Comprehensive quality control of Xue Fu Zhu Yu Pill based on chromatographic fingerprints and antioxidantactivity combined with chemometrics methods
2015-05-14YningGoGuoxingSunPingWngJiyueGoShenyngPhrmceuticlUniversityShenyng110016Chin
Yning Go, Guoxing Sun*, Ping Wng, Jiyue GoShenyng Phrmceuticl University, Shenyng 110016, Chin;
bJiangsu Hengrui Medicine Co., Ltd., No.7 Kunlunshan Road, Lianyungang Eco & Tech Development Zone, 222047, Jiangsu Province, China
Regular article
Comprehensive quality control of Xue Fu Zhu Yu Pill based on chromatographic fingerprints and antioxidant
activity combined with chemometrics methods
Yaning Gaoa, Guoxiang Suna*, Ping Wangb, Jiayue GaoaaShenyang Pharmaceutical University, Shenyang 110016, China;
bJiangsu Hengrui Medicine Co., Ltd., No.7 Kunlunshan Road, Lianyungang Eco & Tech Development Zone, 222047, Jiangsu Province, China
High-performance liquid chromatographic fingerprint technique was adopted to monitor the quality of traditional Chinese medicine (TCM) Xue Fu Zhu Yu Pill (XFZYP). The novel 24 levels of systematic quantified fingerprint methods (SQFMs) were set up, and 230 nm HPLC fingerprints as well as 6 maker components were determined and identified, in combination of antioxidant activity detected by DPPH. Chromatographic fingerprints resolution information indexRIwas employed to screen the optimized condition for sample analysis, and chemometrics was used to extract more information from the data. By 24 levels SQFMs, the samples show accepted quality of 1~4. With help of multi-criteria decision making ranking methods (MCDM) PROMETHEE (Preference Ranking Organization Method for Enrichment Evaluation) and GAIA (Geometrical Analysis for Interactive Aid), the XFZYP samples from two manufactures were efficiently differentiated and classified. Furthermore, the antioxidant activity results expressed as IC50were further analyzed by PLSR (Partial Least Squares Regression) to establish spectrum-efficacy relationship and to predict the IC50. The work indicates that the proposed method is sensitive, efficient and reliable for fast determining complex chemicals, which is practical and possible for controlling quality of XFZYP.
Xue Fu Zhu Yu Pill; 24 levels of SQFMs; antioxidant activity; RI; PROMETHEE and GAIA; PLSR
1 Introduction
Xue Fu Zhu Yu Pill (XFZYP), a famous traditional Chinese medicine (TCM) formula, is first recorded in book of Yi Lin Gai Cuo [Correction of Medical Errors, 1830, by Wang Qingren (1768-1831)] [1], composing of 11 herbs:Prunus persica(L.) Batsch (Taoren),Angelicae sinensis(Oliv.) Diels (Danggui),LigusticumichuanxiongHort. (Chuanxiong),Carthamus tinctoriusL. (Honghua),Paeonia lactifloraPall. (Chishao),Rehmannia glutinosaLibosch. (Dihuang),Citrus aurantiumL. (Zhiqiao),Bupleurum chinenseDC. (Chaihu),Platycodon grandiflorum(Jacq.) A. DC. (Jiegeng),Achyranthes bidentataBl. (Niuxi) andGlycyrrhiza uralensisFisch. (Gancao) [2]. XFZYP is widely used for the prevention and treatment of diseases induced by static blood, suchas headache or chest pain, insomnia, dreaminess and palpitations and have demonstrated protective effects on cardiovascular diseases, especially for atherosclerosis and coronary heart diseases [3-6].
The therapeutic effects of traditional Chinese medicines (TCMs) are integrative action derived from their multiple pharmacologically active constituents, which makes the quality assurance a prime challenge [7]. For XFZYP, there are some publications associated with the qualitative and quantitative analyses [8-14], however, they may be inadequate, insensitive and unreliable due to the highly complicated constituents of XFZYP. To facilitate the quality control approaches, it is necessary and urgent to set up a reliable, accurate and comprehensive methodology to differentiate the XFZYP samples and control their quality validly.
Now, chromatographic fingerprint technique plays an important role in the quality assurance of TCM for the systemic characterization of compositions [15]. The quality of XFZYP is closely related to the contents of the chemical constituents. Considering the highly complicated chemicals, we cannot select only one or several specific components as essential criterion. In light of this, chromatographic fingerprint is developed in the quality assessment of XFZYP.
Free radicals, when produced in excess, can act as oxidants causing some severe chronic diseases such as cancer, hypertension, cardiac infarction and arteriosclerosis [16]. To prevent oxidation, there has been a growing interest regarding antioxidant activity of medicines in recent years. A prevalent strategy to detect the antioxidant activity of a given compoundin vitrois to directly measure clearance ratio (CLR) of the free radicals. Among the reported methods, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) is one of the most broadly employed to quantify antioxidants due to its simplicity and high-efficiency [17]. Thus, with this mind, in the present work we have chosen DPPH assay to study XFZYP samples for their antioxidant properties.
In this paper, Chlorogenic acid (CGA), Paeoniflorin (PNF), Ferulic acid (FA), Naringin (NG), Hesperidin (HPD) and Neohesperidin (NHP) were chosen as the marker compounds and their structures are shown in Fig. 1. We combined high-performance liquid chromatographic fingerprints with multicomponent quantitative analyses to assess the quality of XFZYP. Besides, the novel 24 levels of systematic quantified fingerprint methods (SQFMs), PROMETHEE (Preference Ranking Organization Method for Enrichment Evaluation) and GAIA (Geometrical Analysis for Interactive Aid) methods were adopted to discriminate the XFZYP samples. In addition, spectrum-efficacy relationship of HPLC fingerprint and the antioxidant activity was also established by PLSR (Partial Least Squares Regression) to efficiently control the quality of XFZYP.
2 The theory of 24 levels of SQFMs
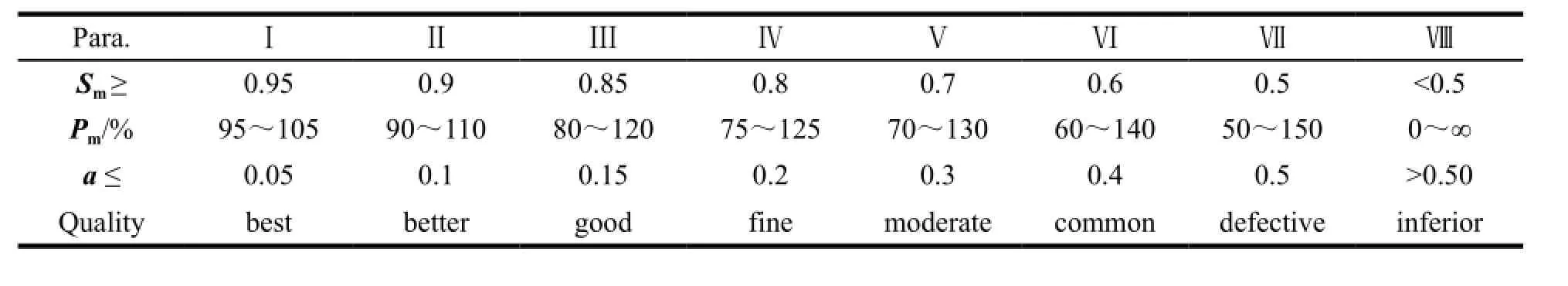
Table 1 The quality grade divided by SQFM
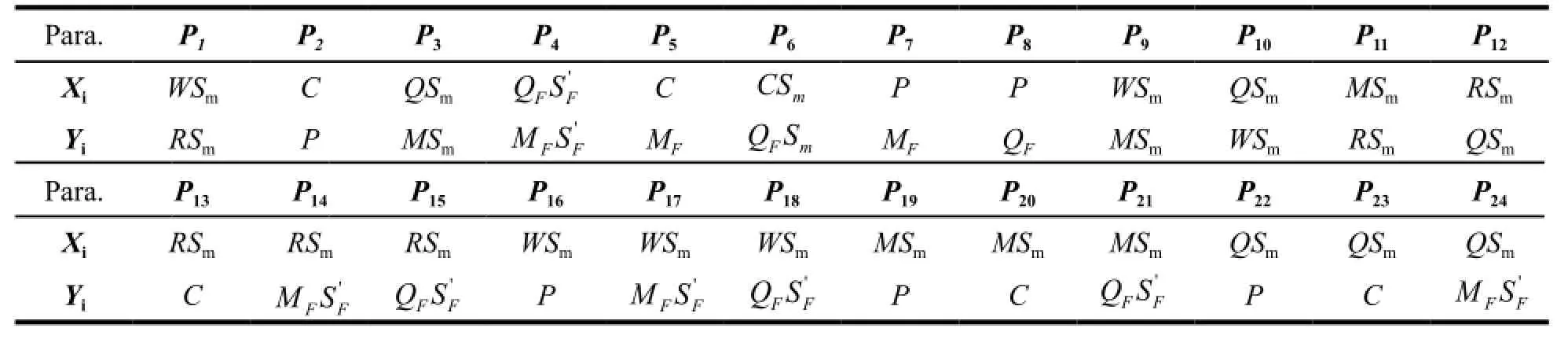
Table 2 The composing ways of the 24 levels of SQFMs
3 Materials and Methods
3.1 Materials
Paeoniflorin (PNF, 110736-201035, 96.5%), Ferulic acid (FA, 110773-201012, 99.6%), Naringin (NG, 110722-201111, 93.2%), Chlorogenic acid (CGA, 110753-200413), Hesperidin (HPD, 110721-201115, 95.3%) and Neohesperidin (NHP, 111857-201102, 99.6%) standards were purchased from National Institutes for Food and Drug Control (Beijing, China). The 2,2-diphenyl-1-picryl-hydrazyl (DPPH) was provided by Sigma. Methanol and acetonitrile were of chromatographic grade and obtained from Yuwang Industry Co., Ltd (Shandong, China), HPLC grade phosphoric acid and analytical grade NaH2PO4were achieved from Kermel Chemistry Reagent Co., Ltd (Tianjin, China) and Shantou Xilong Chemical Factory (Guangdong, China), respectively. Deionized water for HPLC analysis and the other chemicals were of analytical grade.
Twenty-four batches of XFZYP samples were acquired from different pharmacies in Shenyang,China and summarized as follows, manufacture A (S1, 1005114, S2, 1107103, S3, 1108113, S4, 1108134, S5, 1109124, S6, 1109127, S7, 1109131, S8, 1110101, S15, 1002131, S16, 1111129, S17, 1210139, S18, 1211123, S19, 1211145, S20, 1212151, S21, 1301102, S22, 1301159, S23, 1302114, S24, 1302124), manufacture B (S9, 110405, S10, 110603, S11, 110803, S12, 111005, S13, 120201, S14, 121103).
3.2 Instruments
The HPLC analysis was performed with an Agilent 1100 HPLC series (Agilent Technology, CA) equipped with a diode array detector (DAD), a quaternary low pressure mixing pump, an online degasser, an auto-sampler and an Agilent Poroshell 120 SB-C18column (150 mm×4.6 mm, 2.7μm). Software used for data analysis was Agilent HPLC 1100 ChemStation. An AG285 analytic scale (Mettler Toledo, Shanghai, China) and a rotary evaporator of RE-52 (Yarong Biochemistry Instrument Co., Ltd, Shanghai, China) were also used. The antioxidant activity detection was carried on a 752 UV spectrophotometer (Gaomi Caihong Analytical Instrument Co., Ltd, Shandong, China).
3.3 Standard solution preparation
Appropriate amounts of standards CGA, PNF, FA, NG, HPD and NHP were weighted accurately and dissolved in methanol respectively to obtain standard solutions. The standard solutions were diluted with suitable amounts of methanol to provide a series of solutions with gradient concentrations for the calibration curves.
3.4 Sample preparation
A pill (the pill weight was about 9 g) was cut into pieces, weighed accurately, and extracted by refluxing with 50 ml methanol twice, for 60 and 30 min respectively. The extract was filtered, combined and evaporated under reduced pressure. Then the concentrate was diluted with methanol to 25 ml. This solution was again filtered with a 0.45μm filter before use. All of the test solutions were stored at 4°C prior to analysis.
3.5 HPLC conditions
The ideal chromatographic fingerprints of XFZYP samples were achieved on an Agilent Poroshell 120 SB-C18column (150 mm×4.6 mm, 2.7μm) by using a binary gradient elution system consisted of 20 mmol/l solution of NaH2PO4in water (A) and 0.2% solution of phosphoric acid in acetonitrile (B) with the gradient elution program as follows: 0–10 min, 5–14% B; 10–20 min, 14–17% B; 20–35 min, 17–22% B; 35–50 min, 22–45% B; 50–70 min, 45–85% B; 70-85 min, 85% B. A 5μl was injected for HPLC analysis. The column was maintained at (35.0±0.1)°C and the flow rate was kept at 0.5 ml/min. The detect wavelength was 230 nm. All solvents were filtered through a 0.45μm filter prior to HPLC analysis.
3.6 Method validation
3.6.1 Linearity, limit of detection (LOD) and limit of quantitation (LOQ)
The calibration curves from the peak area (y, mAU*s) versus concentration (x, mg/ml) were constructed by running five concentration levels of the marker compounds. The regression equations, correlation coefficients (r), linear ranges, limit of detection (LOD) and limit of quantitation (LOQ) for each analyte were given in Table 3.3.6.2 Specificity, precision, accuracy, repeatability and sample stability analysis
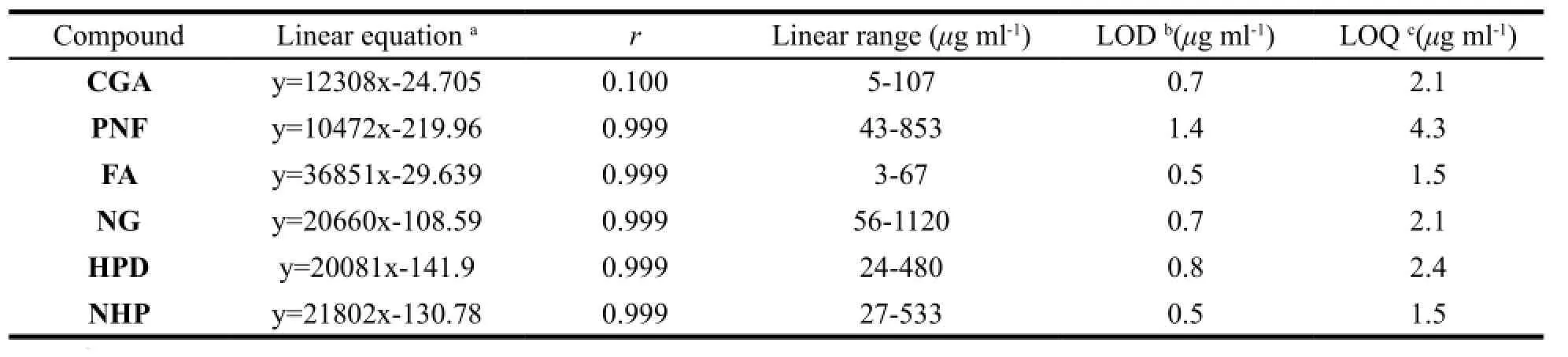
Table 3 Linear equation, correlation coefficient (r), linear range, LOD and LOQ for the marker compounds
The optimized method was validated for specificity with blank solvent and standard solutions, there was no interference from the blank solvent and the retention time (RT) of the 6 marker components in XFZYP sample were consistent with those in standard solutions. The relative retention time (RRT) and relative peak area (RPA) of the HPLC fingerprint common peaks (the ratio of common peak’s retention time and area to the reference, respectively) were chosen to assess the developed method in terms of precision, accuracy, repeatability and sample stability analysis. In our case, the peak of NHP was selected as the reference due to its high response and excellent resolution. The precision was calculated based on six replicate injections of the same sample solution, and the relative standard deviation (RSD) values of RRT and RPA were found to be less than 1.0% and 3.5%, respectively. The accuracy was validated by spike recovery test, and the mean recoveries of the marker compounds were in the range of 95.8%–106.4%. The repeatability was performed by analyzing five independently prepared XFZYP samples, and the RSD values of RRT and RPA were better than 1.1% and 3.5%, respectively. The sample stability was monitored by analyzing the same sample solution for 24 h at an interval of every 4 h, and the RSD values of the RRT and RPA were less than 1.2% and 4.0%, respectively. All these illustrated that the method was precise, accurate and sensitive enough for quantitative determination of the 6 makers simultaneously and assessing the quality of XFZYP effectively.
3.7 Determination of antioxidant activity
The capability to scavenge the stable DPPH free radical can be expressed as a measure of antioxidant activity. DPPH free radical can accept an electron or a hydrogen atom from the antioxidant molecule to form a more stable DPPH molecule, accompanying with a deduction in the absorbance of the solution [21]. During the assay, the absorbance of DPPH free radical solution was reduced by XFZYP extract and the decrease was monitored at 517 nm through UV/Vis spectrophotometer.
The DPPH stock solution was prepared by dissolving a 10 mg DPPH in methanol and diluted to 25 ml. Then a 3.0 ml of the DPPH stock solution was accurately taken into a 25 ml brown volumetric flask and diluted to volume with methanol. At last, 0.2, 0.4, 0.6, 0.8, 1.0 ml of diluent methanol-solution of XFZYP extract and 1.8, 1.6, 1.4, 1.2, 1.0 ml of methanol were added to 2.0 ml of above DPPH solution, respectively. The mixture was measured (in triplicate) after vigorously shaking and protected from light for 40 min. Vc was used as the positive control. The clearance ratio (CLR) was calculated
4 Results and Discussion
The HPLC analytical method described above was subsequently applied in simultaneous determination of the 6 marker components in XFZYP samples. The results are summarized in Table 4. The results indicate that both the sum of the contents and the individual contents of the 6 compounds show some variations among different batches, especially HPD, which is significantly different with a Max/ Min of 9.16 among 24 samples. S5 has the highest content of HPD, while S18 has the lowest content. As HPD derives fromCitrus aurantiumL. (Zhiqiao), the fluctuation may mainly result from the different quality ofC. aurantiumL. (Zhiqiao) raw materials and their production technologies, thus the XFZYP sample quality consistency could be improved by control theC. aurantiumL. (Zhiqiao) properly in the aspect of raw materials and the process of production.4.3 Evaluation of 230 nm fingerprint profiles by 24-level SQFMs
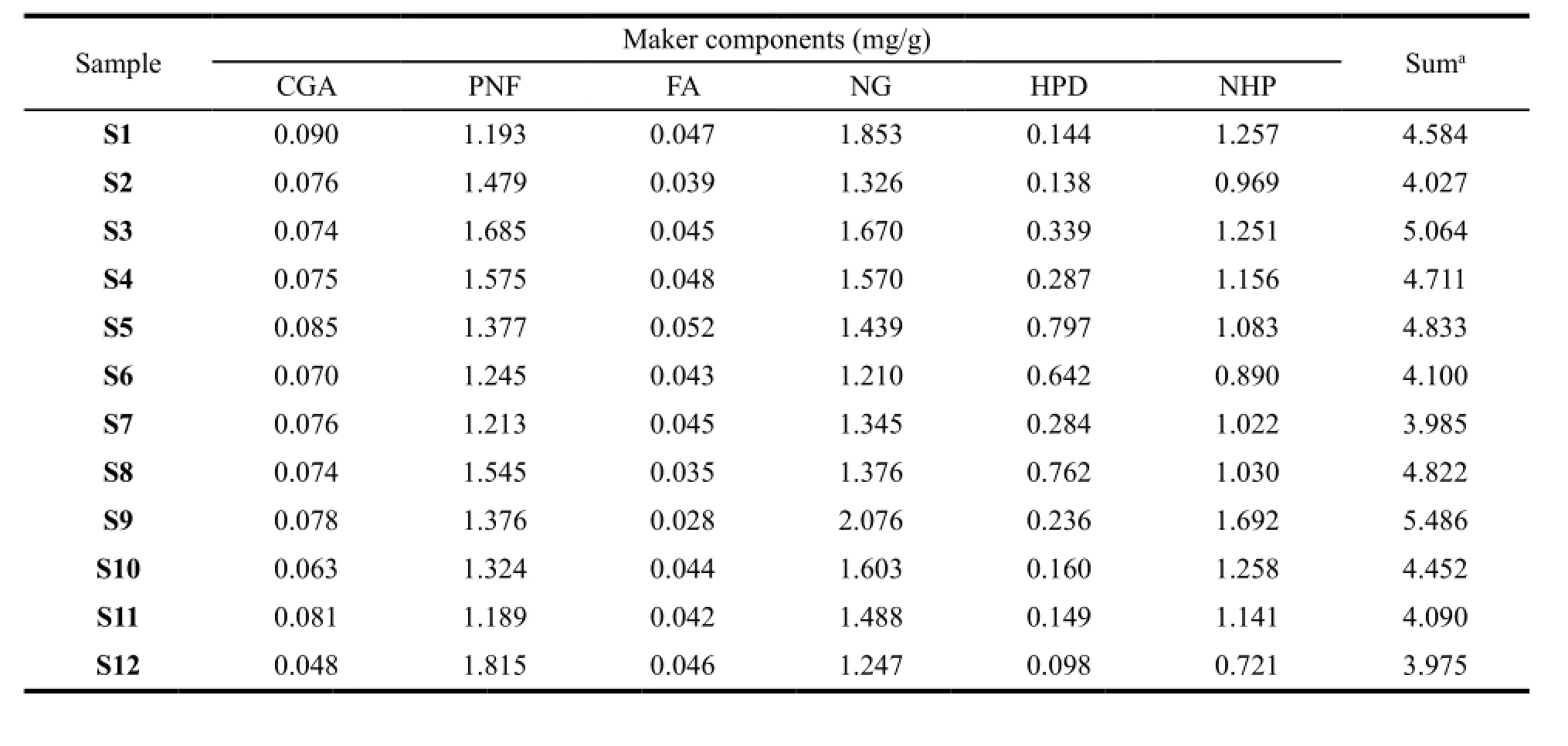
Table 4 Contents of the 6 marker compounds in 24 XFZYP samples
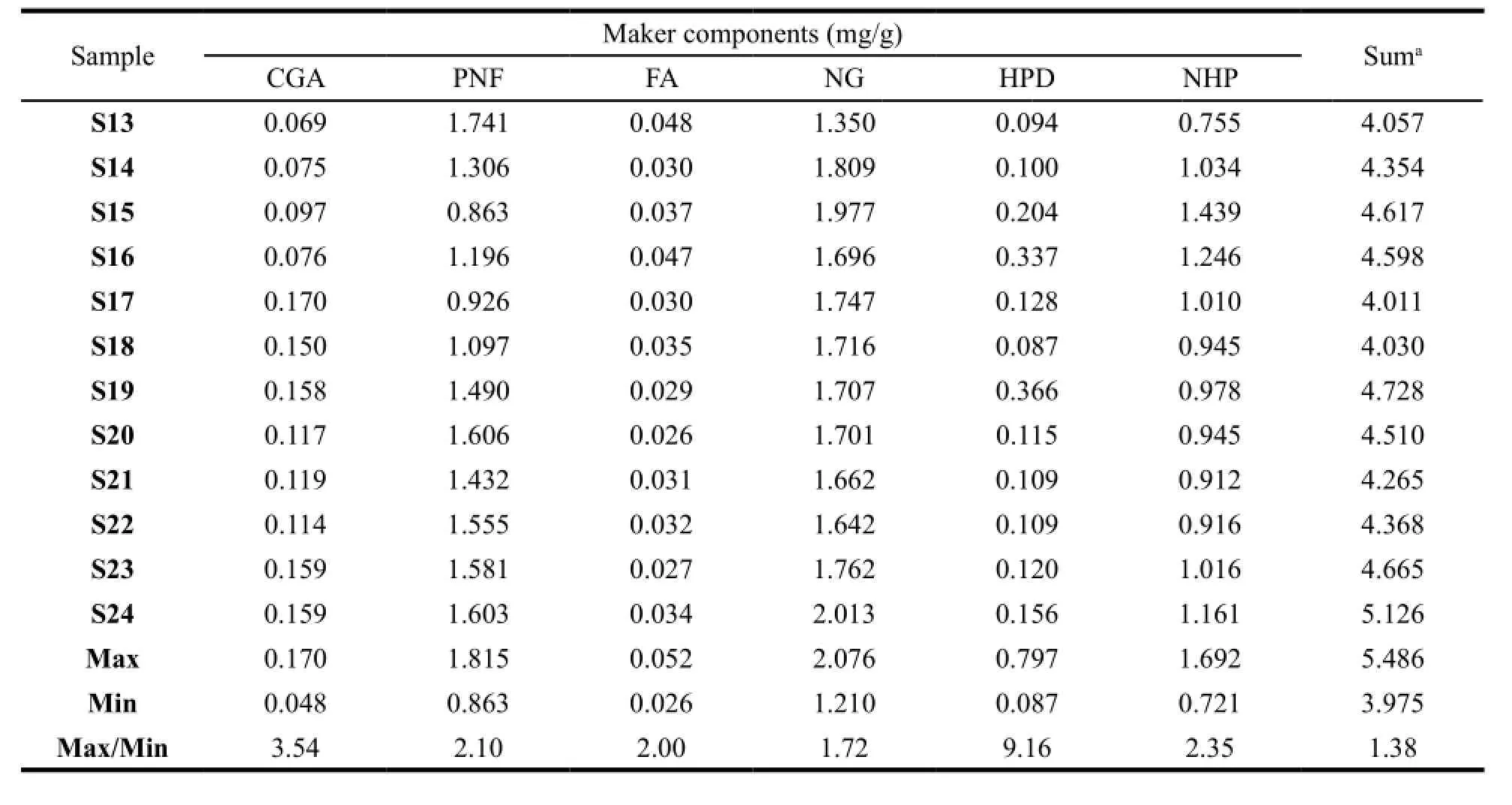
Continued Table 4
The whole chromatographic collection included 24 fingerprints, each corresponding to one sample. By taking average of all the sample chromatograms, the reference fingerprint (RFP) was established as the authoritative reference was unavailable, see Fig. 4 (A). The 6 marker compounds chromatograms are shown in Fig. 4 (B). The fingerprint contains 27 common peaks with reasonable peak height as well as resolution and the Peak 5 (P5), Peak 8 (P8), Peak 9 (P9), Peak 16 (P16), Peak 17 (P17) and Peak 18 (P18) represent CGA, PNF, FA, NG, HPD and NHP, respectively. To assess the quality of the samples comprehensively, 24 levels SQFMs were used based on an in-house developed software Digitized Evaluation System for Super-Information Characteristics of TCM Chromatographic Fingerprints 4.0 (Software certificate No. 0407573, China) invented by Professor Guoxiang SUN, etc.. The calculatedP1%-P24% of the fingerprints were integrated by average algorithm in order to avoid the bias with only one method. Table 5 summarizes the integrated results and the quality of XFZYP samples were evaluated on the basis of the criteria in Table 1. 7 batches of samples (S1, S14, S16, S19, S20, S22, S23) are the best quality (grade 1), 7 samples (S3, S4, S5, S7, S11, S17, S21) better (grade 2), and 9 samples (S6, S8-10, S12, S13, S15, S18, S24) are good (grade 3). S2 is found to have the lowest quality (grade 4). The average quality grade of manufacture A samples is better than B with a smaller average Grade of 2.1, which illustrates that the XFZYP samples from manufacture A may be of better quality than B. Fig. 5 shows the 24 levelsP1%-P24% distribution, S9, S10 and S13 display a significant distinction inP1%-P24%, consistent with Table 5, the quality grade of them are worse than others. Although S2 shows a smaller fluctuation, thePmvalues of it are the lowest. Therefore, as demonstrated in Table 5, S2 is the worst quality among the 24 XFZYP samples supplied by two manufacturers.
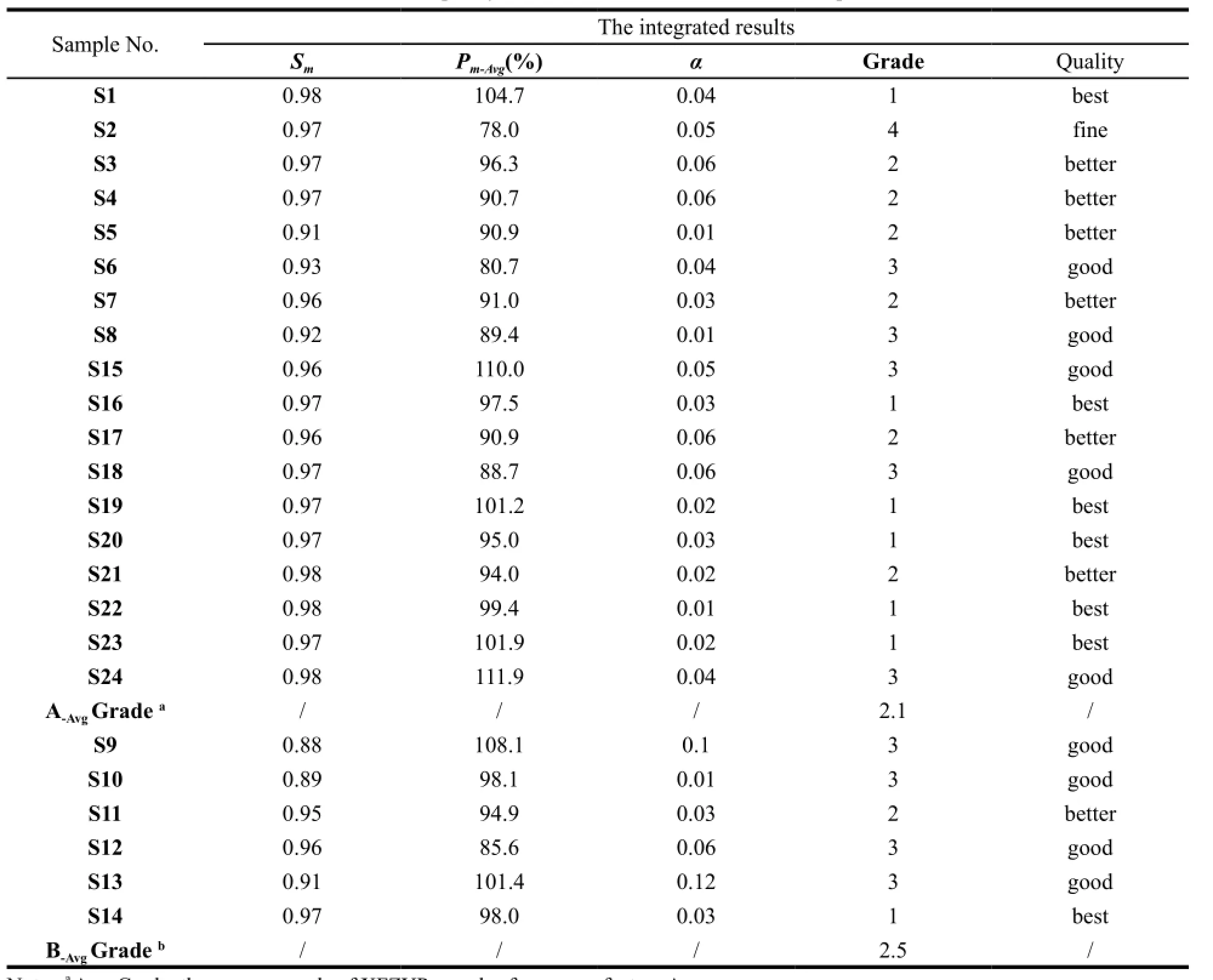
Table 5 The quality evaluation results of 24 XFZYP samples
4.4 POMETHEE and GAIA analysis
PROMETHEE is a non-parametric MCDM (multi-criteria decision making) method, ordering a number of objects (or actions) by a range of variables or criteria. The ranking analysis method has been used in the quality control of TCM based on chromatographic data, and successfully demonstrated its flexibility and effectiveness [23, 24]. The net ranking flow φ is calculated in the procedure, and at the same time, the complete ranking of the objects is also computed by φ value from high to low. As the visual display tool, GAIA is generated from the PROMETHEE ranking results [25].
In this study, principal component analysis (PCA) based on the variation of the common peak areas at 230 nm was performed firstly to extract information from the HPLC fingerprints, and 5 principal components (PCs) were determined, with 75% of total variance explained. The scores of the principal components were used as the multicriteria for the PROMETHEE method in ordering the XFZYP samples [26]. In light of quality grade of XFZYP samples from two manufactures (Table 5), the samples from manufacture A are of better quality than B, moreover, the manufacture A has a bigger sample size. Thus, in our case, the average sample of manufacture A (Avg1) which represented the sample of excellent quality was chosen to be the reference to decide the ranking sense based on its scores on each PC variables. Gaussian was selected as preference function, the computed standard deviation was utilized as the threshold value and the criteria weights were all set to 1.00 [24]. The data matrix consisting of 26 actions and five criteria PC1 to PC5 was submitted for ranking by the PROMETHEE method. As seen in Table 6, The PROMETHEE net outranking flows range from +0.2545 to −0.3909, XFZYP samples from manufacture A appear at the top of the rank order with the higher (φ) values than B except S2 and S14. S14 is embedded in the samples from manufacture A while S2 in B. Thisshows that S14 is more similar to manufacture A samples rather than B, and S2 is on the contrary case, which is consistent with Table 5. The five criteria PC1 to PC5 were used to establish the GAIA plot (Fig. 6), where each sample was represented as a dot. The XFZYP batches from manufacturer A and B appear to be divided into two groups, where samples from manufacturer B are completely separated from A with relative higher negative values on W axis. Fig. 6 reveals that XFZYP samples from different vendors may have different properties casing sample discrimination. This may due to different geographical sources, cultivation conditions, harvest time of raw materials, and manufacturing procedures in different pharmaceutical manufactures. In this paper, the two types of XFZYP samples were successfully discriminated by both PROMETHEE and GAIA.
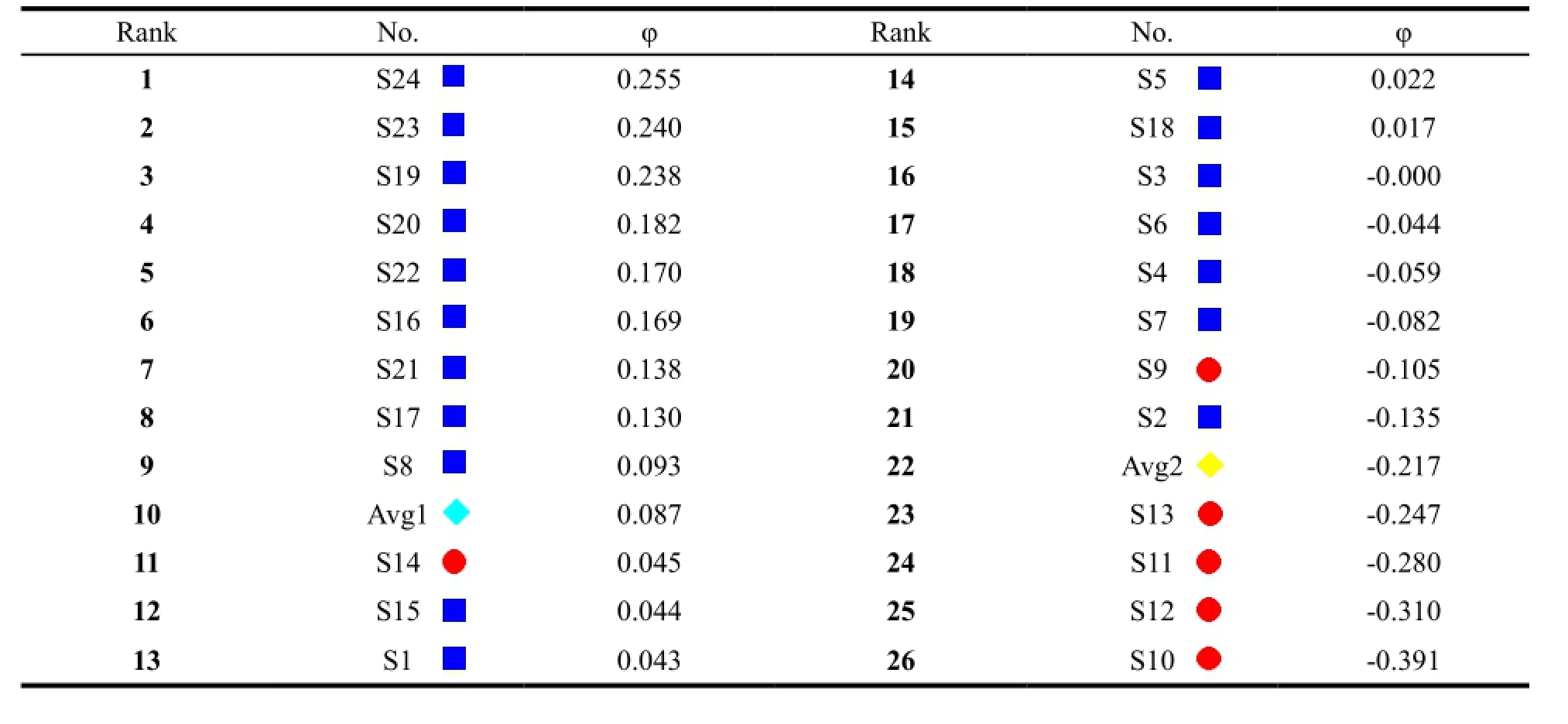
Table 6 PROMETHEE overall ranking (φ) for the comparison of XFZYP samples against the average sample (Avg1) quality criteria
4.5 Antioxidant activity (IC50) prediction by PLSR
When the number of variables exceeds the samples and/or when the variables themselves are highly correlated, the PLSR seems to be suitable for building regression models [27]. By taking not only X (independent variables) variance, but the covariance between X and Y (dependent variables) into consideration, PLSR is used to evaluate the linear relationship between X and Y. The PLS (Partial Least Squares) algorithm attempts to find latent variables (LVs) which maximize the amount of variation explained in X that related for predicting Y [28]. The optimum number of LVs is obtained by minimizing the value of predicted residual error sum of squares (PRESS) in the use of cross-validation technique.
In this assay, the X matrix was composed of the common peak areas of chromatographic profiles at 230 nm while Y vector was constructed with the values of antioxidant activity (IC50) obtained from DPPH trial. The PLSR model was set up to relate antioxidant activity with chromatographic data and a cross-validation was performed. Leave-one-out cross validation technic was used for the searching of the optimal LVs. Five LVs were chosen achieving the minimum PRESS of 0.0787 and all XFZYP samples were employed during the model construction except S16. Fig.7 (A) shows the predicted IC50vs. measured IC50plot that includes a fitting curve and a cross validation curve with a slope of 0.9523, 0.7229, an intercept of 0.04295, 0.2481 and correlation coefficients r of 0.976, 0.848, respectively, indicating that calculated values are well coincident with the measured ones. Fig. 7 (B) shows the standardized correlation coefficients of the fingerprint peaks for the antioxidant activity IC50. Those peaks that have high absolute values of coefficients belong to compounds that are highly related with antioxidant activity. There are 8 peaks exhibiting positive correlation and others negative correlation with IC50. Peak 4 (P4) and Peak 21 (P21) show more significant negative correlation, may have larger contribution to the antioxidant activity than the other 25 peaks. With the help of PLSR model, the IC50can be predicted with the peak areas and the main contributive components to the antioxidant capability of XFZYP can be simultaneously screened. As depicted in Table 7, no significant difference is discovered between the experimental and predicted IC50values for the samples except S16. Overall, XFZYP chromatographic profiles combined with PLSR model have the potential for rapid prediction of antioxidant ability.
5 Conclusion
With use of the 24 levels SQFMs and chemometrics techniques, the XFZYP samples were assessed on the basis of 230 nm HPLC fingerprints accompanied with 6 markers quantitative analysis. Chemometrics was applied in this assay to retrieve more information from the chromatographic data. All the quality grades of 24 XFZYP samples range from 1 to 4, fully satisfy the acceptance criteria within grade 1~5 for TCM, indicating that all samples are waith or above fine quality. Combining with PROMETHEE and GAIA, weefficiently differentiated and classified the XFZYP samples from two manufactures. In addition, the antioxidant activities using the DPPH scavenging effect expressed as IC50were estimated through UV/Vis spectrophotometer, and the results were further analyzed by PLSR to provide supplemental information for the establishment of spectrumefficacy relationship and the prediction of IC50. These results demonstrate that such analytical profiles have the potential to determine the identity, efficacy, and batch-to-batch consistency of XFZYP. In this study, we developed and validated an accurate and reproducible method, by which a reliable overall characterization assessment of XFZYP may be obtained, offering a new method for TCM comprehensive quality control.
[1] Li D. Study on quality control of Xue Fu Zhu Yu Pill and determination of metal elements. Liaoning Province, China. Shenyang Pharmaceutical University, China, 2008.
[2] Drug Specifications Promulgated by Ministry of PublicHealth, P. R. China, Part I. 1989, 72.
[3] Zhou YN, Sun MY, Mu YP,et al. Xuefuzhuyu decoction inhibition of angiogenesis attenuates liver fibrosis induced by CCl4in mice. J Ethnopharmacol, 2014, 153: 659-666.
[4] Lee JJ, Hsu WH, Yen TL,et al.Traditional Chinese medicine, Xue-Fu-Zhu-Yu decoction, potentiates tissue plasminogen activator against thromboembolic stroke in rats. J Ethnopharmacol, 2011, 134: 824-830.
[5] Yang XC, Xiong XJ, Yang GY,et al.Chinese patent medicine Xuefu Zhuyu capsule for the treatment of unstable angina pectoris: A systematic review of randomized controlled trials. ComplemenTherMed, 2014, 22: 391-399.
[6] Song XF, Wang JS, Wang PR,et al.1H NMR-based metabolomics approach to evaluate the effect of Xue-Fu-Zhu-Yu decoction on hyperlipidemia rats induced by high-fat diet. J Pharm Biomed Anal, 2013, 78-79: 202-210.
[7] Li C, Xu F, Cao C,et al. Comparative analysis of two species of Asari Radix etRhizomaby electronic nose, headspace GC-MS and chemometrics. J Pharm Biomed Anal, 2013, 85: 231-238.
[8] Liu ZH, Ye TX, Zhao LL,et al. Identification of Xuefu Zhuyu granule with TLC method. Tianjin Journal of Traditional Chinese Medcine, 2012, 29: 292-294.
[9] Li D, Zuo JL, Bai L,et al.FAAS determination of trace elements in Xuefuzhuyu pills. Chin J Pharm Anal, 2009, 29: 150-152.
[10] Liu ZH, Ye TX, Zhao LL,et al. Simultaneous determination of multicomponent in Xuefuzhuyu granule with different UV-wavelength by HPLC. China Journal of Chinese Materia Medicine, 2010, 135: 2157-2160.
[11] Ye HC, Ye QX, Wang DM,et al.HPLC Determination of Ferulic Acid inXuefu ZhuyuDecoction. Traditional Chinese Drug Research & Clinical Pharmacology, 2009, 20: 356-358.
[12] Gao Y, Gao WY, Dong X,et al.HPLC fingerprint of XuefuZhuyu Capsule. Chinese Traditional Patent Medicine, 2009, 31: 1477-1481.
[13] MA CY. Study on the active constituents in “Xue-Fu-Zhu-Yu capsule” and quantitative analysis of steroid saponins inRhizoma Paridis. Tianjin, China, Tientsin University, 2010.
[14] Zhang L, Zhu L, Wang YF,et al.Characterization and quantification of major constituents of Xue Fu Zhu Yu by UPLC-DAD-MS/MS. J Pharm Biomed Anal, 2012, 62: 203-209.
[15] Liu AH, Lin YH, Yang M,et al.Development of the fingerprints for the quality of the roots ofSalvia miltiorrhizaand its related preparations by HPLC-DAD and LC-MSn. J Chromatogr B, 2007, 846: 32-41.
[16] Al-Dabbas MM, Al-Ismail K, Kitahara K,et al.The effects of different inorganic salts, buffer systems, and desalting ofVarthemiacrude water extract on DPPH radical scavenging activity. Food Chem, 2007, 104: 734-739.
[17] Ameta RK, Singh M. A thermodynamicin vitroantioxidant study of vitamins B (niacin and niacin amide) and C (ascorbic acid) with DPPH through UV spectrophotometric and physicochemical methods. J Mol Liq, 2014, 195: 40-46.
[18] Sun GX, Hu YS, Bi KS. Quality evaluation of Niuhuangjiedu tablets by systematic quantified fingerprint method. Acta Pharm Sin, 2009, 44: 401-405. [19] Sun GX, Wu Y, Liu ZB,et al.Multiple methods were combined to monitor and evaluate the quality of TCM, and make the results more reliable. Anal Methods, 2014, 6: 838-849.
[20] Sun GX, Hou ZF, Zhang CL,et al.Comparison between the qualitative similarity and the quantitative similarity of chromatographic fingerprints of traditional Chinese medicines. Acta Pharm Sin, 2007, 42: 75-80.
[21] Carmona-Jiménez Y, García-Moreno MV, Igartuburu JM,et al.Simplification of the DPPH assay for estimating the antioxidant activity of wine and wine by-products. Food Chem, 2014, 165: 198-204.
[22] Sun GX, Yang TT. Evaluation of the entire quality of Liuweidihuang pills by systematic quantified fingerprint based on chromatographic fingerprint resolution information index. Central South Pharmacy, 2010, 18: 143-148.
[23] Ni YN, Zhang LS, Churchill J,et al.Application of high performance liquid chromatography for the profiling of complex chemical mixtures with the aid of chemometrics. Talanta, 2007, 72: 1533-1539.
[24] Ni YN, Lai YH, Brandes S,et al.Multi-wavelength HPLC fingerprints from complex substances: An exploratory chemometrics study of theCassiaseed example. Analytica Chimica Acta, 2009, 647: 149-158.
[25] Zhang GW, Ni YN, Churchill J,et al.Authentication of vegetable oils on the basis of their physico-chemical properties with the aid of chemometrics. Talanta, 2006, 70: 293-300.
[26] Ni YN, Mei MH, Kokot S. Analysis of complex, processed substances with the use of NIR spectroscopy and chemometrics: Classification and prediction of properties-The potato crisps example. Chemometr Intell Lab, 2011, 105: 147-156.
[27] Cristina RS, Federico M, Luis CR,et al.Quantification of blending of olive oils and edible vegetable oils by triacylglycerol fingerprint gas chromatography and chemometric tools. J Chromatogr B, 2012, 910: 71-77.
[28] Lucio-Gutiérrez JR, Garza-Juárez A, Coello J,et al.Multi-wavelength high-performance liquid chromatographic fingerprints and chemometrics to predict the antioxidant activity ofTurnera diffusaas part of its quality control. J Chromatogr A, 2012, 1235: 68-76.
* Author to whom correspondence should be addressed. Address: School of Shenyang Pharmaceutical University No.103, Wenhua Road, Shenhe District, Shenyang 110016, China; Tel.: +86-24-23986286; Fax: 86-24-24514760; Email: gxswmwys@163.com
Received: 2014-12-18 Accepted: 2015-03-31
