创伤性失血性休克浅低温动物模型的建立
2015-05-08彭罗根赵会民张剑锋
彭罗根,赵会民,张剑锋
创伤性失血性休克浅低温动物模型的建立
彭罗根,赵会民,张剑锋
目的 建立一种简单稳定的创伤性失血性休克浅低温动物模型。 方法 新西兰兔20只随机分为模型组和对照组,每组10只。模型组以颈动脉放血并股骨骨折法建立创伤性失血性休克(traumatic hemorrhagic shock,T/HS)模型,对照组实施假手术。两组均经历麻醉,辅以冷液体及外加冰块等诱导低温过程,分别检测休克前(t0)、休克30 min后(t1)、低温2 h(t2)及复温2 h(t3)的血常规,记录体温(temperature,T)、心率(heart rate,HR)、休克指数(shock index,SI)、平均动脉压(mean arterial pressure,MAP)、血红蛋白(hemoglobin,HGB)及呼吸(respiration,R)等相关生理指标,观察两组成活率。结果 两组动物创伤早期(4 h)的存活率100%。与对照组相比,模型组尿量明显减少(t=3.3,P<0.05)。在t1时间点,模型组MAP(t=22.0,P<0.01)、HGB(t=4.0,P<0.01)水平明显低于对照组;而R(t=2.9,P<0.01)、HR(t=2.1,P<0.05)及SI(t=4.0,P<0.01)水平则显著高于对照组。在t2时间点,模型组MAP(t=4.5,P<0.01)、HGB(t=6.4,P<0.01)仍旧显著低于对照组,差异有统计学意义。在t3时间点,模型组HGB(t=9.8,P<0.01)显著低于对照组,差异有统计学意义。t2时间点与t1时间点相比:模型组MAP(t=20.0,P<0.01)显著升高,对照组HGB(t=19.0,P<0.01)显著升高,差异有统计学意义。对照组MAP、HR、R、SI水平在各时间点均无明显变化(P>0.05)。结论 成功建立兔创伤性失血性休克浅低温模型,为创伤性失血性休克早期浅低温研究提供了动物模型。
创伤性失血性休克;浅低温;动物模型
随着社会进步,人类面临的灾害已从自然灾害(如地震、洪水、泥石流等)向人为灾难(如交通事故、恐怖袭击等)发展。复杂外伤或合并创伤性失血性休克是事发现场早期最常见的死亡原因。而低体温、酸中毒及凝血功能障碍被认为是严重创伤致死的重要危险因素,常规救治主张对T/HS患者进行保暖复温,然而低温复苏在卒中患者器官损伤抑制的益处显而易见,近年来国内外学者试图探讨低温对创伤性休克患者早期复苏的利弊[1-3]。因此,笔者试图建立一种简单的T/HS浅低温模型,为T/HS浅低温研究提供实验动物支持。
1 材料与方法
1.1 材料 健康成年新西兰兔20只(动物许可证号:scxk桂2009-0002),体重(2.5±0.4)kg,SPF级,雌雄不限,按随机数字表法分为模型组(10例)和对照组(10例)。
1.2 创伤性休克动物模型制备及低温复温方法 实验前适应性喂养1周,术前禁食12 h,禁水4 h。实验组操作方法参照Capnoe和Park等[2,4]的研究,略有改进,具体是:(1)建立颈外静脉通路作为麻醉用药及补液通路;以20%乌拉坦(5 ml/kg)静脉麻醉,切开右大腿皮肤,分离肌群,暴露并钳断股骨(以无菌纱块保护创面且便于称重记录失血量);分离左侧颈动脉并置入以枸橼酸钠抗凝的动脉留置管,外接三通阀以供放血并记录失血量及记录心率、血压,在15 min内将MAP降至45 mmHg(1 mmHg=0.133 kPa),放血速率2 ml/(kg·min),本组平均失血量为(38±5.0)ml;低血压稳定持续30 min视为创伤性休克模型成功。(2)以20℃乳酸林格氏液和羟乙基淀粉40氯化钠注射液(体积比2∶1)的液体补液维持MAP在目标血压45 mmHg。(3)辅以乙醇冰水擦浴降温,中心体温(膀胱)(34±0.5)℃维持2 h,以电热毯、红外线照射缓慢复温2 h。对照组仅进行假手术(皮肤切开,不放血不输液,同样经历低温复温过程)。
1.3 采集与指标测定 记录动物存活率及总失血量(注射器采血和纱块称重法并用,为颈动脉失血量和骨折创伤失血量之和)、尿量和补液量;分别于术前(t0)、术后30 min(t1)、降温2 h(t2)及复温2 h(t3)采集血样本,优利特全自动血细胞分析仪(3010型)检测血常规;泰盟(BL-420S)生物机能试验系统实时监测MAP、收缩压(systolic blood pressure,SBP)、R、HR等相关数据;计算SI(脉搏与收缩压的比值);自动电子体温计每30 s记录膀胱温度(T)。实验完毕常规处死尸检。

2 结 果
2.1 模型组与对照组MAP、HGB、SI、HR、R在不同时间点的比较 实验前模型组与对照组比较,MAP、HGB、HR、R及SI差异均无统计学意义(P>0.05)。在t1时间点,模型组MAP(t=22.0,P<0.01)、HGB(t=4.0,P<0.01)显著低于对照组,差异有统计学意义;模型组HR(t=2.1,P<0.05)、R(t=2.9,P<0.01)及SI(t=4.0,P<0.01)显著高于对照组,差异有统计学意义。在t2时间点,模型组MAP(t=4.5,P<0.01)、HGB(t=6.4,P<0.01)仍旧显著低于对照组,差异有统计学意义。在t3时间点,模型组HGB(t=9.8,P<0.01)显著低于对照组,差异有统计学意义。t2时间点与t1时间点相比:模型组MAP(t=20.0,P<0.01)显著升高,对照组HGB(t=19.0,P<0.01)显著升高。对照组MAP、HR、R、SI水平在各时间点均无变化(P>0.05)。见表1。
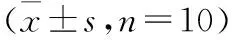
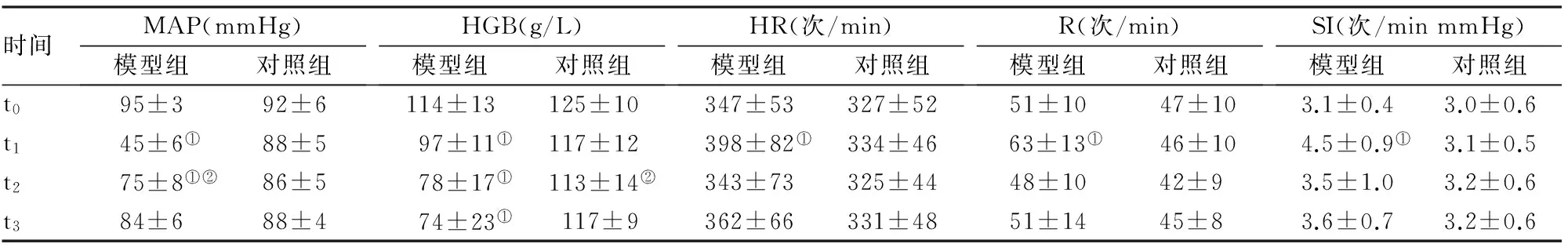
时间MAP(mmHg)模型组对照组HGB(g/L)模型组对照组HR(次/min)模型组对照组R(次/min)模型组对照组SI(次/minmmHg)模型组对照组t095±392±6114±13125±10347±53327±5251±1047±103.1±0.43.0±0.6t145±6①88±597±11①117±12398±82①334±4663±13①46±104.5±0.9①3.1±0.5t275±8①②86±578±17①113±14②343±73325±4448±1042±93.5±1.03.2±0.6t384±688±474±23①117±9 362±66331±4851±1445±83.6±0.73.2±0.6
注:模型组为颈动脉放血并股骨骨折建立创伤性失血性休克模型,对照组为假手术组。①与对照组同时间点比,P<0.05 ;②与同组t1比,P<0.05
2.2 模型组与对照组尿量及补液量比较 记录创伤性休克动物模型制备及低温复温过程两组动物的补液量及尿量得出,模型组与对照组4 h内的补液量分别为(73±10)ml、(18±7)ml,两组比较差异有统计学意义(t=14.0,P<0.01);模型组与对照组4 h内的尿量分别为(12±6)ml、(21±8)ml,两组比较差异有统计学意义(t=3.3,P<0.01)。
3 讨 论
目前,低温疗法已被用于心脏骤停、创伤、出血性休克等,其保护作用也得到广泛认可[4,5]。最近美国匹兹堡大学把低温疗法应用到严重T/HS患者中,为后续抢救性治疗赢得时间,对T/HS低温治疗价值有了新认识。本实验采用开放性长骨骨折及控制性失血,辅以冷液体和物理降温成功复制了T/HS浅低温模型。模型组处于重度休克(SI=4.5),经历浅低温后,休克程度(SI=3.5)明显减轻,且平均动脉压较休克时显著上升,可能与浅低温对T/HS的保护作用有关。本方法构建的模型具有相对稳定性、实用性和合理性,为临床研究T/HS早期(4 h)一线、院前期基础救治研究及低温抗休克等提供了多方面的参考依据,也为T/HS的救护赢得了时间。
近年来,国外研究表明低温可提高失血性休克动物早期存活率,延长救治“黄金时间”,对于失血性休克后全身炎性反应也有一定抑制作用[6]。若温度继续低于34 ℃,死亡率随温度的降低而明显增加[7]。另外,快速诱导低温对心脏骤停、创伤、出血性休克等的保护作用已得到广泛认可[4,5]。传统方法使用冰毯、醇浴等物理降温很难迅速降低至目标体温,反而在降温过程中增加机体耗氧等。另外有学者用“冬眠合剂”加冰毯也能成功快速诱导低温,但不良反应也不可避免,如引起血压下降、抑制体温及呼吸中枢等[8]。本实验以快速输注低温液体(乳酸林格氏液和羟乙基淀粉40氯化钠注射液体积比2∶1)总量少于2倍失血量,降温至目标温度,辅以乙醇擦浴大血管冰块降温维持中心体温(膀胱)目标温度。本实验目标温度为34 ℃,输注液体温度为20 ℃,复温以电热毯、红外线照射缓慢复温,能较好地控制温度。文献[9,10]报道,限制性液体复苏能够降低重度失血性休克早期的死亡率,小剂量补液较快速大量补液更有利于抑制酸中毒进展及高钾血症。
相比于传统的创伤性休克模型常用方法,如①拍击肢体法、②转筒法、③钝器冲击腹部脏器致创伤(肝、脾、肾等)等[11],本实验采用开放性长骨骨折及控制性失血制作T/HS浅低温模型,较好地量化了创伤和休克程度[12-15]。前期预实验MAP按Park等[16]方法降至40 mmHg左右,再予以创伤和低温后,在不输液时动物死亡率非常高(3/4),给予输液后勉强能维持在40 mmHg,但输液量较多(多于失血量的4倍),这很大程度影响了内环境。若MAP在45 mmHg左右,同样的操作后不需干预也能维持平稳血压,又能有典型的休克体征。本实验主要观察创伤性失血性休克早期(4 h)浅低温的病理生理变化及救治,更长时间的研究和后续治疗有待于进一步研究。
[1] Huang C H,Chiang C Y,Pen R H,etal. Hypothermia treatment preserves mitochondrial integrity and viability of cardiomyocytes after ischaemic reperfusion injury[J].Injury,2014-10-31.[Epub ahead of print]
[2] Capone A C, Safar P, Stezoski W,etal. Improved outcome with fluid restriction in treatment of uncontrolled hemorrhagic shock[J]. Am Coll Surg,1995,180(1):49-56.
[3] Murphy L D,Green R S. A case of commotio cordis treated with therapeutic hypothermia[J].J Emerg Med,2014,46(5):149-153.
[4] Park K H, Lee K H, Kim H,etal. Effect of hypothermia on coagulatory function and survival in Sprague-Dawley rats exposed to uncontrolled haemorrhagic shock[J].Injury,2013,44(1):91-96.
[5] Bemard S A, Gray T W, Buist M D,etal. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia[J]. N Engl J Med,2002,346(8):557-563.
[6] Klenter R, Rollwagen F M, Prueckner S,etal. Effects of mild hypothermia on survival and serum cytokines in uncontrolled hemorrhagic shock in rats[J]. Shock,2002,17(6):521-526.
[7] Iwamoto S, Takasu A, Sakamoto T,etal. Therapeutic mild hypothermia: effects on coagulopathy and survival in a rat hemorrhagic shock model[J]. Trauma,2010,68(3):669-675.
[8] 郝艳玲,衷凤刚,杨 龙,等. 冬眠合剂对小鼠缺氧耐受力的影响[J]. 齐齐哈尔医学院学报,2011,32(10):1540-1541.
[9] 杜鹏飞,朱海彬,赵会民.不同补液强度对重度失血性休克早期血清乳酸及钾的影响[J]. 中华急救医学,2013,33(5):462-464.
[10] Subeq Y M, Hsu B G, Lin N T,etal. Hypothermia caused by slow and limited-volume fluid resuscitation decreases organ damage by hemorrhagic shock[J]. Cytokine, 2012,60(1):68-75.
[11] 胡同增,张自云. 实验外科学[M].2版. 北京:人民卫生出版社,2000:287-296.
[12] Shan Y J, Xiao D H, Jian A W,etal. Therapeutic mild hypothermia improves early outcomes in rabbits subjected to traumatic uncontrolled hemorrhagic shock[J].J Surg Res,2013,179(1):145-152.
[13] Soller B, Zou F, Dale Prince M,etal. 2014 Military Supplement: Comparison of Noninvasive pH and Blood Lactate as Predictors of Mortality in a Swine Hemorrhagic Shock with Restricted Volume Resuscitation Model[J]. Shock,2014,42(1):44-51.
[14] Frohlich M, Hildebrand F, Weuster M,etal. Induced hypothermia reduces the hepatic inflammatory response in a swine multiple trauma model[J]. J Trauma Acute Care Surg,2014,76(6):1425-1432.
[15] Stern S, Rice J, Philbin N,etal. Resuscitation with the hemoglobin-based oxygen carrier, HBOC-201,in a swine model of severe uncontrolled hemorrhagic and traumatic brain injury[J]. Shock,2009,31(1):64-79.
[16] Park K H, Lee K H, Kim H,etal. Effect of hypothermia on coagulatory function and survival in Sprague-Dawley rats exposed to uncontrolled haemorrhagic shock[J]. Injury, 2013,44(1):91-96.
(2014-12-02收稿 2014-01-07修回)
(责任编辑 张亚丽)
Establishment of hypothermia animal model of traumatic haemorrhagic shock
PENGLuogen,ZHAOHuimin,andZHANGJianfeng.
DepartmentofEmergencyMedicine,ThefirstAffiliatedHospital,GuangxiMedicalUniversity,GuangxiZhuangAutonomousRegion,Nanning530021,China
ZHAOHuimin,E-maill:hmzhao2006@163.com
Objective To establish animal model of hypothermia of traumatic hemorrhagic shock. Methods 20 rabbits were randomly divided into control group and model group, 10 cases each. Carotid artery bloodletting and femoral fracture were applied to model group to establish traumatic hemorrhagic shock(T/HS) model, while sham operation was used in control group. Both of them experienced anesthesia and low temperature process induced by cold liquid and ice. Then the routine blood, temperature (T), heart rate (HR), shock index (SI), mean arterial pressure (MAP), hemoglobin (HGB), respiration(R)and related physiological indexes were recorded at different time including before shock(t0), 30 minutes after shock(t1), 2 hours after hypothermia(t2) and 2 hours after resuscitation(t3), and survival rates of two groups were also observed. Results Survival rates in both groups were 100%. Compared with control group, urine volume of model group was significantly decreased(t=3.3,P<0.05). 30 minutes after shock, in model group, the level of MAP(t=22.0,P<0.01) and HGB(t=4.0,P<0.01) were significantly lower, while R(t=2.9,P<0.01), HR(t=2.1,P<0.05), and SI(t=4.0,P<0.01) were significantly higher than that of control group. 2 hours after hypothermia, the level of MAP(t=4.5,P<0.01)and HGB(t=6.4,P<0.01) of model group were still lower than those of control group. 2 hours after resuscitation, the level of HGB(t=9.8,P<0.01) of model group were lower than those of control group. The level of MAP(t=20.0,P<0.01) in model group and HGB(t=19.0,P<0.01) in control group 2 hours after hypothermia were increased significantly than that 30 minutes after shock. The level of MAP, HR, R and SI of control group didn’t change significantly in all of the time points. Conclusions The rabbit T/HS model was established by this way successfully, and provide an animal model of hypothermia with T/HS for further researching.
traumatic hemorrhagic shock; hypothermia; animal model
10.13919/j.issn.2095-6274.2015.01.004
广西青年基金项目(NO.桂科青0832040)
彭罗根,硕士,住院医师,E-mail:pengluogen2012@163.com
530021 南宁,广西壮族自治区广西医科大学第一附属医院急诊科
赵会民,E-mail:hmzhao2006@163.com
R361
