Synthesis, Crystal Structure and Thermal Behavior of 3,4-Bis(3-nitrofurazan-4-oxy)furazan
2015-05-10ZHAILianjieWANGBozhouHUOHuanHUHuaimingSUPengfeiFANXuezhongLIHui
ZHAI Lian-jie, WANG Bo-zhou, HUO Huan, HU Huai-ming, SU Peng-fei, FAN Xue-zhong, LI Hui
(1. Xi′an Modern Chemistry Research Institute, Xi′an 710065, China; 2. College of Chemistry & Material Science, Northwest University, Xi′an 710069, China)
1 Introduction
In the field of high-energy materials, the synthesis and development of new energetic materials continues to focus on new heterocycles with high densities, high heats of formation, and good oxygen balance. Furazan ring has attracted considerable amount of attention as building block for the synthesis of a high-energy materials due to their high standard enthalpy of formation, high nitrogen content, high energy density, good thermal stability, and low melting point[1-4]. In addition to having furazan groups, furazanyl ether compounds have ether bonds. The introduction of ether bonds between furazan rings tends to lower melting point but contributes markedly to the overall energetic performance. In recent years, more furazanyl ether compounds, such as 3,3′-dicanyodifurazanyl ether, 3,3′-dinitrodifurazanyl ether, 3,3′-bis(fluorodinitromethyl)difurazanyl ether and bifurazano[3,4-b:3′,4′-f] furoxano [3″,4″-d] oxacyclohetpatriene, were developed[5-11]. 3,4-Bis(3-nitrofurazan-4-oxy)furazan was reported by Aleksei B. Sheremetev with the density of 1.91 g·cm-3and melting point of 38-40 ℃[12], which suggest that it would be a potential candidate for high energy plasticizer.
In this paper, 3,4-bis(3-nitrofurazan-4-oxy)furazan was synthesized from 3,4-diaminofurazan by Caro′s acid oxidation, hydrolysis, neutralization and substituent reactions, and its structure was characterized by the means of IR, NMR and elemental analysis. In order to confirm the molecular structure of the title compound, its single crystals were obtained firstly and then characterized by X-ray diffraction analysis.
2 Experimental
2.1 Materials and measurements
Melting point was measured on XT4A Melting-Point Apparatus with Microscope and uncorrected.1H NMR and13C NMR were obtained in DMSO-d6on Bruker AV500 NMR spectrometer. Infrared spectra were obtained from KBr pellets on Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Elemental analyses (C, H and N) were performed on VARI-El-3 elementary analysis instrument. Differential scanning calorimetry (DSC) were carried out in a platinum sample container using Q-200 at a rate of 10 ℃·min-1. Thermogravimetric (TG) were carried out in a platinum sample container using Nicolet TA 2950.
3,4-Diaminofurazan was purchased from commercial sources and recrystallized from water for further use. 3,4-Dinitrofurazan was prepared[13]. Acetonitrile was chromatographical pure. Other chemicals were analytically pure and obtained from commercial sources.
2.2 Synthesis and characterization
The title compound (1) was synthesized from Scheme 1.

Scheme 1 Synthesis of the title compound
Preparation of compound 4: To a solution of compound 3 (6.4 g, 40 mmol) in tetrahydro furan(THF) (40 mL) was added dropwise a solution of potassium hydroxide (16 g, 0.4 mol) in water (80 mL) at room temperature. The reaction mixture was heated to 40 ℃ for 1 h, diluted with water (60 mL), acidified with an appropriate concentrated hydrochloric acid and extracted with Et2O (4×80 mL). The combined organic layer was dried over magnesium sulfate, filtered and the solvent was removed to give 3.77 g compound 4 (92.5%) and a purity of 99.3%(HPLC). m. p.: 152-153 ℃. IR (KBr,ν/cm-1): 3559, 3388 (—OH), 1590, 1421, 1266(furazan ring), 1020(C—OH);1H NMR (DMSO-d6, 500 MHz)δ: 12.386 (s, 2 H, OH);13C NMR (DMSO-d6, 125 MHz)δ: 156.106 (C—OH); Anal. calcd for C2H2N2O3: C 23.54, H 1.98, N 27.45; found C 23.35, H 2.12, N 27.29.
Preparation of compound 5: Ethanol (8 mL) of sodium ethylate (1.43 g, 21 mmol) was added dropwise to solution compound 4 (1.02 g, 10 mmol) in 15 mL ether. The mixture was stirred at room temperature for 1 h. The precipitate was collected, washed with MeOH and Et2O to furnish of 1.38 g compound 5 (94.6%) and a purity of 98.8%(HPLC). IR (KBr,ν/cm-1): 1597, 1584 , 1350, 971 (furazan ring);13C NMR (DMSO-d6, 125 MHz)δ: 165.596(C—ONa); Anal. calcd for C2N2O3Na2: C 16.45, N 19.19; found C 16.63, N 19.08.(CAUTIONS:Thiscompoundishighexplosiveandmaybesensitivetoshockorheatingandmustbehandledwithappropriateprecautions).
Preparation of compound 1: To a solution of 5 (1.48 g, 10 mmol) in absolute MeCN (20 mL) was added a solution of 3,4-dinitrofurazan (2.56 g, 16 mmol) in absolute MeCN at 30 ℃ under N2. The reaction mixture was stirred at this temperature for 30 min, diluted with Et2O (50 mL), washed with water and dried over magnesium sulfate, filtered and the solvent was evaporated. 1.65 g (yield 51.0%) Compound 1 was obtained with a purity of 99.8%(HPLC) by silica gel flash chromatograpy using pentroleum/ethyl acetate(V/V=7/1) as eluent. m.p.: 72-73 ℃(lit. 38-40 ℃)[12]. IR (KBr,ν/cm-1): 1602, 1543, 1489 , 1263, 1202(furazan ring), 1034 (ether bond), 1584, 1365 (—NO2);13C NMR (DMSO-d6, 125 MHz)δ: 152.860(O—C—C—O), 152.987(C—NO2), 154.833(C—C—O); Anal. calcd for C6N8O9: C 21.96, N 34.15; found C 21.64, N 33.45.
2.3 Crystal structure determination
The title compound was dispersed into solution of petroleum/diethyl ether (V/V=5/3) with a stirrer, and the mechanical impurities were eliminated by filtration. The saturated solution of title compound 1 was put in a conical flask for two weeks at room temperature to evaporate slowly the mixed solvent and obtain single crystal of title compound.
A colorless single crystal of the title compound with dimensions of 0.36 mm×0.28 mm×0.20 mm was selected for X-ray diffraction analysis, and the data collection was performed on a Bruker SMART Apex-II CCD X-ray diffractometer equipped with a graphite-monochromatized MoKαradiation (λ=0.71073Å) using theφ-ωscan mode (1.51°≤θ≤25.10°) at 296(2) K. 11509 Reflections were collected, of which 4166 were independent (Rint=0.0464) and 2736 withI>2σ(I) were considered to be observed and used for the refinement.
The structure was solved by direct methods and refined by full-matrix least squares techniques onF2using SHELXS-97 and SHELXL-97 programs[14-15]. All non-hydrogen atoms were refined anisotropically. The atomic scattering factors and anomalous dispersion corrections were taken from International Table for X-ray Crystallography[16]. Crystal data and structural refinement parameters for compound 1 are listed in Table 1.
Further information on the crystal-structure determinations can be obtained free of charge at www.ccdc.ac.uk/conts/retrieving.html or from the Cambridge Crystallograpnic Data Centre with CCDC No. 942434.
Table 1 Crystal data of the title compound

formulaC6N8O9formulaweight/g·mol-1328.14crystalsystemmonoclinicspacegroupP21/ca/Å15.256(3)b/Å11.579(3)c/Å14.981(3)crystalsize/mm0.36×0.28×0.20volume/Å32344.7(9)Z8ρcalcd/g·cm-31.859F(000)1312T/K296(2)λ/Å0.710732θmax/(°)50.20reflectionscollected/unique11509/4166parameters416GOFonF21.015finalRindexes(I>2σ(I))0.0433finalRindexes(alldata)0.0755largestdiff.peakandhole/e·Å-30.243and-0.189
3 Results and Discussion
3.1 Improvement of synthesis of compound 5 and 13C NMR analysis of title compound
Compound 5 is an important intermediate for compound 1. It is a high explosive and may be sensitive to shock or heating. So NaOEt was selected as reaction reagent instead of sodium in order to make the operation in the preparation of compound 5 simpler and safer. In addition, THF was employed instead of MeCN as reaction medium in the acidification of compound 4 in order to reduce the toxicity of reaction system and avoid hydrolysis of MeCN. The elemental analysis, IR and13C NMR of the product are all in good agreement with the assumed structure. However, it was found that a few characteristic results of compound 1 performed in our laboratory were different from those reported by Sheremetev. For example, the melting point we determined was 72-73 ℃, which was 38-40 ℃ in the literature[12]. The13C NMR data of the title compound obtained in our study were 152.98 (C1), 154.83 (C2) and 152.86(C3), which were different from the data of 153.6 (C1), 156.3 (C2) and 154.2 (C3)[12](Scheme 2).
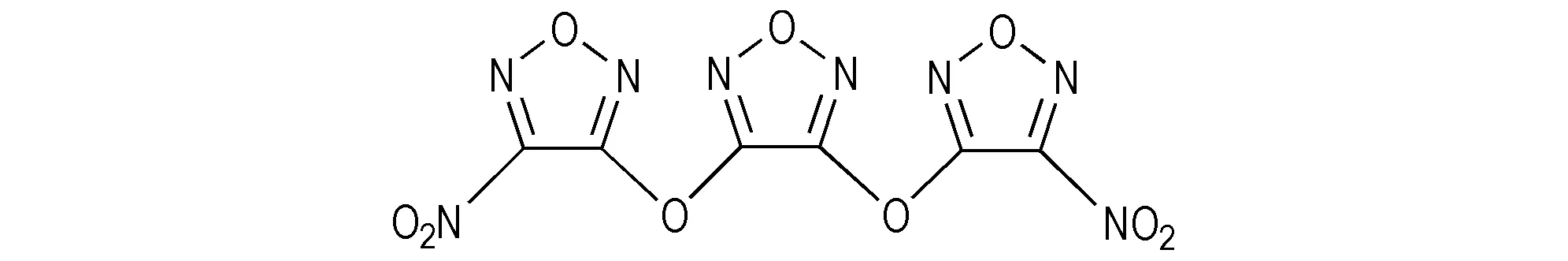
Scheme 2
3.2 Crystal structure
The single crystal X-ray diffraction analysis shows that the title compound crystallizes in monoclincP21/cspace group and there are two crystallographic independent host molecules in the asymmetric unit, as shown in Figure 1. These two independent host molecules have different conformations (Ⅰ and Ⅱ), in which the bond angles and torsion angles show significant difference. The selected bond lengths, bond angles and dihedral angles are summarized in Table 2.
In the crystal, the bond lengths and bond angles in the furazan rings are generally normal and each ring is almost planar[17-18]. The average bond length of C—N of nitro group and furazan ring is 1.441 Å, which is good agreement with the corresponding values in related nitrofurazan derivatives[8]. Because of thep-πconjugative effect with O atom of ether bond between furazan rings, the bond distances of C(2)—O(4) [1.347(3) Å], C(3)—O(4) [1.358(3) Å], C(4)—O(6) [1.353(3) Å], C(5)—O(6) [1.354(3) Å], C(2A)—O(4A) [1.349(3) Å], C(3A)—O(4A) [1.344(3) Å], C(4A)—O(6A) [1.368(3) Å] and C(5A)—O(6A) [1.347(3) Å] are shorter than those of the normal C—O single bond (1.41 Å)[19]. The bond angles C(2)—O(4)—C(3) [120.2(2)°], C(4)—O(6)—C(5)[118.3(2)°], C(2A)—O(4A)—C(3A)[121.2(2)°], C(4A)—O(6A)—C(5A)[116.26(19)°] indicate ansp2hybridization nature of O atom. However, the whole molecular structure is not planar. Both nitro groups are twisted slightly relative to their adjacent furazan rings. The torsion angles of O(1)—N(1)—C(1)—N(2), O(9)—N(8) —C(6)—N(7), O(1A)—N(1A)—C(1A)—N(2A), O(8A)—N(8A)—C(6A)—N(7A) in conformation Ⅰ and Ⅱ are 10.6(4)°, -17.7(4)°, -159.1(3)°, -13.4(4)°, respectively. Both furazan rings are also twisted relative to the central furazan ring. The dihedral angles between the central furazan ring (C(3)—N(4)—O(5)—N(5)—C(4)) and terminal furzan rings (C(1)—N(2)—O(3)—N(3)—C(2) and C(5)—N(6)—O(7)—N(7)—C(6)) in conformation Ⅰ are 15.95° and 66.23°, respectively. In conformation Ⅱ, the corresponding dihedral angles are 7.81° and 69.38°, respectively. Obviously, the terminal furazan ring (C(1A)—N(2A)—O(3A)—N(2A)—C(1A)) and the central furazan ring (C(3A)—N(4A)—O(5A)—N(5A)—C(4A)) in conformation Ⅱ exhibit better conjugation than that in conformation Ⅰ, which causes a bigger dihedral angle between the other terminal furazan ring (C(5A)—N(6A)—O(7A)—N(7A)—C(6A)) and the central furazan ring (C(3A)—N(4A)—O(5A)—N(5A)—C(4A)) in conformation Ⅱ than that in conformation Ⅰ.
In the crystal structure of compound 1, there are several weak intermolecular contacts. In conformation Ⅰ: the short contacts of O(6)…O(8i) (2.890 Å, symmetric code i: -x, 2-y, -z) link two molecules into dimer, as shown in Figure 2. In conformation Ⅱ: the short contacts of O(3A)…N(6Aii) (3.021 Å) and N(3A)…C(5Aii) (3.164 Å, symmetric code ii: x, 0.5-y, 0.5-z) connect the molecules into one-dimensional chain, as depicted in Figure 3. A three-dimensional supramolecular structure is formed through the short contacts between the dimers and the one-dimensional chains (O(1)…N(1Aiii), 2.895 Å, O(2)…O(5Aiv), 2.887 Å, symmetric code iii: 1-x, 0.5+y, 0.5-z; iv: x, 1+y, z). These intermolecular short contacts as well as absence of hydrogen atoms in the molecules lead to a sufficiently dense structure (ρcalcd=1.859 g·cm-3, which is higher than that of TNT[20]).
Table 2 Selected bond lengths and bond angles/torsion angles of compound 5

bondlength/Åbondlength/Åbondlength/ÅN(1)—O(1)1.209(3)C(2)—C(1)1.406(4)O(4)—C(2)1.347(3)N(1A)—O(1A)1.206(3)C(2A)—C(1A)1.402(4)O(4A)—C(2A)1.349(3)N(1)—O(2)1.196(3)C(2)—N(3)1.296(3)O(4)—C(3)1.358(3)N(1A)—O(2A)1.207(3)C(2A)—N(3A)1.291(3)O(4A)—C(3A)1.344(3)N(1)—C(1)1.441(4)O(3)—N(2)1.363(3)O(6)—C(4)1.353(3)N(1A)—C(1A)1.437(4)O(3A)—N(2A)1.357(3)O(6A)—C(4A)1.368(3)C(1)—N(2)1.287(3)O(3)—N(3)1.387(3)O(6)—C(5)1.354(3)C(1A)—N(2A)1.284(3)O(3A)—N(3A)1.388(3)O(6A)—C(5A)1.347(3)bondangle/(°)bondangle/(°)bondangle/(°)O(1)—N(1)—O(2)125.7(3)O(3)—N(2)—C(1)104.9(2)O(1)—N(1)—C(1)—N(2) 10.6(4)O(1A)—N(1A)—O(2A)125.9(3)O(3A)—N(2A)—C(1A)104.8(2)O(1A)—N(1A)—C(1A)—N(2A)-159.1(3)O(1)—N(1)—C(1)118.1(3)C(2)—O(4)—C(3)120.2(2)N(2)—C(1)—C(2)—N(3)180.0(2)O(1A)—N(1A)—C(1A)115.9(3)C(2A)—O(4A)—C(3A)121.2(2)N(2A)—C(1A)—C(2A)—N(3A)-0.5(3)O(2)—N(1)—C(1)116.2(3)C(4)—O(6)—C(5)118.3(2)N(3)—C(2)—O(4)—C(3)6.5(4)O(2A)—N(1A)—C(1A)118.2(3)C(4A)—O(6A)—C(5A)116.26(19)N(3A)—C(2A)—O(4A)—C(3A)0.5(4)N(2)—C(1)—C(2)110.0(3)O(8)—N(8)—C(6)116.0(2)N(6)—C(5)—O(6)—C(4)34.7(4)N(2A)—C(1A)—C(2A)109.8(3)O(8A)—N(8A)—C(6A)118.3(3)N(6A)—C(5A)—O(6A)—C(4A)-3.7(4)N(2)—O(3)—N(3)111.6(2)O(9)—N(8)—C(6)118.5(2)O(8)—N(8)—C(6)—C(5)-17.3(4)N(2A)—O(3A)—N(3A)111.9(2)O(9A)—N(8A)—C(6A)115.4(3)O(8A)—N(8A)—C(6A)—C(5A)164.9(3)

Fig.1 Molecular structure of the title compound

Fig.2 Dimer structure linked by short contacts between the molecules with conformation Ⅰ

Fig.3 One-dimensional chain connected by short contacts between the molecules with conformation Ⅱ
3.3 Thermal behavior
The DSC and TG-DTG curves measured with a linear heating rate of 10 K· min-1under nitrogen atmosphere are shown in Figures 4 and 5, respectively. In Fig.4, curve 1 was measured at general pressure and curve 2 in a sealed condition at a pressure of 3.0 MPa, respectively. In the curve 1, there are two main endothermic processes from 50 ℃ to 240 ℃. The first endothermic process is an intense melting process that starts at 67.0℃, the peak temperature is 72.9 ℃ and this is the melting point of compound 1. The second endothermic process is a very tardy volatile process that starts at 156.4 ℃ and ends at 238.5 ℃, which indicates that compound 1 is rather volatile in melten state. In Fig.4, the curve 2 shows that the endothermic process is a little different from curve 1 owing to increase of the pressure. The successive exothermic decomposition step begins at 245.1 ℃ with the exothermic decomposition peak temperature at 305.9 ℃. TG-DTG curves also show that compound 1 has volatilized before decomposition in accordance with the tardy endothermic processes in the curve 1. Thermal studies reveal that compound 1 has a very high thermal stability.

Fig.4 DSC curves of compound 1

Fig.5 TG-DTG curves of compound 1
4 Conclusion
The title compound was synthesized using THF instead of CH3CN and NaOEt instead of sodium. X-ray single-crystal diffraction analysis indicated that there are two host molecules of different conformation. It is also found that several different weak intermolecular contacts lead to a high density and stable structure. The title compound has low melting point(72.9 ℃), and high computing density(1.859 g·cm-3,cacluated). It could be used as a high energy plasticizer in the future.
[1] Shereemetev A B. Chemistry of furazans fused to five-menbered rings[J].JHeterocyclChem, 1995, 32: 271-385.
[2] Shereemetev A B, Yudin I L. Advances in the chemistry of furazano[3, 4-b]pyrazines and their analogues[J].RussChemRev, 2003,72: 87-100.
[3] Olofson R A, Michelman J S. Furazan[J].JournalofOrganicChemistry, 1965,30: 1854-1859.
[4] Sikder A K, Sikder N A. Review of advanced high performance, insensitive and thermally stable energetic materials emerging for military and space applications[J].JHazardMater, 2004, 112: 1-15.
[5] Pivina T S, Sukhachev D V, Evtushenko A V. Comparative characteristic of energy content calculating methods for the furazan series as an example of energetic materials[J].Propellants,Explosives,Pyrotechnics, 1995, 20: 5-10.
[6] FAN Yan-jie, WANG Bo-zhou, LAI Wei-Peng, et al. Synthesis, characterization and quantum chemistry study on 3,3′-Dicyanodifurazanyl Ether (FOF-2)[J].ChinJOrgChem, 2009, 29: 614 -620.
[7] Sheremetv A B, Mantseva E V. Hydroxyfurazans: Outlook to using[C]∥32thInternational Annual Conference of ICT, Karlsruhe, Germany, 2001: 103/1-4.
[8] WANG Xin-jie, LIAN Peng, GE Zhong-xue. Synthesis, crystal structure and theoretical research of 3,3′-dinitrodifurazanyl ether (FOF-1)[J].ActaChimSinica, 2010, 68: 557-563 .
[9] Novikova T S, Melnikova T M, Kharitonova O V. Novel synthesis of 3,4-dicyanofuroxan[J].MendeleevCommun, 2001,11: 30-31.
[10] Sheremetev A B. 3,3-Bis(1-fluoro-1,1-dinitromethyl)difurazanyl ether[C]∥29thInternational Annual Conference of ICT, Karlsruhe, Germany. 1998: 58/1-6.
[11] ZHOU Yan-shui, WANG Bo-zhou, WANG Xi-jie, et al. Synthesis and quantum chemistry study of novel bifurazano[3,4-b :3′,4′-f] furoxano [3″,4″-d] oxacyclohetpatriene[J].ChinJSynChem, 2012, 20: 147-152.
[12] Sheremetev A B, Kulagina V O, Aleksandrova N S, et al. Dinitro trifurazans with oxy, azo and azoxy bridges[J].Propellants,Explosives,Pyrotechnics, 1998, 23: 142-149.
[13] Novikova T S, Melnikova T M, Kharitonova O V, et al. An effective method for the oxidation of aminofurazans to nitrofurazans[J].MendeleevCommun, 1994 (4): 138-140.
[14] Sheldrick G M. SHELXS-97, Program for Crystal Structure Solution[CP], University of Göttingen, Germany 1997.
[15] Sheldrick G M. SHELXL-97, Program for Crystal Structure Refinement[CP], University of Göttingen, Germany 1997.
[16] Siemens. Area Detector Control and Integration Software[CP], USA 1996.
[17] JIA Si-yan, WANG Bo-zhou, FAN Xue-zhou, et al. 4-[4-(4-Amino-1,2,5-oxadiazol-3-yl)-1,2,5-oxadiazol-3-yl]-1,2,5-oxadiazol-3-amine[J].ActaCryst, 2012, E68, o1573.
[18] Sheremetev A B, Ivaanova E A, Dmtriev D E, et al. Synthsis of macrocycles incorporating azo-bis(azofurazan) framework[J].JHeterocyclicChem, 2005, 42: 803-810.
[19] LIU Yan-qiu, ZHANG Zhi-wen, LI Dan-dan, et al. Synthesis, crystal structure and theoretical studies of (E)-1-((9-(2-(2-methoxythoxy)ethyl)-9H-c arbazol-3-yl)methylene)thiosemicarbazide[J].ChinJStructChem, 2013, 32: 659-666.
[20] Eaton P E, Gilardi R L,Zhang Mao-Xi. Polynitrocubanes: advanced high-density, high-energy materials[J].AdvMater, 2000, 12: 1143-1148.
