Facile Synthesis and Crystal Structure of 3,4-Bis(1H-5-tetrazolyl)furoxan
2015-05-10ZHAILianjieFANXuezhongWANGBozhouBIFuqiangHUOHuanLIYananLIXiangzhi
ZHAI Lian-jie, FAN Xue-zhong, WANG Bo-zhou, BI Fu-qiang, HUO Huan, LI Ya-nan, LI Xiang-zhi
(Xi′an Modern Chemistry Research Institute, Xi′an 710065, China)
1 Introduction
The rational design of new energetic materials with high performance properties has been becoming one of the most challenging tasks in the field of advanced materials[1-3]. At the early stages of energetic materials, scientists mainly focused on designing and synthesizing the polynitro compounds, such as 1,3,5,7-tetranitrotetraazacycloctane (HMX), 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracy-clododecane (CL-20), and octanitrocubane (ONC). Although these materials exhibit excellent detonation properties, the synthesis is often expensive and includes multiple steps which make the industrial scale-up and practical use infeasible. Along with growing concerns about the environment and safety issues, considerable effort has been devoted to pursuing the integrated performance of HEDMs, for example, high density and energy, good thermal stability, low sensitivity, positive oxygen balance, environmentally bengin decomposition products, and low cost[4-7]. However, in most cases, the requirements of insensitivity and high detonation velocity and pressure are often contradictory to each other, which makes the design and synthesis of new generation HEDMs an interesting and enormous challenge.
Five-membered nitrogen-containing azole compounds are traditional sources of energetic materials due to their high nitrogen content and the thermal stability which results from the aromatic ring system. Owing to the high positive heats of formation resulting from the large number of N—N and C—N bonds and the high level of environmental compatibility, triazole and tetrazole compounds have been studied over the last couple of years with growing interest[8-9]. Furthermore, a further way of azole functionalization is the introduction of the heterocycles containing oxygen (e.g. oxadiazoles and nitro-substituted heterocyles). This type is strikingly represented by 3,4-bis(1H-5-tetrazolyl)furoxan, which was firstly reported by Huang et al[10]. It has a high decomposition temperature and high-nitrogen compound with better oxygen balance than those containing only C, H, and N, which suggest that it would be a potential candidate for green energetic materials.
In this paper, 3, 4-bis(1H-5-tetrazolyl)furoxan was synthesized by a new method from 3,4-dicyanofuroxan with a higher yield (91%), and its structure was characterized by IR, NMR and elemental analysis. In order to confirm the molecular structure of the title compound, the crystal structure was characterized by X-ray diffraction analysis.
2 Experimental
2.1 Materials and Instruments
1H NMR and13C NMR were obtained in DMSO-d6with a Bruker AV500 NMR spectrometer. Infrared spectra were obtained from KBr pellets with a Nicolet NEXUS870 Infrared spectrometer in the range of 4000-400 cm-1. Elemental analyses were performed with a VARI-El-3 elementary analysis instrument. Differential scanning calorimetry (DSC) experiments were carried out in a platinum sample container using a Q-2000 at a rate of 10 ℃·min-1.
3, 4-Dicyanofuroxan was prepared according to the literature procedure[13]. Other chemicals were obtained from commercial sources and used without further purification.
2.2 Synthesis and Characterization
The title compound was synthesized from Scheme 1.
To a solution of 3,4-dicyanofuroxan (1.36 g, 10 mmol) and NaN3(1.95 g, 30 mmol) in water (30 mL) ZnCl2(1.36 g, 10 mmol) was added at room temperature. The reaction mixture was heated to refluxing temperature and stirred for 5 h, cooled to room temperature, and acidified with an appropriate concentrated hydrochloric to pH 1-2. Then, the precipitated product was filtered, washed with water, dried under vacuum and finally to yield a white solid (2.02 g) with a purity of 98.6% (HPLC). DSC (10 ℃·min-1): 229-230 ℃.1H NMR (DMSO-d6, 500 MHz),δ: 8.28 (s, 2H, NH);13C NMR (CDCl3, 125 MHz),δ: 149.01, 146.18(C—CN), 146.05, 106.76; IR(KBr,ν/cm-1): 3139, 2990, 2905, 2752, 2667, 2514, 1622, 1581, 1458, 1419, 1387, 1281, 1234, 1204, 1182, 1130, 1094, 1071, 1026, 1015, 1004, 960, 935, 824, 782, 736, 704, 544; Anal.calcd For C4H2N10O2: C 21.63, H 0.91, N 63.06; Found C 21.56, H 1.08, N 62.79.
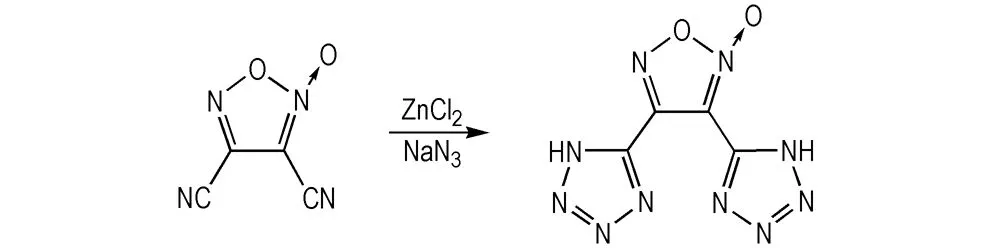
Scheme 1 Synthetic route of the title compound
2.3 Determination of the Single Crystal Structure
The title compound was dispersed into hot water (60 ℃) with a stirrer, and the mechanical impurities were eliminated by filtration. The saturated solution of the title compound was put in a conical flask for two weeks at room temperature to evaporate slowly the solution and obtain single crystals of title compound.
A colorless single crystal of the title compound with dimensions of 0.35 mm×0.30 mm×0.23 mm was selected for X-ray diffraction analysis, and the data collection was performed on a Bruker SMART Apex-II CCD X-ray diffractometer equipped with a graphite-monochromatized MoKαradiation (λ=0.71073 Å) using theφ-ωscan mode (2.55°≤θ≤25.09°) at 296(2) K. A total of 4143 reflections were collected, of which 1498 were independent (Rint=0.0431) and 1378 withI>2σ(I) were considered to observed and used for the refinement.
The structure was solved by direct methods and refined by full-matrix least squares techniques onF2using SHELXS-97 and SHELXL-97 programs[11-12]. All non-hydrogen atoms were refined anisotropically. Crystal data and structural refinement parameters for the title compound are listed in Table 1.
Further information on the crystal-structure determinations can be obtained free of charge atwww.ccdc.ac.uk/conts/retrieving.html or from the Cambridge Crystallograpnic Data Centre with CCDC No.1402690.
3 Results and Discussion
3.1 Synthesis
3, 4-Bis(1H-5-tetrazolyl)furoxan was synthesized from 3,4-dicyanofuroxan, which was in turn prepared according to a literature procedure[13]. 3,4-Dicyanofuroxan was treated with sodium azide and ZnCl2in water according to the method reported by Demko and Sharpless[14]. 3, 4-Bis(1H-5-tetrazolyl)furoxan was successfully obtained in high yield (91.0%), which is catalyzed by Zn2+in water. However, 3, 4-bis(1H-5-tetrazolyl)furoxan was catalyzed by NH4Cl inN,N-dimethylformamide (DMF) in the literature, and the reaction mixture has to be diluted with water, extracted with ethyl acetate, washed with brine, and evaporated process[10]. Those procedures are not good for environment protection and reducing the cost. Furthermore, the yield of the target compound was increased significantly compared to the literature′s method.
Table 1 Crystal data and structure refinement details

FormulaC4H2N10O2Formulaweight/g·mol-1222.16T/K296(2)crystalsystemOrthorhombicspacegroupP212121a/Å6.1172(14)b/Å9.657(2)c/Å14.220(3)α/(°)90.0β/(°)90.0γ/(°)90.0crystalsize/mm0.35×0.30×0.23volume/Å3840.0(3)Z4Dc/g·cm-31.76F(000)448λ/Å0.710732θmax/(°)50.18Indexranges-6≤h≤7-11≤k≤9-16≤l≤16reflectionscollected/unique4134/1498parameters145GOFonF21.031finalRindexes(I>2σ(I))R1=0.0800,wR2=0.2523finalRindexes(alldata)R1=0.0884,wR2=0.2626largestdiffpeakandhole/e·Å-30.477and-0.431
The infrared vibrational spectra of 3, 4-bis(1H-5-tetrazolyl)furoxan show the presence of the N—H (3139 cm-1group. Other vibrations also suggest the presence of the tetrazole ring and furoxan rings. In the1H NMR spectra 3,4-bis(1H-5- tetrazolyl)furoxan shows resonance at 7.63, which is in the expected N—H range. The13C NMR spectrum of 3,4-bis(1H-5-tetrazolyl)furoxan shows two signals with chemical shifts of 112.6 and 123.1, which correspond to the tetrazole ring carbon atoms and the exocylic carbon atoms of the nitrile groups. However, since the chemical shifts of both are very similar, an assignment has not been made.
3.2 Crystal Structure
Crystals of 3, 4-bis(1H-5-tetrazolyl)furoxan suitable for X-ray analysis were obtained from water. Its structure is shown in Fig. 1 and the selected bond lengths, bond angles and dihedral angles are summarized in Tables 2 and 3. Single-crystal X-ray diffraction analyses reveal that 3,4-bis(1H-5-tetrazolyl)furoxan is orthorhombic, space groupP212121with a calculated density of 1.76 g·cm-3. It was found that the whole molecular structure tends to be coplanar. Both tetrazole rings are slightly twisted relative to the central furoxan ring, with dihedral angles between them of 9.52° and 11.59°, respectively. The oxygen atom outside of the furoxan ring is approximately coplanarwiththering,andthedihedralangleis178.3° (O(1)—N(5)—C(2)—C(3)). The N—O bond length (O(1)—N(5): 1.214 Å) outside of the furoxan ring is much shorter than the N—O distances in the furoxan ring (O(2)—N(5): 1.462 Å, O(2)—N(6): 1.371 Å). Meanwhile, the O(2)—N(5) bond length is the largest, such that cleavage of this bond may trigger decomposition of compound 3. The N—N bond lengths in the tetrazole ring of the title compound vary from 1.305(42) to 1.371(78) Å and lie between those of N—N single bonds (1.454 Å) and NN double bonds (1.245 Å), which is good agreement with the corresponding values in related tetrazole derivatives[15-17].
The final three dimensional networks (as shown in Fig.2) are formed by extensive weak intermolecular contacts. One molecule was connected with four adjacent molecules through four contacts of N…N (2.75-2.92 Å). These intermolecular short contacts between the molecules not only stabilize the structure, but also lead to a sufficiently dense structure (Dc=1.76 g·cm-3).
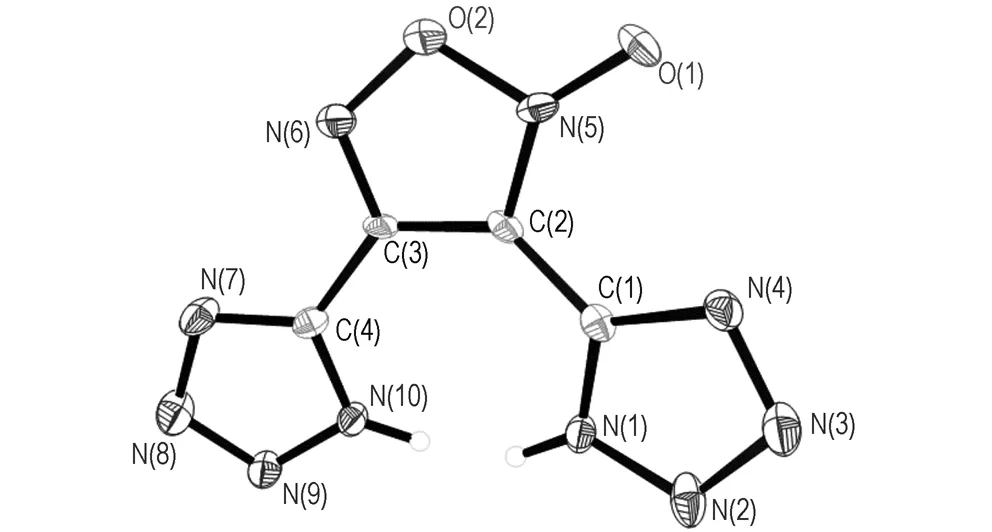
Fig.1 Molecular structure of the title compound
Table 2 Selected bond lengths and bond angles of the title compound
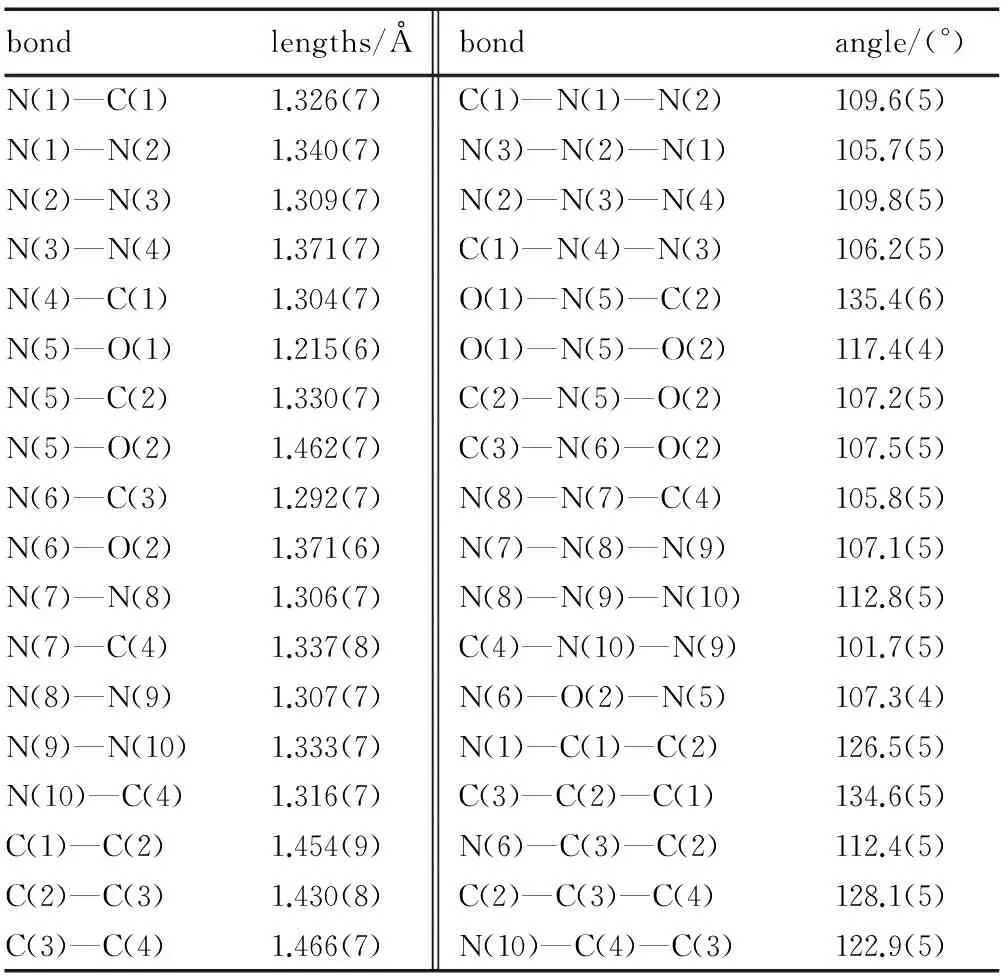
bondlengths/Åbondangle/(°)N(1)—C(1)1.326(7)C(1)—N(1)—N(2)109.6(5)N(1)—N(2)1.340(7)N(3)—N(2)—N(1)105.7(5)N(2)—N(3)1.309(7)N(2)—N(3)—N(4)109.8(5)N(3)—N(4)1.371(7)C(1)—N(4)—N(3)106.2(5)N(4)—C(1)1.304(7)O(1)—N(5)—C(2)135.4(6)N(5)—O(1)1.215(6)O(1)—N(5)—O(2)117.4(4)N(5)—C(2)1.330(7)C(2)—N(5)—O(2)107.2(5)N(5)—O(2)1.462(7)C(3)—N(6)—O(2)107.5(5)N(6)—C(3)1.292(7)N(8)—N(7)—C(4)105.8(5)N(6)—O(2)1.371(6)N(7)—N(8)—N(9)107.1(5)N(7)—N(8)1.306(7)N(8)—N(9)—N(10)112.8(5)N(7)—C(4)1.337(8)C(4)—N(10)—N(9)101.7(5)N(8)—N(9)1.307(7)N(6)—O(2)—N(5)107.3(4)N(9)—N(10)1.333(7)N(1)—C(1)—C(2)126.5(5)N(10)—C(4)1.316(7)C(3)—C(2)—C(1)134.6(5)C(1)—C(2)1.454(9)N(6)—C(3)—C(2)112.4(5)C(2)—C(3)1.430(8)C(2)—C(3)—C(4)128.1(5)C(3)—C(4)1.466(7)N(10)—C(4)—C(3)122.9(5)
Table 3 Selected torsion angles of the title compound

bondangle/(°)C(1)—N(1)—N(2)—N(3) 0.1(6)N(3)—N(4)—C(1)—C(2) -179.5(5)N(1)—N(2)—N(3)—N(4) 0.2(7)N(2)—N(1)—C(1)—N(4) -0.4(6)N(2)—N(3)—N(4)—C(1) -0.4(7)N(2)—N(1)—C(1)—C(2) 179.6(5)C(4)—N(7)—N(8)—N(9) -0.9(7)O(1)—N(5)—C(2)—C(3) 178.3(6)N(7)—N(8)—N(9)—N(10) 0.5(7)O(2)—N(5)—C(2)—C(3) -0.1(5)N(8)—N(9)—N(10)—C(4) -1.2(6)N(1)—C(1)—C(2)—N(5) -172.1(5)C(3)—N(6)—O(2)—N(5) -177.9(5)N(1)—C(1)—C(2)—C(3) 12.2(10)C(2)—N(5)—O(2)—N(6) 0.8(6)O(2)—N(6)—C(3)—C(2) 1.2(6)N(3)—N(4)—C(1)—N(1) 0.5(6)O(2)—N(6)—C(3)—C(4) 180.0(5)

Fig.2 Molecular packing diagram of the unit cell of the title compound
3.3 Electrostatic Potential (ESP)
To obtain a better understanding of structure for target compound, electrostatic potential (ESP) calculations was carried out based upon the B3LYP/6-31+g(d, p) method with optimized structure. Fig.3 shows the ESP for the 0.001 electron/bohr3isosurface of the electron density evaluated at the B3LYP level of theory. It has recently been found, and is extensively used, that the computed ESP is generally related to the impact sensitivity of the bulk energetic materials[18-20]. In contrast to non-energetic organic molecules for which the positive potential is larger but weaker in strength, in energetic compounds usually more extensive regions with larger and stronger positive potentials are observed which can be related to increased impact sensitivities. In Fig. 3, it can be clearly seen that the positive ESP region of BBF is larger and also a higher charge separation. This is in good accord with the experimental result of relatively high impact sensitivity of BBF[8].
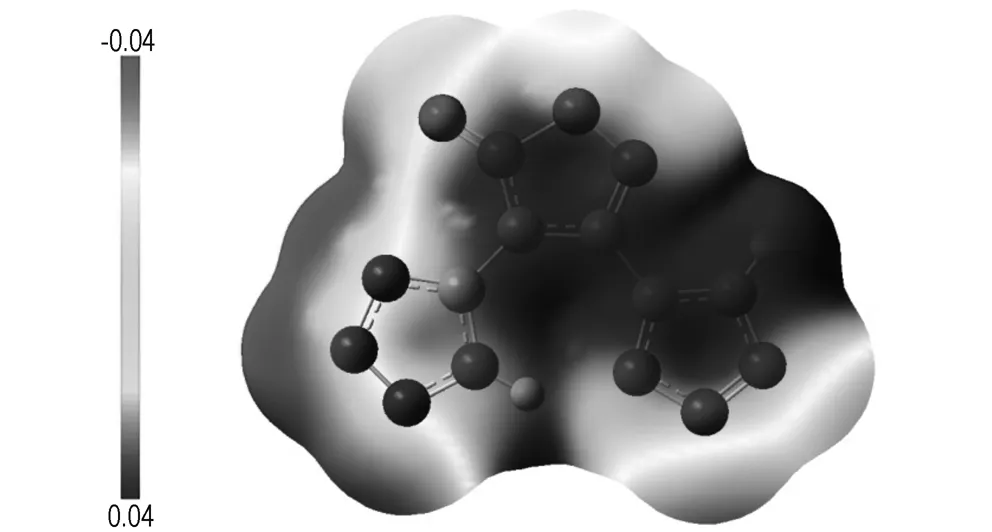
Fig.3 Calculated [B3LYP/6-31+G(d,p)] electrostatic potential of the title compound. The 3D isosurface of electron density is shown between -0.04 hartree (electron-rich regions) and +0.04hartree (electron-poor regions)
4 Conclusions
3,4-Bis(1H-5-tetrazolyl)furoxan was prepared using 3,4-dicyanofuroxan and sodium azide, and the reaction is catalyzed by Zn2+. This method has many advantages, such as high yield, simple post-processing steps. The crustal structure was determined using single crystal X-ray diffraction. The title compound is almost planar with dihedral angles of 9.52° and 11.59°, respectively. There are extensive weak intermolecular contacts leading to a high density and good thermal stability. These properties make it suitable as a promising green energetic material for solid propellant.
[1] Badgujar D M, Talawar M B, Asthana S N, et al. Advances in science and technology of modern energetic materials: an overview[J].JournalofHazardousMaterials, 2008, 151(2): 289-305.
[2] Dippold A A and Klapötke T M. A study of dinitro-bis-1,2,4-triazole-1,1′-diol and derivatives: design of high-performance insensitive energetic materials by the introduction of N—Oxides[J].JournaloftheAmericanChemicalSociety, 2013, 135(26): 9931-9938.
[3] Kim T K,Choe J H, Lee B W, et al. Synthesis and characterization of BNFF analogues[J].BulletinoftheKoreanChemicalSociety, 2012, 33(8): 2765-2768.
[4] Zhang Min, Bi Fu-qiang, Xu Cheng, et al. Synthesis of 2-dinitromethyl-5-alkoxytetrazole[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2015, 23(4): 401-404.
[5] Li Hui, Yu Qian-qian, Wang Bo-zhou, et al. Synthesis and thermal properties of 3,3′-bis(tetrazol-5-yl)-4,4′-azofruazan and its
energetic salts[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2015, 23(4): 401-404.
[6] Zhang Qing-hua,Shreeve J M. Ionic liquid propellants: future fuels for space propulsion[J].Chemistry-AEuropeanJournal, 2013, 19(46): 15446-15451.
[7] Fu Zhan-da, He Cheng, Chen Fu-xue. Synthesis and characteristics of a novel, high-nitrogen, heat-resistant, insensitive material (NOG2Tz)[J].JournalofMaterialsChemistry, 2012, 22(1): 60-63.
[8] Gao Hai-xiang, Shreeve J M. Azole-based energetic salts[J].ChemicalReviews, 2011, 111(11): 7377-7436.
[9] Li Sheng-hua, Shi Hong-gang, Sun Cheng-hui, et al. Synthesis and crystal structure of nitrogen-rich compound: 2,5,2′-triazi- do-1,1′-azo-1,3,4-triazole[J].JournalChemistryCrystallography, 2009, 39: 13-16.
[10] Huang Hai-feng, Zhou Zhi-ming, Liang Li-xue, et al. Nitrogen-Rich Energetic Monoanionic Salts of 3, 4-bis(1H-5- tetrazolyl)furoxan[J].Chemistry-AnAsianJournal. 2012, 7(4): 707-714.
[11] Sheldrick G M. SHELXS-97, Program for crystal structure solution[CP], University of Göttingen, Germany 1997.
[12] Sheldrick G M. SHELXL-97, Program for crystal structure refinement [CP], University of Göttingen, Germany 1997.
[13] Parker C O, Emmons W D, Rolewicz H A, et al. Chemistry of dinitroacetonitrile—I reraration and properties of dinitroacetonitrile and its salts[J].Tetrahedron, 1962, 17(1): 79-87.
[14] Demko Z P, Sharpless K B. Preparation of 5-substituted 1H-tetrazoles from nitriles in water[J].JournalofOrganicChemistry, 2001, 66(24): 7945-7950.
[15] Liang Li-xuan, Wang Kai, Bian Cheng-ming, et al. 4-Nitro-3-(5-tetrazole)furoxan and its salts: synthesis, characterization, and energetic properties[J].Chemistry-AEuropeanJournal, 2013, 19(44): 14902-14910.
[16] Dachs M, Dippold A A, Gaar J, et al. A comparative study on insensitive energetic derivatives of 5-(1,2,4-triazol-C-yl)- tetrazoles and their 1-hydroxy-tetrazole analogues[J].ZeitschriftfürAnorganischeundAllgemeineChemie, 2013, 639(12): 2171-2180.
[17] Klapötke T M,Sabate′ C M. New energetic compounds based on the nitrogen-rich 5,5′-azotetrazolate anion ([C2N10]2-)[J].NewJournalofChemistry, 2009, 33(7): 1605-1617.
[18] Fischer D, Klapötke T M,Stierstorfer J. Synthesis and characterization of diaminobisfuroxane[J].EuropeanJournalofInorganicChemistry, 2014, 34: 5808-5811.
[19] Zhang Jia-heng, Shreeve J M. 3,3′-Dinitroamino-4,4′-azoxyfurazan and its derivatives: an assembly of diverse N-O building blocks for high-performance energetic materials[J].JournaloftheAmericanChemicalSociety, 2014, 136(11): 4437-4445.
[20] Klapötke T M, Nordheider A,Stierstorfer J. Synthesis and reactivity of an unexpected highly sensitive 1-carboxymethyl-3-diazonio-5-nitrimino-1,2,4-triazole[J].NewJournalofChemistry, 2012, 36(7): 1463-1468.
杂志排行
含能材料的其它文章
- Non-isothermal Decomposition Kinetics,Specific Heat Capacity and Adiabatic Time-to-explosion of Cu(pn)2(FOX-7)2
- 《含能材料》2015年(第23卷)总目次
- Molecular Dynamics Simulations of Crystalline δ-HMX with Void Defect
- Synthesis and Characterization of Two New Energetic Polyamino and Nitro Pyridine Derivatives
- Synthesis and Properties of N,N-Bis((3,5-dinitro-1H-1,2,4-triazol-1-yl)methyl) nitramine
- Comparison with Molecular Surface Electrostatic Potential and Thermal Reactivity of Nitramines
