliquiddroplettoagasiscomparabletothetransfer of molecules from a solid nanocrystal to a liquid dispersion medium.Therefore,the Kelvin equation is also applicable to explain the relation between the dissolution pressure and the curvature of the solid particles in liquid.The dissolution pressureisequivalenttothevaporpressure.Atsaturationsolubility state,the dissolving molecules and recrystallizing molecules areequilibrium.Thedissolutionpressurecanbeincreasedwith increasing curvature(decreasing particle size).Therefore, the equilibrium is shifted toward dissolution,and thus the saturation solubility increases.The curvature is especially immense when the particle size is in the nanometer range.
where Pris the dissolution pressure of a particle with the radius r,P∞is the dissolution pressure of an inf i nitely large particle,γ is the surface tension,R is the gas constant,T is the absolute temperature,r is the radius of the particle,Mris the molecular weight,ρ is the density of the particle.
The Ostwald-Freundlich equation(Eq.(2))directly describes the relation between the saturation solubility of the drug and the particle size.
where Csis the saturation solubility,Cαis the solubility of the solid consisting of large particles,σ is the interfacial tension of substance,V is the molar volume of the particle material,R is the gas constant,T is the absolute temperature,ρ is the density of the solid,r is the radius.
FromtheOstwald-Freundlichequation,itobviously shows that the saturation solubility(Cs)of drug increases with a decrease in the particle size(r).However,this effect is not substantial for larger particles but will be pronounced for materials that have a mean particle size of less than 1-2 μm, especially well under 200 nm[2-4,8-11].
2.2.An increase in dissolution velocity
Nanocrystals possess an increased dissolution velocity that can be explained by the Noyes-Whitney equation(Eq.(3)).
where dX/dt is the dissolution velocity,D is the diffusion coeff i cient,A is the surface area,hDis the diffusional distance,Csis the saturation solubility,Ctis the concentration around the particles.
The dissolution velocity(dX/dt)of drug nanocrystals increases due to the greater surface area(A)and the increase in saturation solubility(Cs)of the compound.The size reduction of nanocrystals leads to an increased surface area and thus according to the Noyes-Whitney equation the dissolution velocity is increased[12].Furthermore,the size reduction of nanocrystals also leads to an increased saturation solubility which can provide two advantages.Firstly,dissolution velocity is further enhanced due to an increased in concentration gradient(Cs-Ct)/hD,according to Noyes-Whitney equation. Secondly,an increasein saturationsolubilitycontributesto an increase in concentration gradient between gut lumen and blood;therefore,the permeation and absorption by passive diffusion is further promoted.
Anotherimportantfactor isthediffusionaldistancehD,as a part of the hydrodynamic boundary layer hH,which is also strongly dependent on the particle size as shown by Prandtl equation(Eq.(4)):
where hHis the hydrodynamic boundary layer thickness,k denotes a constant,L is the length of the particlesurface in the direction of f l ow,V is the relative velocity of the f l owing liquid surrounding the particle.
In accordance with Prandtl equation,the particle size reduction leads to a decreased diffusional distance hDand consequently an increased dissolution velocity,as described by Noyes-Whitney equation[2-4,8-11].
2.3.An increased adhesiveness to surface/cell membranes
Comparingwithmicroparticles,drugnanocrystalshaveanother outstanding feature because they can distinctly increase adhesivenesstosurface/cellmembranes.Anincreasedadhesiveness of nanomaterials is usually due to an increased contact area of small particles versus large particles(at identical total particle mass).Similar to other nanoparticles,drug nanocrystals show an increased adhesiveness to tissue which lead to an improvement of oral absorption of poorly soluble drugs apart from the increased saturation solubility and dissolution rate[2,3].This aspectwillbefurtherdiscussedintopic“Invivoperformancesof drug nanocrystals in oral administration routes”.
An additional feature of nanocrystals is an advantage of amorphous state drug nanocrystals.The drug nanocrystals in the amorphous state possess higher saturation solubility comparedtoequallysizeddrugnanocrystalsinthe crystalline form.Therefore,to obtain the highest saturation solubility,a combination of nanometer size and amorphous state is ideal.The process to produce drug nanocrystals may induce the transformation of crystalline structure,increasing an amorphous fraction in the particle or even creating completely amorphous particles that make the drugs dissolve more rapidly.However,the utilization of the amorphous state in pharmaceutical products has to be concerned that it can maintain the amorphous state for the shelf life of the product[4].Furthermore,the high drug loading of nanocrystals is also one aspect that makes nanocrystals to be very ef fi cient in transporting drug to or into cells,reaching a suffi ciently high therapeutic concentration for pharmacological effect[6,10].
Another special feature of drug nanocrystals is a longterm stability.The nanosuspension could provide the good physical stability by an absence of aggregation and Ostwald ripening phenomenon.The prevention of aggregation may be achieved by the addition of surface active agent including ionic surfactants,non-ionic surfactants,and polymers which can provide an electrostatic and steric repulsion between the nanocrystals.It was reported that a combination using of electrostatic and steric stabilizer usually had a better effectiveness for stabilizing drug nanocrystals.Ostwald ripening phenomenon[13]is the incident that the solute concentration in the vicinity of smaller particles is higher than the large particles due to the higher saturation solubility of small particles.Therefore,the molecules surrounding of the small particles will diffuse to surround the large particles driven by the concentration gradient.Then,the recrystallization on the surface of the larger particles is occurred and leads to the formation of microparticles.A narrow size distribution of drug nanocrystals can avoid the different in saturation solubility due to the different particle sizes.In general,to achieve an absence of Ostwald ripening phenomenon,the narrow size distribution of drug nanocrystals should be concerned.Besides the physical stability,drug nanocrystals can be used for a chemical stabilization of chemically labile drug.The increased stability of drug nanocrystals can be explained by a shield effect of surfactants and a monolayer of degraded drug molecules which acts as the surface of drug nanocrystals for protecting the drug underneath its surface from degradation.Paclitaxel formulated in nanosuspension can preserve drug from degradation which can be exemplifi ed for this incident[14].Another good example is omeprazole,the chemically labile drug.The stability of omeprazole nanosuspension was distinctly increased when compared to an aqueous solution[15].Additionally,the same result was found for ascorbyl palmitate nanocrystals.The chemical stability of ascorbyl palmitate could be improved when formulated as nanosuspension,in comparison to its methanolic solution and other colloidal carrier systems[16].
Research studies on bene fi ts of nanocrystals are exemplifi ed as follows.Mauludin et al.developed an oral rutin nanocrystal-loaded tablet via high pressure homogenization method.They found that the dissolution velocity of the rutin nanocrystal-loaded tablet was superior compared to the rutin microcrystal-loaded and the marketed tablets.The improving dissolution behavior of the rutin nanocrystal-loaded tablet led to a better bioavailability of the poorly soluble rutin in thebody[17].Andrej et al.prepared the crystalline nano-sized celecoxib by the emulsion-diffusion method using three different stabilizers(Tween 80,polyvinyl pyrrolidone K30 (PVP K30)and sodium dodecyl sulfate(SDS)).The result showed that nanocrystals had a dramatic increase in dissolution rate and extent compared to micronized form[18].In the study by Kocbek et al.,they developed the formulation of ibuprofen as a nanosuspension by melt emulsif i cation and solvent diffusion method.The results demonstrated that the combination of Tween 80 and PVP K25 used as stabilizers yielded nanosuspensions of the smallest average particle size.The nanosuspensions,either in the form of lyophilized powder or granules,were very successful in enhancing dissolution rate as shown that more than 65%of the drug was dissolved within the f i rst 10 min compared to less than 15%of the micronized drug.They concluded that the increase in in vitro dissolution rate might favorably affect bioavailability and improve safety for the patient by decreasing gastric irritancy[7].
In conclusion,the properties of drug nanocrystals that should be concerned and the given benef i ts over the microsizedparticlesaresummarizedinTable1[4].Alternatively,the nanotoxicity of drug nanocrystals cannot be neglected.In recent years,a concern of nanotoxicity of nanoparticles is increased due to the fact that nanoparticles have ability to enter the cell and cause damage to single cells.Hence,the interaction of nanoparticles with cells and its uptaking should be considered when the nanoparticles are developed.The nanotoxicity should be aware in nanoparticles with particle size below 100 nm and prepared by non-degradable polymer. Normally,the nanoparticles in range of 100 nm up to 1000 nm can only be taken up by quite limit number of cells with phagocytic activity and are not easy to access.Therefore,the toxic risk is limited.In contrast,the nanoparticles with size below100nmcanbetakenupbyallcellsbyendocytosiswhich leads to the high risk of toxicity.Additionally,the persistency of nanoparticles in the body after administration also affects the risk of toxicity.It should be considered that the nanoparticles can be degraded in the body or at least be eliminated, otherwise they are biopersistent.The non-biodegradable nanoparticles cannot be easily eliminated because they are too large for renal clearance.Normally,they stay within the cells,cannot exocytose,and remain as a waste.Therefore,the non-biodegradable nanoparticles are not acceptable in pharmaceuticalproducts.Ananotoxicologicalclassif i cationsystem(NCS)is applied to arrange the toxicity risk of nanoparticles.The size and persistency related risks are combined to classify the NCS of nanoparticles as follows.Class I is classif i ed for the nanoparticles with size above 100 nm and biodegradable,Class II is for the nanoparticles with size above 100 nm and non-biodegradable,Class III is for the nanoparticles with size below 100 nm and biodegradable,and Class IV is for the nanoparticles with size below 100 nm and nonbiodegradable.Normally,the nanocrystals belong to the low risk class of nanoparticles,because their particle size can be madetobehigherthan100nmandtheyarealsobiodegradable (the dissolution occurs when the water is suff i cient).However, they can cause undesired systemic effects in the body.When the nanoparticles are taken up by the cells of the immune system,they can trigger an immune response and irritate the immune system.Hence,the development of nanocrystals requires the carefully investigation to provide the potential effects and less toxicity[6].
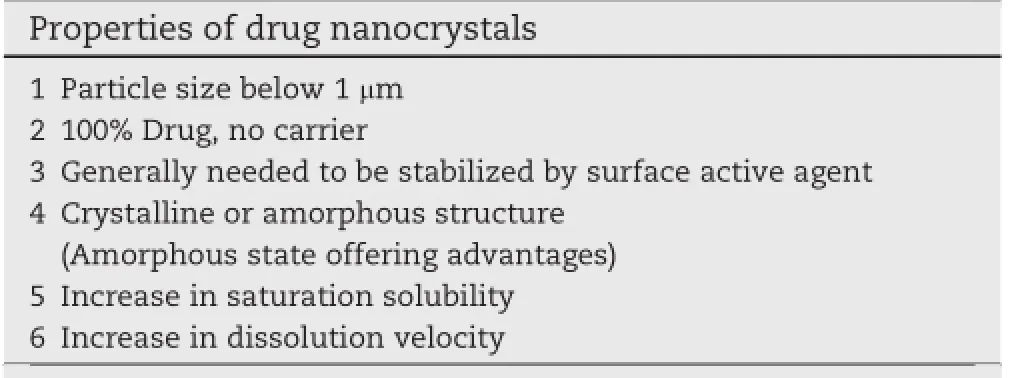
Table 1-Properties of drug nanocrystals that should be concerned and the given bene fi ts over the microsizedparticles.
3.Preparation of drug nanocrystals
Several preparation methods for drug nanocrystals have been investigated.The techniques to produce drug nanocrystals can be divided in two basic approaches,namely the bottom up and the top down technologies.To obtain nanoparticles of drugs,the top down processes involve a breaking down of larger particles by milling or homogenization,while the bottom up processes associate with an assembling and controlling of precipitations at nanometer scale.An overview of drug nanocrystals for oral administration which were prepared by different techniques in current marketed and during pharmaceutical researches was shown in Table 2.
3.1.Bottom up processes[2,4,6,19]
Starting from the molecules in solution,the molecules are aggregated to form particles that can be crystalline or amorphous form.This technique may be called‘a classical precipitation process’(in latin:via humida paratum).In this technique,the drug is completely dissolved in a solvent.Then the solvent solution is added to a non-solvent,causing precipitation of the drug.Importantly,it is necessary to control the structure of the particles and to avoid the growth of the particles to the micrometer size range by controlling inf l uence factors and adding stabilizers such as surfactants.Other bottomuptechnologiesincludesonocrystallization,the high gravity controlled precipitation technology,conf i ned impinging liquid jet precipitation and multi-inlet vortex mixing.Bottom up processes open the ways of interesting possibilities to incorporate multiple active ingredients in a single nanocarrier and to tailor nanoparticle surface functionality. However,a basic disadvantage of many precipitation processes is the use of organic solvent which is needed to be removed,leading to the high cost of production.Particularly, in case oflowwaterand organicsolventsolubledrug,thelarge solvent volumes are required.Hence,in pharmaceutical industry,the bottom up processes has not been employed for the production of the marketed drug.
3.2.Top down processes[1-4,6,20,21]
One starts from large crystals in the micrometer range and goes down to the nanodimension by diminuting the crystals; such as performing a milling process and using high pressure homogenization[5,22,23].
For the milling method,dry milling(e.g.jet milling)is not eff i cienttoobtainasizeinthenanometerrange;therefore,wet milling is applied.Wet milling is a means that the drug particles are dispersed in a surfactant/stabilizer solution and the obtainedmacrosuspensionisthensubjectedtomillingenergy. The classical milling process is the pearl mill(bead mill),being the NanoCrystal™technology.Milling media,dispersion medium(generally water),stabilizer and drug are f i lled into the milling chamber.Shear force of impact,generated by the movement of milling media,leads to the particle size reduction.The pearls or balls used as milling media consist of ceramics,stainless steel,glass or highly crosslinked polystyrene resin-coated beads[20,22,24].This technology is an important particle size reduction technology which has been used to produce four FDA-approved drugs such as Rapamune®, Emend®,Tricor®,and Megace ES®[25].The common problem of this technology is an erosion of milling material during the milling process.To solve this problem,coated milling beads are used to reduce the impurities caused by erosion of milling media.Another problem is an adherence of product to the inner surface area of the mill(consisting mainly of the surface of the milling pearls and the surface of the mill itself).
For the homogenization method,there are three important technologiestoproducenanocrystalswhichareMicrof l uidizer technology(IDD-P™technology),Piston-gap homogenization in water(Dissocubes®technology)and Piston-gap homogenization in water mixtures or in nonaqueous media(Nanopure®technology).Themicrof l uidizertechnologycangeneratesmall particles by a frontal collision of two f l uid streams under pressure up to 1700 bar.This leads to particle collision,shear forcesand also cavitation forces.This methodcan be achieved with jet stream homogenizer such as the microf l uidizer. Unfortunately,for the suff i cient particle size reduction,it is required a relatively high number of cycles(50-100 passes). The Dissocubes®technology employs piston-gap homogenizersthat can produce thenanoparticle suspensions in water at room temperature.A drug powder is dispersed in an aqueous surfactant solution and subsequently forced by a piston through the tiny homogenization gap with pressure up to 4000 bar,typically 1500-2000 bar.The resulting high streaming velocity of the suspension causes an increase in the dynamic pressure which is compensated by a reduction in the static pressure below the vapor pressure of the aqueous phase (according to Bernoulli's law).The simplif i ed form of Bernoulli's law is shown below.
where p0is total pressure,p is static pressure,q is dynamic pressure.
Formation of gas bubbles occurs because the water starts boiling at room temperature.The gas bubbles collapse immediately when the liquid leaves the homogenization gap being again under normal air pressure of 1 bar.The phenomenon of formation and implosion of the gas bubbles iscalled cavitation resulting in shockwaves.The drug particles are reduced in size due to high shear forces,turbulent f l ow and the enormous power of these shockwaves.However,the use of water leads to many disadvantages such as hydrolysis of water-sensitive drugs and problem during subsequent drying steps.Another approach using the piston-gap homogenizer is the Nanopure®technology.The dispersion media with a low vapor pressure(e.g.oils,PEG or hot-melted polyethylene glycols)and optionally homogenization at low temperatures are used in this technology.The cavitation in the homogenization gap is very little or nonexistent.Even without cavitation,the size diminution to achieve nanoparticles is suff i cient by the remaining shear forces,particle collisions and turbulences.A low temperature while homogenizing makes this process suitable for temperature labile drug.Also, it is possible to carry out the whole process in nonaqueous media to protect the drug from hydrolysis.The obtained suspensions from Nanopure®technology can directly be f i lled into soft gelatin capsules or into hard gelatin or HPMC capsules which are then being sealed.In addition,drug nanocrystals in solid PEG can be used as powder for tablet production[26].
To obtain an optimized formulation for the homogenization method,the following process parameters that inf l uence on properties of nanocrystals must be considered such as:
1.Applied pressure
2.Number of homogenization cycles
3.Temperature
Usually,the homogenizer can handle varying pressures, ranging from 100 to 1500 bar for most lab-scale ones.Therefore,an effect of homogenization pressure on the particle size should be investigated to optimize the fi nal formulation.The pressure is provided by the pump converting the kinetic energy of the fl uid in the gap.The higher homogenization pressure,the higher velocity of the fl uid in the gap is.The static pressure will drop to a larger extent leading to generating more bubbles and then higher energy to comminute the particles.This is consistent with the law of conservation of energy.Therefore,it is anticipated that the higher homogenization pressure,the smaller particle sizes are obtained. Usually for the production of the drug nanocrystals,a maximum pressure(for most lab homogenizers this value is 1500 bar)is required.The fl uid passing through the gap is performed instantaneously,generally within several milliseconds.The energy generated in such short time is not suffi cienttocomminuteallparticlesintouniformdrug nanocrystals even at the highest applied pressure 1500 bar; thus more homogenization cycles are needed to perform.The increased cycle numbers provide more energy to break down the crystals.Therefore,homogenization is often performed in fi ve,ten,or more cycles depending on the hardness of drug and the desired particle size.Apart from reducing the particle size,more cycles lead to more homogenous nanocrystal suspensions,i.e.a narrow size distribution.Because the fl ow rate of fl uid in the gap is not identical among different zones and the fl uid in central zone of the pipe has the higher velocity than the fl uid near the wall,the energy dispersed among the fl uid is not uniform,leading to an inhomogeneous particle size distribution.By increasing number of cycles,the probability that larger particles pass the zone of high-power density in the middleof the gap increases;thus theseparticlesare also diminished.Therefore,the particle size is a function of pressure and number of cycles.The desired particle size can be achievedby adjusting theprocedureparameters,pressureand cycle number.Temperature is also an important parameter which should be strictly controlled when the drug is temperature sensitive.High pressure processing increases the temperature of the sample(approximately 10°C at 500 bar).An increasing temperature in the homogenization process is not favorable to temperature-sensitive drugs.In that case,the temperature can be promptly reduced by placing a heat exchanger ahead of the homogenizer valve.In general,the sample temperature can be maintained at about 10°C and evenbelowsothattheprocessisapplicabletothe temperature-sensitive drugs.
High pressure homogenization is a simple technique. When an optimized procedure is achieved after adjustment of the production parameters,high quality nanosuspensions with little batch-to-batch variation can be obtained.An important advantage is that a considerably high productivity can be obtained with very low microparticle content in the product.In addition,compared with pearl milling technique, the contamination due to the erosion from the wall of the homogenizer is at a lower level.Müller et al.investigated the metal contamination of the nanosuspensions under a harsh production condition,i.e.20 cycles at a maximum pressure of 1500 bar.The most dominant iron ion in steel was analyzed in nanosuspensions and was found to be below 1 ppm,which was an uncritical level and safe even for a chronic therapy.
3.3.Other techniques for the production of drug nanocrystals[2,4,6,11,20,21]
Milling,high pressure homogenization,and precipitation are main methods employed for the production of drug nanocrystals.However,there is an intensive research for new technologies leading to many other approaches for the production of drug nanocrystals.The combination technologies combine generally a pre-treatment step followed by a high energy process,such as the NanoEdge™technology.In the fi rst step,crystals are precipitated;and the obtained suspension is then subjected to a high energy process,typically used high pressure homogenization.SmartCrystal technology is not only one technology but a number of different processes that are combined either to accelerate production by reducing the number of passes through the homogenizer or to obtain very small nanocrystals below 100 nm.Such small nanocrystals aredif fi cultto producevia pearlmillingor simplehigh pressure homogenization,especially in large scale industrial production.The combination process H69 is a parallel fl ow precipitation and subsequent high pressure homogenization (HPH)in which the precipitation takes place in the cavitation zone or just before the cavitation zone of the homogenizer (cavi-precipitation).In the H42 process,spray-drying and high pressure homogenization are combined.Moreover,in H96 process,the most effective combination technology,the lyophilization(bottom up)and the high pressure homogenization(top down)are combined to yield nanocrystals of thesize signif i cantly smaller than 100 nm.Recently,a novel tricombination technology as“Precipitation-lyophilization-homogenization(PLH)method”for preparation of nanocrystals had been proposed by Janyaprasert group[27].This combination technology composed of precipitation,lyophilization and homogenization techniques,respectively.First step was the precipitation process which was used to reduce an initial particle size of the drug.In this step,the drug was dissolved in an organicsolventand added intoan aqueousphase,resulting in a precipitation of preferably friable and small crystals.The organic solvent was carefully removed from the nanosuspensions to avoid its cosolvent action which may result in particle growth.Afterward,the secondlyophilization step was applied which led to modif i cation of the starting material and removal of the organic solvent used in the precipitation step. Finally,in the last step,high pressure homogenization was applied to break the crumbly particles into the nanometer range.Diagram of preparation step of PLH technique is shown in Fig.1.The results showed that PLH technique could provide an effective reduction of particle size of clarithromycin nanocrystals to approximately of 400 nm with homogeneity size distribution after only the f i fth cycle of homogenization whereas the same size was attained after 30 cycles by the normal high pressure homogenization(HPH)technique[27]. Among other technologies,the following supercritical f l uid methods are also mentioned to produce nanocrystals such as rapid expansion of supercritical solution(RESS),rapid expansion from supercritical to aqueous solution(RESAS), solution-enhanceddispersionbythesupercriticalf l uids (SEDS),spray freezing into liquid(SFL),evaporative precipitation intoaqueoussolution(EPAS),and aerosol solvent extraction(ASES).
4.In vivo performances of drug nanocrystals in oral administration routes
In contrast to other nanoparticle systems,drug nanocrystals consist mainly of pure active drugs.Drug nanocrystals exhibit many advantages including high eff i ciency of drug loading, easyscale-upformanufacture,relativelylowcostfor preparationandapplicabilitytovariousadministration routes,such as oral[28,29],parenteral[30,31],ocular[32-34], pulmonary[35-37]and dermal[38-41]delivery.
As known,the oral route is the most important and the f i rst choice for drug delivery because of its several advantages including convenience,safety,inexpensive,etc.Poorly watersoluble drugs for oral administration often show many problems in bioavailability including,a low/variable bioavailability,a retarded onset of action,a variation in bioavailability resulting from fed/fast state and a large oral dose usage.The production of drug nanocrystals offers many advantages for oral drug delivery and provides a solution to these problems. Additionally,at present,the formulations of drug nanocrystal in the market are mostly used for oral delivery.
Drug nanocrystals could improve an absorption of drug due to two major mechanisms via f i rstly,an improvement of solubility and dissolution rate and secondly,the bioadhesion to the intestinal wall.For the f i rstly aspect,drug absorption in oral administration is involved with the process that drug is dissolved from the formulation into aqueous digestive f l uid and then it is transported across the GI epithelium into the blood circulation.The dissolution is generally consideredto be the rate-limiting process in oral delivery of the drugs in BSC class II.Drug with poor solubility and dissolution rate will provide a slow and erratic dissolution that limits the in vivo absorption and is unable to reach an effective therapeutic concentration.The formulation of drug nanocrystals can impressively improve the bioavailability of perorally administered poorly soluble drugs as shown by changes in pharmacokineticparametersofbloodprof i lesincluding,an increase in area under the blood concentration-time curve (AUC),an increase in maximum plasma concentration(Cmax), a decrease in time to maximum plasma concentration(Tmax). For example,Liversidge and Cundy reported that danazol,a gonadotropin inhibitor,showed the absolute bioavailability of marketed danazol microsuspension(200 mg,10 μm)only 5.1±1.9%.Meanwhile,the absolute bioavailability of danazol nanosuspension(200 mg,169 nm)was 82.3±10.1%which was equal to 16-fold increase in bioavailability.Additionally,the Tmaxwas reduced and the Cmaxwas 15-fold increased[28]. Amphotericin B was formulated as a nanosuspension for thetreatment of visceral leishmaniasis.After oral administration (5 mg kg-1)in BALB/c mice,amphotericin nanosuspension could signif i cant reduce liver parasite numbers in the liver by 28.6%compared to untreated controls.While,the micronized amphotericin B did not show any curative effect[42].Additionally,the formulation of drug nanocrystals can provide advantage whenever a quick onset of a poorly soluble drug is required.For instance,an analgesic naproxen was formulated as nanosuspension(270 nm)for oral administration.Besides the nanosuspension of naproxen was approximately 3-fold increased in AUC when compared to an unmilling suspension(20 μm),it could be concurrently reduced in Tmax.The data showed that the time for nanosuspension to reach Cmaxwas only about 8 min whereas the unmilling naproxen suspension was achieved the Cmaxat 33.5 min.It was suggested that an increase in 4-fold faster absorption rate of nanosuspension when compared to unmilling suspension was contributed to the increased solubility and dissolution rate of nanocrystals[43].In the study by Li et al.,revaprazan hydrochloride was developed in form of nanosuspensions.The in vivo evaluation showed that revaprazan hydrochloride nanosuspensions exhibited signif i cant increase in AUC0-t(45% and 36%higher),Cmax(87%and 98%higher)and decrease in Tmax(185 and 315 min shorter),MRT(114 and 157 min shorter) when compared to a coarse suspension[44].Nitrendipine nanosuspensionswerepreparedbyprecipitationultrasonication method to enhance the dissolution rate and oral bioavailability of the drug.The in vivo test demonstrated that the Cmaxand AUC0-12values of nanosuspensions in rats were approximately 6.1-fold and 5.0-fold greater than that of commercial tablets,respectively[45].These examples obviously demonstrated that nanocrystals formulation could increase dissolution velocity and saturation solubility of poorly soluble drugs.Therefore,the fast and complete drug dissolution,animportantprerequisitefordrugabsorption,is achieved.
The second mechanism of nanocrystals that can improve the drug absorption is due to the mucoadhesion to biological mucosa(GI mucosa)which can positively inf l uence the oral bioavailability.Owing to the adhesiveness of nanocrystals to GI mucosa,drugs can provide the higher concentration gradient and prolonging residence and contact time in the GIT.The mucoadhesion mechanism of nanoparticles could be explained by many theories including,the electronic theory (electrostatic attraction forces between the surfaces of particles and mucus),the adsorption theory(hydrogen and van der Waals bond between the surfaces of particles and mucus),the diffusion theory(interpenetration and physical entanglement of the mucus protein and polymer chains),and the trapping theory(retention of nanoparticles by the uneven mucosa surface).Due to the benef i ts of mucoadhesion,some researchers wereinterestedin an enhancement of adhesiveness between nanocrystals and GI mucosa by modifying the surface of drug nanocrystals with cationic polymers or incorporation of drug nanocrystals into mucoadhesive polymers. Additionally,theutilizedmucoadhesive polymers can prevent thedrugfrom degradation.The antibiotic buparvaquone,used for treatment of Cryptosporidium parvum(C.parvum),has very low oral bioavailability due to its low solubility.Nanosuspension of buparvaquone cannot only increase drug solubility but it can also perform a mucoadhesion to the gut wall.In addition,an incorporation of buparvaquone nanosuspension into mucoadhesive polymers can enhance the mucoadhesiveness and show more effectively clear C.parvum from the GIT when compared to the unmodif i ed nanosuspension[46].
Another problem of poorly soluble drug is a variation in bioavailability resulting from fed/fast state.Poorly soluble drugs usually shows an increased or accelerated absorption when intake with food.Drug bioavailability is increased due to the food effect because of the enhanced dissolution rate in GIT caused by several factors including larger volume of the gastric f l uid,delayed gastric emptying,increased bile secretion,increased gastric pH(for acidic drugs),and increased splanchnic blood f l ow[47].When poorly soluble drugs are formulated as a uniform nanosuspension,the variation in bioavailability resulting from fasted/fed state can be minimized.The nanocrystals could signif i cantly increase dissolutionratebecauseoftheincreaseinsolubilityand enormous particle surface.The dissolution rate of nanocrystals is fast enough even under the fasted state.Therefore, the absorption in both fasted and fed state can be a permeability-limit,and the absorption difference between the fasted and fed conditions due to the dissolution difference is eliminated.For example,the formulation of cilostazol nanocrystals(220 nm)could signif i cantly reduce fed-fasted ratios of the Cmax,AUC,Tmaxand MRT as compared to a microsized dispersion(13 and 2.4 μm)when given in beagle dogs.Therefore,the fasted/fed variation in bioavailability was almost eliminated[47].The study of Wu et al.showed that the nanocrystals dispersion of aprepitant(MK-0869),the active ingredient in Emend®,could eliminate the food effect on oral absorption.The fed-fasted ratio was reduced and the bioavailability was improved in the beagle dogs at a dose of 2 mg/kg[48].The same result was also found in the study by Sauron et al.The food effect on bioavailability of a new tablet formulationcontainingfenof i bratenanoparticleswas accessed in human.It was demonstrated that the peak and overall exposures from the 145 mg nanoparticle fenof i brate tablet were not affected by food and the result was concluded that the nanoparticle fenof i brate tablet can be taken regardless of the timing of meals[49].
Poorly soluble drugs usually provide more troublesome in the safety issue because of the use of a large amount of organic cosolvent or solubilizer that will result in an unwanted side effect or toxicity.Drug nanocrystals are generally reported as a safe and well tolerated formulation in many administration route compared with the conventional products.Several safety advantages of drug nanocrystals in oral delivery include i)f i ne particle size,ii)safe composition,and iii)tolerance to various sterilizations.The f i ne particle size of drug nanocrystals can increase the distribution uniformity in the gastrointestinal f l uid and avoid the high and prolonged local concentration[43].Nanocrystals are also benef i cial to a better toleration in the mucosa delivery by reduction in the occurrence of the local irritation or gritty feel.For example, the study by Liversidge and Conzentino demonstrated that naproxen nanosuspensions showed not only the faster onset of action but also a reduction in the gastric irritancy[43].Drug nanocrystals can provide an opportunity to escalate dose andreduce solvent-related adverse effect because of the safe compositionsincenanosuspensionformulationsdonot require organic solvent or extreme pH ranges for solubilization of poorly soluble drug[22].Additional bene fi t of drug nanocrystals in safety issue is the tolerance to various sterilizations.Several sterilization approaches can be successfully applied to nanosuspensions including gamma radiation, fi ltration sterilization,and thermal sterilization.
Concerning the fi nal formulations of drug nanocrystals, most drug nanocrystals in the in vivo experiments are aqueous dispersions.In clinical application,liquid dosage forms might be suitable for some groups of patients,e.g.children or elderly patients,but not for normal patients.In general,solid dosage forms are usually more preferred.Therefore,the liquid nanosuspensions should be transformed into dry powders which are then used for production of tablets,capsules,or pellets.There are several methods that can be used for solidi fi cation this nanosuspension.In case of drug nanosuspensions in pure water or in water containing mixture, nanosuspensions may be used as a granulation fl uid for further production of tablets.The nanosuspension is admixed to binders and other excipients,and the granules are then fi nely compressed into the tablets[50].Furthermore,nanosuspensions can also be produced as matrix pellets or layering dispersion in fl uidized bed process[51-53].In case of drug nanosuspensions produced in nonaqueous media such as liquid/solid PEG,the use of melted PEG which is solidi fi ed at room temperature for the dispersion of nanosuspension is interesting.After solidi fi cation of PEG,the nanocrystals containing mass can be ground and fi lled into the capsules. Additionally,the other approval methods for solidi fi cation of nanosuspension are such as spray-drying and lyophilization. Spray-drying process is the cost effective approach to transform the nanosuspensions into dry products under appropriate conditions.Lyophilization process is recommended for intravenous product in order to avoid aggregation or caking of settled drug nanocrystals.However,during the drying process,the particle aggregation should be considered since the bene fi ts of nano-sized particles will be lost if the particle aggregation occurs.Therefore,an addition of protectants (usually sugars)may reduce the growth of particle size during a solidi fi cation process.Besides the transformation to dry powder of nanosuspensions,the redispersion of solid drug nanocrystals in gastrointestinal fl uid should be concerned. The stabilizers attached to the nanocrystal surfaces that provide ef fi cient ionic or steric repulsion and have no effect from the GIT environment should be used.
Regarding micromeritic aspects,drug nanocrystals provide high saturation solubility and consequently increase dissolution velocity.However,in some applications,drug nanocrystals are essentially combined with traditional controlled release technology(e.g.coated pellets)to avoid excessively high plasma peaks and premature time to reach maximum plasma concentration(Tmax),and to achieve prolonged blood levels.Besides an optimal drug nanocrystal size and crystalline/amorphous state that are taken into account for the production of drug nanocrystals,the other factors including the required blood pro fi le,administration route,and stability of the amorphous state during shelf life of the product should be in consideration[4].
In addition to oral administration,drug nanocrystals also play a benef i cial role on other administration routes.They can create supersaturated systems with high thermodynamic activity for dermal delivery;create systems with prolonged retentiontimesforophthalmicadministration;create mucoadhesive systems for mucosal administration of nasal, vaginal and pulmonary.Furthermore,an administration of drug nanocrystal suspensions as parenteral formulation is also feasible.The surfaced-modif i ed drug nanocrystals can be preferentially adsorbed onto blood proteins for site specif i c localization that is applied as a targeted drug delivery.
5.Conclusion
Nanocrystal technology is evidently suitable for drugs with poor solubility.Drug nanocrystals can be applied to all poorly soluble drugs to overcome their solubility and bioavailability problems.The decrease in particle size to nanometer range contributes to the increased particle surface,curvature, saturation solubility,dissolution velocity and further acceptable bioavailability.Various applied and combination technologiesaredevelopedfortheproductionofdrug nanocrystals.Many reports on drug nanocrystals within recent years exhibit excellent in vivo performances of drug nanocrystalsindifferentadministrationroutes.Inoral administration,drug nanocrystals offer great benef i ts of enhanced drug bioavailability.Moreover,drug nanocrystals allow the quickly absorption due to the fast dissolution that is suitable for the required fast onset drug.The increased solubility of drug nanocrystals also eliminates the food effect to drug absorption.Therefore,drug in nanocrystal formulations perform similar absorption in fed and fasted conditions. Another benef i t of drug nanocrystals is that it can provide smaller dose administration to achieve moderate blood level and thus reduce the side effect from given larger dosage. Furthermore,drug nanocrystals can be applied to various administration routes such as oral,parenteral,ocular,pulmonary and dermal delivery.The liquid nanosuspensions can be employed as a liquid dosage form or transformed into solid dry powder for further production of tablets,capsules,or pellets dosage forms.Several techniques can be used to solidify the nanosuspension including the preparation as the granulation f l uid for tablet production,the layering dispersion in f l uidized bed process,the use of solid/liquid PEG,spray drying and lyophilization.At present,drug nanocrystals are paid increasing more attention as a promising approach owing to many reasons such as an increasing number of poorly soluble drugs in drug development process,pharmacoeconomic value,easier production,safer composition and other advantages that are previously mentioned.However, there is a lack of cytotoxicity studies and the details of intracellular fate of the nanocrystals.The particle size and persistency in the cells of nanocrystals are important parameters that determine the interaction between nanocrystals and the cells and their risk of toxicity.Additionally,the nanoparticles can lead to an irritation of the immune systems.Therefore, the nanotoxicity should be concerned when the nanocrystals are prepared.Moreover,the additional knowledge about intracellular fate of nanocrystals might open the way for newapplication.In the future,the development of stealth nanocrystals and active targeting nanocrystals modif i ed with functionalized surface will be the next important part of work for drug nanocrystals.The surface modif i cation of nanocrystals affects the protein adsorption pattern and determines the cellular aff i nity.This aspect of nanocrystals can be employed as the new approach for the targeted delivery.
Acknowledgments
The authors wish to thank the Thailand Research Fund through Thai Basic Research Grant(BRG5680020 to V.B.J.),and Ph.D.scholarship from the Royal GoldenJubilee Ph.D.Program and Mahidol University(PHD/0258/2550 to B.M.)for f i nancial supports.
REFERENCES
[1]Magdalene R.Pure drug nanoparticles for the formulation of poorly soluble drugs.NewDrugs 2001;3:62-68.
[3]Gao L,Zhang D,Chen M.Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system.J Nanopart Res 2008;10:845-862.
[4]Junghanns JUAH,Müller RH.Nanocrystal technology,drug delivery and clinical appications.Int J Nanomedicine 2008;3(3):295-309.
[5]Chen H,Khemtong C,Yang X,et al.Nanonization strategies for poorly water-soluble drugs.Drug Discov Today 2011;16(7/ 8):354-360.
[6]Müller RH,Gohla S,Keck CM.State of the art of nanocrystals -special features,production,nanotoxicology aspects and intracellular delivery.Eur J Pharm Biopharm 2011;78:1-9.
[7]Kocbek P,Baumgartner S,Kristl J.Preparation and evaluation of nanosuspensions for enhancing the dissolution of poorly soluble drugs.Int J Pharm 2006;312:179-186.
[8]Buckton G,Beezer AE.The relationship between particle size and solubility.Int J Pharm 1992;82:R7-10.
[10]Gulsun T,Gursoy RN,Oner L.Nanocrystal technology for oral delivery of poorly water-soluble drugs.FABAD J Pharm Sci 2009;34:55-65.
[11]Keck CM,Müller RH.SmartCrystals-review of the second generation of drug nanocrystal.In:Torchilin VP,Amiji MM, editors.Handbook of materials for nanomedicine.Singapore: Pan Stanford;2010.p.555-580.
[12]Kesisoglou F,Panmai S,Wu Y.Nanosizing-oral formulation development and biopharmaceutical evaluation.Adv Drug Deliv Rev 2007;59(7):631-644.
[13]Jacobs C,Kayser O,Müller RH.Nanosuspensions as a new approach for the formulation of the poorly soluble drug tarazepide.Int J Pharm 2000;196:161-164.
[14]Merisko-Liversidge E,Wei L.Stabilization of chemical compounds using nanoparticulate formulations.US 2001; 952032 20010914.CAN 138:243327,US 2003054042 A1.2003.
[16]Teeranachaideekul V,Junyaprasert VB,Souto EB,et al. Development of ascorbyl palmitate nanocrystals applying the nanosuspension technology.Int J Pharm 2008;354:227-234.
[17]Mauludin R,Müller RH,Keck CM.Development of an oral rutin nanocrystal formulation.Int J Pharm 2009;370:202-209.
[18]Dolenc A,Kristl J,Baumgartner S,et al.Advantages of celecoxib nanosuspension formulation and transformation into tablets.Int J Pharm 2009;376:204-212.
[21]Keck CM,Müller RH.Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation.Eur J Pharm Biopharm 2006;62:3-16.
[22]Merisko-Liversidge E,Liversidge GG.Nanosizing for oral and parenteral drug delivery:a perspective on formulating poorly-water soluble compounds using wet mediamilling technology.Adv Drug Deliv Rev 2011;30:427-440.
[23]Eerdenbrugh BV,den Mooter GV,Augustijns P.Top-down production of drug nanocrystals:nanosuspension stabilization,miniaturization and transformation into solid products.Int J Pharm 2008;364:64-75.
[24]Niwa T,Miura S,Danjo K.Universal wet-milling technique to prepare oral nanosuspension focused on discovery and preclinical animal studies-development of particle design method.Int J Pharm 2011;405:218-227.
[25]Merisko-Liversidge E,Liversidge GG.Drug nanoparticles: formulating poorly water-soluble compounds.Toxicol Pathol 2008;36(1):43-48.
[26]Bushrab FN,Müller RH.Nanocrystals of poorly soluble drugs for oral administration.NewDrugs 2003;5:20-22.
[27]Morakul B,Suksiriworapong J,Leanpolchareanchai J,et al. Precipitation-lyophilization-homogenization(PLH)for preparation of clarithromycin nanocrystals:inf l uencing factors on physicochemical properties and stability.Int J Pharm 2013;457:187-196.
[28]Liversidge GG,Cundy KC.Particle size reduction for improvement of oral bioavailability of hydrophobic drugs:I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs.Int J Pharm 1995;125(1):91-97.
[29]Kesisoglou F,Mitra A.Crystalline nanosuspensions as potential toxicology and clinical oral formulations for BCS II/ IV compounds.AAPS J 2012;14:677-687.
[30]Peters K,Leitzke S,Diederichs JE,et al.Preparation of a clofazimine nanosuspension for intravenous use and evaluation of its therapeutic eff i cacy in murine Mycobacterium avium infection.J Antimicrob Chemother 2000;45(1):77-83.
[31]Ganta S,Paxton JW,Baguley BC,et al.Formulation and pharmacokinetic evaluation of an asulacrine nanocrystalline suspension for intravenous delivery.Int J Pharm 2009;367:179-186.
[32]Rosario P,Claudio B,Ferrara P,et al.Eudragit RS100 nanosuspensions for the ophthalmic controlled delivery of ibuprofen.Eur J Pharm Sci 2002;16:53-61.
[33]Kassem MA,Abdel Rahman AA,Ghorab MM,et al. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs.Int J Pharm 2007;340:126-133.
[34]Ali HSM,York P,Ali AMA,et al.Hydrocortisone nanosuspensions for ophthalmic delivery:a comparative study between microf l uidic nanoprecipitation and wet milling.J Control Release 2011;149:175-181.
[35]Jacobs C,Müller RH.Production and characterization of a budesonide nanosuspension for pulmonary administration. Pharm Res 2002;19(2):189-194.
[36]Sultana S,Talegaonkar S,Ali R,et al.Inhalation of alendronate nanoparticles as dry powder inhaler for the treatment of osteoporosis.J Microencapsul 2012;29(5):445-454.
[37]Zhang J,Lv H,Jiang K,et al.Enhanced bioavaiability after oral and pulmonary administration of baicalein nanocrystal.Int J Pharm 2011;420:180-188.
[38]Shaal LA,Shegokar R,Müller RH.Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation.Int J Pharm 2011;420:133-140.
[39]Mitri K,Shegokar R,Gohla S,et al.Lutein nanocrystals as antioxidant formulation for oral and dermal delivery.Int J Pharm 2011;420:141-146.
[40]Zhai X,Lademann J,Keck CM,et al.Nanocrystals of medium soluble actives-novel concept for improved dermal delivery and production strategy.Int J Pharm 2014;470:141-150.
[41]Mishra PR,Shaal LA,Müller RH,et al.Production and characterization of hesperetin nanosuspensions for dermal delivery.Int J Pharm 2009;371:182-189.
[42]Kayser O,Olbrich C,Yardley V,et al.Formulation of amphotericin B as nanosuspension for oral administration. Int J Pharm 2003;254:73-75.
[43]Liversidge GG,Conzentino P.Drug particle size reduction for decreasing gastric irritancy and enhancing absorption of naproxen in rats.Int J Pharm 1995;125:309-313.
[44]Li W,Yang Y,Tian Y,et al.Preparation and in vitro/in vivo evaluation of revaprazan hydrochloride nanosuspension.Int J Pharm 2011;408:157-162.
[45]Xia D,Quan P,Piao H,et al.Preparation of stable nitrendipine nanosuspensions using the precipitation-ultrasonication method for enhancement of dissolution and oral bioavailability.Eur J Pharm Sci 2010;40:325-334.
[46]Kayser O.A new approach for targeting to Cryptosporidium parvum using mucoadhesive nanosuspensions:research and applications.Int J Pharm 2001;214:83-85.
[47]Jinno J,Kamada N,Miyake M,et al.Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug,cilostazol,in beagle dogs.J Control Release 2006;111:56-64.
[48]Wu Y,Loper A,Landis E,et al.The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869:a Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human.Int J Pharm 2004;285:135-146.
[49]Sauron R,Wilkins M,Jessent V,et al.Absence of a food effect with a 145 mg nanoparticle feno fi brate tablet formulation. Int J Clin Pharmacol Ther 2006;44(2):64-70.
[50]Kirkof N.Creation and characterization of nanoparticles.In: 32nd annual meeting and exposition of the controlled release society Miami;2005.
[52]Mo¨schwitzer J,Müller RH.Spray coated pellets as carrier system for mucoadhesive drug nanocrystals.Eur J Pharm Biopharm 2006;62(3):282-287.
[54]Food and drug administration,Center for drug evaluation and research.Orange book:approved drug products with therapeutics equivalence evaluations.29th ed Rockville:MD. http://www.accessdata.fda.gov/scripts/cder/ob/default.cfm [Updated May 17,2013.Accessed May 23,2014].
[55]de Waard H,Frijlink HW,Hinrichs WL.Bottom up preparation techniques for nanocrystals of lipophilic drugs. Pharm Res 2011;28:1220-1223.
[56]Vergote GJ,Vervaet C,Driessche IV,et al.In vivo evaluation of matrix pellets containing nanocrystalline ketoprofen.Int J Pharm 2002;240:79-84.
[57]Müller RH,Runge S,Revelli V,et al.Oral bioavailability of cyclosporine:solid lipid nanoparticles(SLN)versus drug nanocrystals.Int J Pharm 2006;317:82-89.
[58]Lungguth P,Hanafy A,Frenzel D,et al.Nanosuspension formulations for low-soluble drugs:pharmacokinetic evaluation using spironolactone as model compound.Drug Dev Ind Pharm 2005;31:319-329.
[59]Mou D,Chen H,Wan J,et al.Potent dried drug nanosuspensions for oral bioavailability enhancement of poorly soluble drugs with pH-dependent solubility.Int J Pharm 2011;413:237-244.
*Corresponding author.Department of Pharmacy,Faculty of Pharmacy,Mahidol University,Rajathevee,Bangkok 10400,Thailand.Tel.: +66 2 644 8677-91;fax:+66 2 644 8694.
E-mail address:varaporn.jun@mahidol.ac.th(V.B.Junyaprasert).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2014.08.005
1818-0876/©2015 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Bioavailability
Poorly water-soluble drugs
杂志排行
Asian Journal of Pharmacentical Sciences的其它文章
- Delivery systems for siRNA drug development in cancer therapy
- Evaluation of chitosan-anionic polymers based tablets for extended-release of highly watersoluble drugs
- Preparation and evaluation of tamsulosin hydrochloride sustained-release pellets modif i ed by two-layered membrane techniques
- Enhancement of solubility and therapeutic potential of poorly soluble lovastatin by SMEDDS formulation adsorbed on directly compressed spray dried magnesium aluminometasilicate liquid loadable tablets:A study in diet induced hyperlipidemic rabbits
- Degradation kinetic study of lysine in lysine hydrochloride solutions for injection by determining its main degradation product
- Targeted delivery of docetaxel to the metastatic lymph nodes:A comparison study between nanoliposomes and activated carbon nanoparticles
