Highly Effectively Evaluating the quality consistency ofDihuang Pills by Quantified both HPLC and IR profiles that assisted by antioxidant activity
2015-05-14XinxinChenGuoxingSunJiyueGoLeiWngYueZouShenyngPhrmceuticlUniversity103WenhuRodShenyngLioning110016Chin
Xinxin Chen, Guoxing Sun*, Jiyue Go, Lei Wng, Yue ZouShenyng Phrmceuticl University, 103 Wenhu Rod, Shenyng, Lioning 110016, Chin
Regular article
Highly Effectively Evaluating the quality consistency of
Dihuang Pills by Quantified both HPLC and IR profiles that assisted by antioxidant activity
Xinxin Chena, Guoxiang Suna*, Jiayue Gaoa, Lei Wanga, Yue ZouaaShenyang Pharmaceutical University, 103 Wenhua Road, Shenyang, Liaoning 110016, China
A high performance liquid chromatography (HPLC) method was developed for fingerprint profiling to evaluate the quality consistency of DHPs. Method validation results demonstrate that desired precision, repeatability, and a sample stability test was achieved. The HPLC fingerprints of samples were evaluated by PCA and the similarity analysis introduced by us. The infrared spectral fingerprint (IR-FP) was also achieved as a supplement of qualitative information. At last, DPPH assay was performed to assess the antioxidant activity of DHPs,invitro, which was correlated to the fingerprint components. This study demonstrates that HPLC fingerprint combined with IR-FP and antioxidant ability provides a reliable and efficient methodology for the assessment of quality of the TCM and herbal preparations.
Liuwei Dihuang pill; HPLC fingerprint; IR-FP; antioxidant activity; quality control
1 Introduction
Liuwei Dihuang Pill (DHP), has been practised for thousands of years in China and now is used all over the world to prevent and cure many diseases, especially the disorder of immune and endocrine systems such as diabetes, backache, alopecia, menoxenia, sore waist and knees, etc. [1, 2]. It is prepared fromRadix Rehmanniae Preparata,Rhizoma Dioscoreae,Fructus Corni,Cortex Moutan,Rhizoma AlismatisandPoria.There are many components in DHP and some of them have been determined for their special activities [3, 4], but most of them are still unidentified. According to the theory of traditional Chinese medicine (TCM), the therapeutic effects of herbal medicines are based on synergistic effects of many kinds of ingredients. Therefore, it is insufficient to select single or multiple chemical markers or bioactive constituents to assess the quality of complex herbal preparations [5, 6].
Fingerprint profiling has been internationally accepted as an indispensable technique for the quality control of complex analytes [7-11]. Since then, fingerprint analysis can be observed in China and other countries all over the world. Fingerprints can be obtained by spectroscopic or separation (mainly chromatography) techniques [12]. Chromatographic fingerprints are performed on
2 Theory
3 Experimental
All the data were analyzed by the professional software called Digitized Evaluation System for Super-Information Characteristics of TCM Fingerprints 4.0 (Software certificate NO. 0407573, China) which was developed by Professor Guoxiang Sun, and SIMCA software (Demo version 13.0.3, U metrics AB).
3.3 Apparatus and chromatographic conditions
An Agilent 1100 series LC system equipped with a low pressure mix quater nary pump, an online degasser, an autosampler, a thermostatted column compartment, and a DAD detector (Agilent Technology, CA) was used to perform HPLC fingerprints. The chromatographic separation was carried out on a Poroshell 120SBC18column (150 ×4.6 mm, 2.7μm), maintained at 35±0.15°C. The mobile phase was 0.2% phosphoric acid Acetonitrile solution (A) and 0.2% phosphoric acid aqueous solution (B) with a gradient program as follows: 0-10 min, linear gradient 2-10% A; 10-20 min, linear gradient 10-15% A; and 20-50 min, linear gradient 15-40% A; 50-70 min, linear gradient 40-80% A; 70-80 min, isocratic 80% A, at a flow rate of 0.5 ml·min-1. The UV absorbance was monitored at 236 nm using DAD. All injection volumes of sample and standard solutions were 5μl. The antioxidant activity analysis was achieved on a 722S vis spectrophotometer (Shanghai Precision Instrument Co., Ltd, Shanghai, China).
3.4 Method validation
Among these active components, loganin indicates a high and stable component. Therefore, we choose it as the reference substance. For method validation of fingerprint analysis, all common peaks’relative retention time (the ratio of peak retention time of sample constituents to the reference standard, RT) and relative peak area (the ratio of peak area of sample constituents to the reference standard, RA) were calculated firstly. The precision test performed with five replicate injection, RSD of RT and RA
4 Result and discussion
4.2 Principle component analysis (PCA) of the HPLC fingerprints of DHPs
PCA was performed using ‘common peaks’areas as input. The two-dimensional matrices (22 ×28) consisted of 22 objects and 28 variables, the objects are represented by the samples, and variables are represented by the areas of fingerprints at 236 nm. PCA was performed by SIMCA13.0.3, and we could achieve the variance of PC1 and PC2, representing the areas as high as 61.7% and 26.7%, respectively, and the cumulative ratio is more than 85% from the total variance explained. On the basis of cumulative ratio >80%, the first two principal components were used to assess the consistency of the data sets, shown in Fig. 3. And the samples were classified into two groups, which were marked group 1 and group 2. S3, S4, S5, S6, S7 and S8 were classified into group 1, while the rest of all samples were into another. There’s no appearance of abnormal sample at 95% confidence limit.
4.3 Similarity analysis based on Sm, P9and α
To evaluate the quality of the drug samples, the values ofSm,P9andαwere calculated according to eqn (1)–(7) by importing the fingerprint signals to the TCM Fingerprint software. The values ofSm,P9andαwere summarized in Table 2. All samples haveSmvalues above 0.94 andαvalues below 0.10, which indicate all samples have similar chemical composition. The values ofSmandαwere accepted. Then the quantitative similarity parameter,P9should be calculated. TheP9value was a major index to reflect the total content of sample, providing a great potential to link to medicinal effects in clinics. All samples haveP9values lower than 116.3% and higher than 83.5%, which were accepted. Higher values ofP9indicate higher overall component contents in these samples, as S6, S7 and S8 (P9, ≥110%). In comparison, lower values ofP9indicate lower overall component contents in these samples, as S1, S2, S15 and S17 (P9, ≤ 90%).
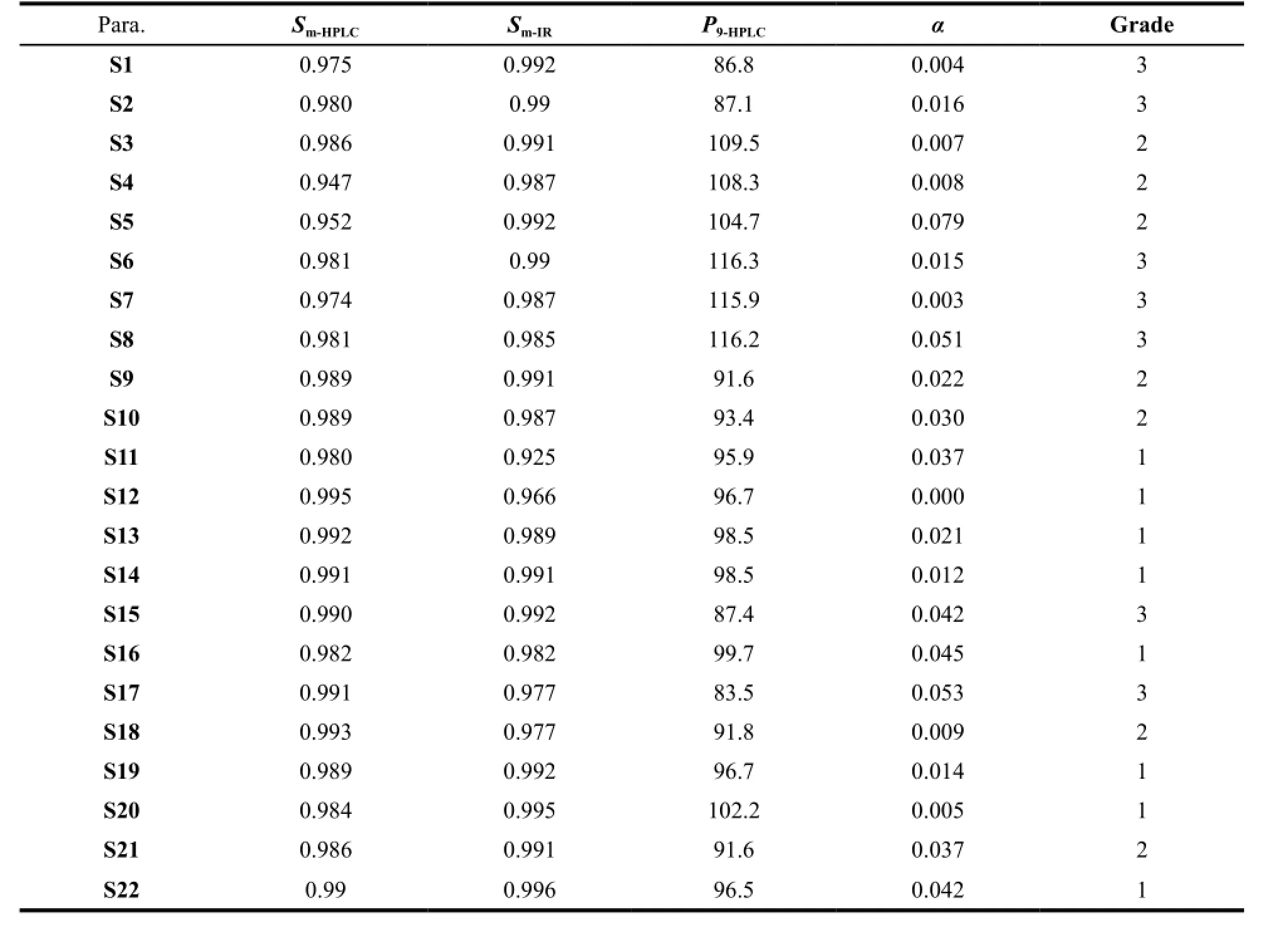
Table 2 Quality level results of 22 batches of DHPs assessed by similarity analysis based on 236 nm HPLC fingerprints and IRFPs
Then, HCA was performed usingSmandP9as input. 22 batches of DHPs were classified into two groups, shown as Fig. 4. S3, S4, S5, S6, S7, S8 and S20 were classified into group 1, and the rest of all samples were into another.
4.4 Similarity analysis of IR-FP
When the absorption points of IR used as input, similarity of IR-FP could also be calculated. Similarity of IR-FP could be a supplement of qualitative information, making the result more credible. In this study, the values ofSmof IR-FPs were calculated and shown in Table 2. All samples haveSm-IRvalues above 0.925, which indicates that all samples have similar chemical composition. The value ofSm-IRis a basis for further quantitative analysis.
4.5 The detection of AOP
It is reported that antioxidant is one of important activities of DHPs. DPPH radical is widely used to measure antioxidant capacity of food and medicine. In this study, IC50were used as the indicator for the antioxidant activity for the 22 drug batches. The values of IC50were in the range of 133.4µg·ml-1to 192.3µg·ml-1. When ascorbic acid was used as positive control, the antioxidant activity of 100µg·ml-1of DHPs was equal to 2.08µg·ml-1ascorbic acid.
For further investigation of the compositions of AOP, an efficiently statistical algorithm, PLS analysis was performed in SIMCA software (Demo version 13.0.3, U metrics AB). In total, 28 fingerprint peaks and IC50were chosen as input values. In the final accepted model, 10 peaks were retained as descriptors. They proved to be significantly related to IC50. With the 10 peaks, PLS analysis could achieved three principal components by cross validation, and provided a good fit between the observed and predicted IC50, see Fig. 5. The variance of 10 peaks and IC50could be explained as high as 86.3% and 83.1% respectively. The cumulative predicted fraction of IC50(Q2cum) was calculated to be 0.693. When Q2cumis greater than 0.50, the corresponding model is considered to be internally robust and predictive [19]. With PLS analyses, the coefficients of 10 peaks were calculated, shown in Fig. 6. Peaks 2, 3, 20 and 28 were found to have higher negative correlation with IC50, respectively present GA, 5-HMF, PNF and POL. The result is consistent with the literature [20-23]. Peaks 5, 17, 22 and 26 also had higher negative correlation with IC50. And peaks 10 and 15 were positive correlated with IC50.
By the first two principal components, the variance of 10 peaks and IC50could be explained as high as 71.8% and 75.3% respectively. The first two principal components were used to assess the consistency of the data sets, shown in Fig. 7. And the samples were also classified into two groups, group 1 contains S3, S4, S5, S6, S7 and S8, group 2 contains all samples out of group 1. There’s no appearance of abnormal sample at 95% confidence limit.
5 Conclusion
In this study, the HPLC fingerprints were established and combined with PCA and similarity analysis respectively to evaluate the quality consistency of DHPs. By both the two methods, there’s no appearance of abnormal sample. And 22 batches of DHPs were classified into two similar groups by both the two methods. But similarity
analysis could give more visual information about the similarity between sample fingerprint with reference fingerprint. When reference fingerprint comes from standard product, the quality of sample could be evaluated by the similarity between sample fingerprint with reference fingerprint. Moreover the AOP of samples were correlated with the fingerprint components, which provided important supplemental information for the quality control of the DHPs. HPLC fingerprint combined with IRFP and antioxidant ability provides a reliable and practical methodology for the assessment of quality of the TCM and herbal preparations.
Acknowledgements
This research work was financially supported by National Natural Science Foundation of China (Accession No.90612002).
[1] Chen YL, He JL, Chen WY,et al. The exploration and clinical application of Liuwei Dihuang pill (decoction). Clinical Journal of Chinese Medicine, 2011, 12: 41-42.
[2] Song LL, Zhang Y, Zhang DL. Studies on immunopharmacology and antitumour pharmacology of the liuweidihuang compound. Chinese Traditional Patent Medicine, 2001, 23: 910-913.
[3] The state commission of Chinese pharmacopoeia, pharmacopoeia of People's Republic of China, The Medicine Science and Technology Press of China, Beijing, Part I, 2010, 589.
[4] Zhao XF, Wang Y, Sun YQ. Simultaneous determination of four bioactive constituents in Liuwei Dihuang Pills by micellar electrokinetic chromatography. J Pharm Biomed Anal, 2007, 44:1183-1186.
[5] Ye J, Zhang X, Dai WX,et al. Chemical fingerprinting of Liuwei Dihuang Pill and simultaneous determination of its major bioactive constituents by HPLC coupled with multiple detections of DAD, ELSD and ESI-MS. J Pharm Biomed Anal, 2009, 49: 638-645.
[6] Xie BG, Gong T, Tang MH,et al. An approach based on HPLC-fingerprint and chemometrics to quality consistency evaluation of Liuwei Dihuang Pills produced by different manufacturers. J Pharm Biomed Anal, 2008, 48:1261-1266.
[7] World health organization, guidelines for the assessment of herbal medicines, Munich, 28.6. WHO, Geneva. 1991.
[8] FDA guidance for industry—botanical drug products (draft guidance), US Food and Drug Administration, Rockville, 2000, 4.
[9] Note forguidance on quality of herbal medicinal products, European Medicines Agency, London, 2001, 6.
[10] Zhang JX, Guan SH, Yang M,et al. Simultaneous determination of 24 constituents in Cortex Lycii using high-performance liquid chromatography–triple quadrupole mass spectrometry. J Pharm Biomed Anal, 2013, 77: 63-70.
[11] Requirements for studying fingerprints of traditional Chinese medicine injection, Drug Administration Bureau of China, Beijing, China, 2002.
[12] Alaerts G, Erps JV, Pieters S,et al. Similarity analyses of chromatographic fingerprints as tools for identification and quality control of green tea. J Chromatogr B, 2012, 910: 61-70.
[13] Tarachiwin L, Katoh A, Koichi U,et al. Quality evaluation of Angelica acutiloba Kitagawa roots by1H NMR-based metabolic fingerprinting. J Pharm Biomed Anal, 2008, 48: 42-48.
[14] Sun GX, Hu YS, Bi KS. Evaluating the quality of Niuhuangjiedu tablets by the systematic quantified fingerprint method. Acta Pharmaceutica Sinica, 2009, 44: 401-405.
[15] Sun GX, Zhang JX. Evaluating authentic quality of longdanxiegn pill by systematic quantified fingerprint method. Chinese Journal of Analytical Chemistry, 2009, 37: 1183-1187.
[16] Sun GX, Wu Y, Liu ZB,et al. Multiple methods were combined to monitor and evaluate the quality of TCM and make the results more reliable. Anal Methods, 2014,6: 838-849.
[17] Prevc T, Šegatin N, Ulrih NP,et al. DPPH assay of vegetable oils and model antioxidants in protic and aprotic solvents. Talanta, 2013, 109: 13-19.
[18] Mandade1 R, Sreenivas SA, Choudhury A. Radical scavenging and antioxidant activity ofCarthamus tinctoriusextracts. Free Radicals and Antioxidants, 2011, 1: 87-93.
[19] Gu CG, Goodarzi M, Yang XL,et al. Predictive insight into the relationship between AhR binding property and toxicity of polybrominated diphenyl ethers by PLS-derived QSAR. Toxicol Lett, 2012, 208: 269-274.
[20] Tsai Y, Tsai HH, Wu CP,et al. Preparation, characterisation and activity of the inclusion complex of paeonol with b-cyclodextrin. Food Chem, 2010, 120: 837-841.
[21] Asnaashari M, Farhoosh R, Sharif A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem, 2014, 159: 439-444.
[22] Choi EM, Suh KS, Rhee SY,et al. Inhibitory effect of paeoniflorin on methylglyoxal-mediated oxidative stress in osteoblastic MC3T3-E1 cells. Phytomedicine, 2014, 21: 1170-1177.
[23] Ya BL, Zhang L, Zhang L,et al. 5-hydroxymethyl-2-furfural prolongs survival and inhibits oxidative stress in a mouse model of forebrain ischemia. Neural regeneration research, 2012, 7: 1722-1728.
* Author to whom correspondence should be addressed. Address: College of Pharmacy, Shenyang Pharmaceutical University, 103 Wenhua Road, Shenyang, Liaoning 110016, China; Tel.: +86-24-23986286; Email: gxswmwys@163.com
Received: 2014-12-20 Accepted: 2015-03-31
