大空间位阻的2,9-双噻吩-1,10-菲咯啉-5,6-二酮铜(Ⅱ)配合物
2015-04-01金超王银歌钱惠芬黄伟
金超 王银歌 钱惠芬*,,2 黄伟
(1南京工业大学理学院,南京210009)
(2南京大学化学化工学院,南京微结构国家实验室,配位化学国家重点实验室,南京210093)
大空间位阻的2,9-双噻吩-1,10-菲咯啉-5,6-二酮铜(Ⅱ)配合物
金超1王银歌1钱惠芬*,1,2黄伟*,2
(1南京工业大学理学院,南京210009)
(2南京大学化学化工学院,南京微结构国家实验室,配位化学国家重点实验室,南京210093)
基于1,10-菲咯啉-5,6-二酮2,9位双噻吩的扩展策略,设计合成了2个化合物1和2。有趣的是,在大位阻双齿螯合配体2的配位化学研究中发现,只有铜(Ⅱ)离子生成了稳定的配合物3·H2O。此外,对2,2·CHCl3和3·H2O的X-射线单晶结构研究表明,为了克服空间位阻以满足中心铜(Ⅱ)离子的配位构型要求,1,10-菲咯啉-5,6-二酮及其2,9位取代的2个噻吩环之间的二面角分别从自由配体中的1.9(2)°、5.2(6)°和25.3(3)°、34.9(3)°增加到了铜(Ⅱ)配合物中的5.6(2)°、6.5(6)°和27.2(3)°、38.2(3)°。
铜(Ⅱ))配合物;1,10-菲咯啉-5,6-二酮;噻吩衍生物;偶联反应
0 Introduction
During the past decades,coordination compounds haveattractedmuchattentionbecauseoftheir potentialapplications in magnetism,catalysis,molecular sensing,opticsandmolecularadsorption[1-5].Particularly, coordinationchemistryof1,10-phenanthroline derivatives having extended π system has become a hot topic of research,and many attempts have been made on the introduction of different donor/acceptorunits to different substituted positions of 1,10-phenanthroline ring[6].The resultant 1,10-phenanthroline based compounds and their metal complexes can be finelytunedindifferentaspects,suchassize, symmetry,conformation,dihedralanglebetween adjacent aromatic heterocycles,solubility,reaction activity and optoelectronic properties[7-11].
Inthiophenesubstituted1,10-phenanthroline compounds,the thiophene ring is π electron rich because of the presence of one sulfur atom in the structure,whereasthephenanthrolinepartisπ electron deficient due to the existence of two nitrogen atoms[12-13].The formation of distinguishable delocalized π system for the whole donor-acceptor molecule can be achieved by altering the substituted positions of 1,10-phenanthrolineandthiopheneunits.Our previous research in this area has been focused mainly on the linear 3,8-and planar 5,6-extended 1,10-phenanthrolinebasedheterocyclicaromatic fluorescentcompoundsshowingsemiconducting, photoresponsive and chemosensing properties[14-21].In this work,we have introduced two thiophene rings into 2 and 9 positions of 1,10-phenanthroline-5,6-dione to build a new donor-acceptor molecule(1)and explore the coordination chemistry of this 2 and 9 thiophenesubstituted1,10-phenanthroline-5,6-dione bedentate ligand with large steric hindrance.
1 Experimental
1.1 Materials and instruments
Allmeltingpointsweremeasuredwithout correction.The reagents of analytical grade were purchased directly from commercial sources and used without any further purification.The starting material was prepared according to the previously reported approachshowninSchemeSI1.Column chromatography was carried out on silica gel(300~400 mesh)and analytical thin-layer chromatography (TLC)was performed on glass plates of silica gel GF-254 with detection by UV light.Standard techniques for synthesis were carried out under argon atmosphere. Elemental analyses were measured with a Perkin-Elmer 1400C analyzer.Infrared spectra(4 000~400 cm-1)were collected on a Nicolet FT-IR 170X spectrophotometer at 25℃using KBr plates.Ultraviolet-Visible(UV-Vis)spectrawererecordedona Shimadzu UV-3100 double-beam spectrometer using a quartz glass cell with a path length of 10 mm at room temperature.
1.2 Synthesis of compound 1
Anhydrous K2CO3(1.379 g,9.98 mmol),Pd(PPh3)4(0.150 g,0.13 mmol)and 6,9-dibromo-2,2-dimethyl-1, 3-dioxolo[4,5-f][1,10]-phenanthroline-5,6-dione(1.853 g,4.52 mmol)were dissolved in a mixture of H2O/ DMF(20 mL,1∶1,V/V)and transferred to a roundbottom flask under N2atmosphere.Then 2-thiophenylboric acid(1.276 g,9.97 mmol)was carefully added and the mixture was kept to react for 4 h at 110℃with constant stirring.Thereactionmixturewas quenched with saturated NH4Cl aqueous solution, extracted with CHCl3,washed with brine,and purified by silica gel column chromatography(VCHCl3∶Vn-hexane=1∶1).Yellow crystalline solid 1 was obtained in a yield of 3.239 g(78%)after removal of the solvent and drying in vacuo.m.p.206~207℃.Anal.Calcd.for C61H31Cl3N6O6S6(%):C,58.96;H,2.51;N,6.76. Found:C,58.87;H,2.65;N,6.70.Main FT-IR absorptions(KBr pellets,cm-1):3 410,2 990,2 931, 1 648,1 427,1 368,1 221,1 044,823,697.1H NMR (300 MHz,CDCl3):δ 8.24(s,1H),8.22(s,1H),8.01 (s,1H),7.98(s,1H),7.85(s,1H),7.83(s,1H),7.54 (s,1H),7.52(s,1H),7.23~7.18(m,2H),1.90(s,6H). ESI-MS in methanol(positive mode),m/z:439.42 (100%),[M+Na]+.UV-Vis in CH3OH:λmax=242,257, 310,391 and 412 nm.
1.3 Synthesis of compound 2
Compound 1(1.250 g,3.00 mmol)was dissolved in a mixture of H2O/trifluoroacetic acid(TFA)(15 mL, 1∶2,V/V)solution and heated at 50℃under N2for 5 h.After being cooledtoroomtemperature,the resultant mixture was washed with water and the residue was extracted with CHCl3(5×30 mL).The organic layer was separated,dried by anhydrous sodium sulfate,and evaporated to dryness.The reaction mixture was extracted with CHCl3,washed with brine, and purified by silica gel column chromatography(VCHCl3∶Vn-hexane=1∶2).Red crystalline solid 2 was finally obtained in a yield of 0.784 g(70%)after removal of the solvent and drying in vacuo.m.p.255~256℃. Anal.Calcd.for C20H10N2O2S2:C,64.15;H,2.69;N, 7.48%.Found:C,64.05;H,2.60;N,7.40%.Main FT-IR absorptions(KBr pellets,cm-1):3 444,1 671, 1 578,1 437,1 118,715,537.1H NMR(500 MHz, DMSO-d6):δ 8.41(d,J=10.1 Hz,2H),8.18(d,J=8.3 Hz,2H),8.11(d,J=3.6 Hz,2H),7.93(d,J=4.9 Hz, 2H),7.30(m,2H).ESI-MS in methanol(positive mode),m/z=396.24(100%),[M+Na]+;UV-Vis in CH3OH:λmax=234,259 and 322 nm.The red single crystals of 2 and 2·CHCl3suitable for X-ray diffraction determination were grown from a mixture of CH3CN and CHCl3by slow evaporation in air at room temperature,respectively.
1.4 Synthesis of complex 3·H2O
A mixture of CuSO4·5H2O(0.025 g,0.10 mmol) and 2(0.047 g,0.10 mmol)was dissolved in a mixture of CH3CN/CH3Cl(15 mL,2∶1,V/V).The mixture was refluxed for 2 h and cooled to room temperature,and then the red block single crystals of 3·H2O were collected in a yield of 0.037 g(46%) (based on 2)after slow evaporation in air for five days.Anal.Calcd.for C40H22CuN4O9S5(%):C,51.85; H,2.39,N,6.05.Found:C,51.78;H,2.51,N,5.99. Main FT-IR absorptions(KBr pellets,cm-1):3 404, 1 688,1 572,1 266,1 092,801.UV-Vis in CH3OH: λmax=323 nm.
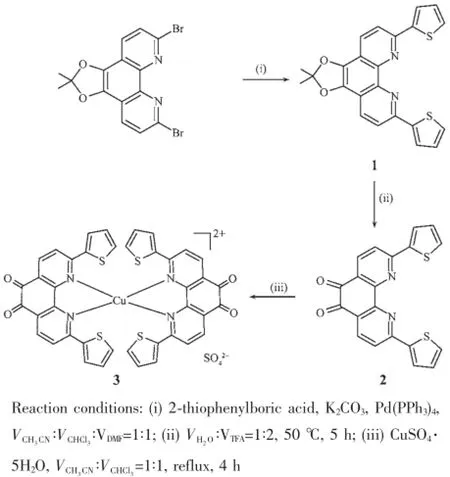
Scheme 1Synthetic route of compound 1~3
1.5 X-ray crystallography
Single-crystal samples of 2,2·CHCl3and 3·H2O were covered with glue and mounted on glass fibers for data collection with Mo Kα radiation(λ=0.071 073 nm)on a Bruker SMART 1K diffractmeter equipped with a CCD camera.Data collection was performed by using SMART program and cell refinement and data reduction were made with the SAINT program[22].The crystal systems were determined by Laue symmetry and the space groups were assigned on the basis of systematic absences using XPREP,and then the structures were solved by direct method and refined by least-squares method on F2by using full-matrix least squares methods with SHELXTL version 6.10[23]. All non-H atoms were anisotropically refined,and all hydrogen atoms except two water protons in 3·H2O were inserted in the calculated positions assigned fixed isotropic thermal parameters at 1.2 times the equivalent isotropic U of the atoms to which they are attached and allowed to ride on their respective parent atoms.Twowaterprotonswerelocatedinthe difference synthesis first and their positions were fixed geometrically and the distances to oxygen atom were set as 0.085 nm.Furthermore,the two protons were assigned fixed isotropic thermal parameters at 1.5 times the equivalent isotropic U of the oxygen atom. Two oxygen atoms(O5 and O7)of sulfate anion in 3· H2O are refined disorderly with the site occupancy factors of 0.706(12)and 0.294(12).In addition,three sulfur atoms(S1,S3 and S4)of four thiophene rings in 3·H2O are refined disorderly with the site occupancy factors of 0.646(5)∶0.354(5),0.311(5)∶0.689(5)and 0.700(5)∶0.300(5).All calculations were carried out by the SHELXTL PC program package and molecular graphics were drawn by using XSHELL and DIAMOND software[24].The summary of the data collection and refinement for 2,2·CHCl3and 3·H2O is given in Table 1.
CCDC:1013452,2;1013453,2·CHCl3;1013454, 3·H2O.
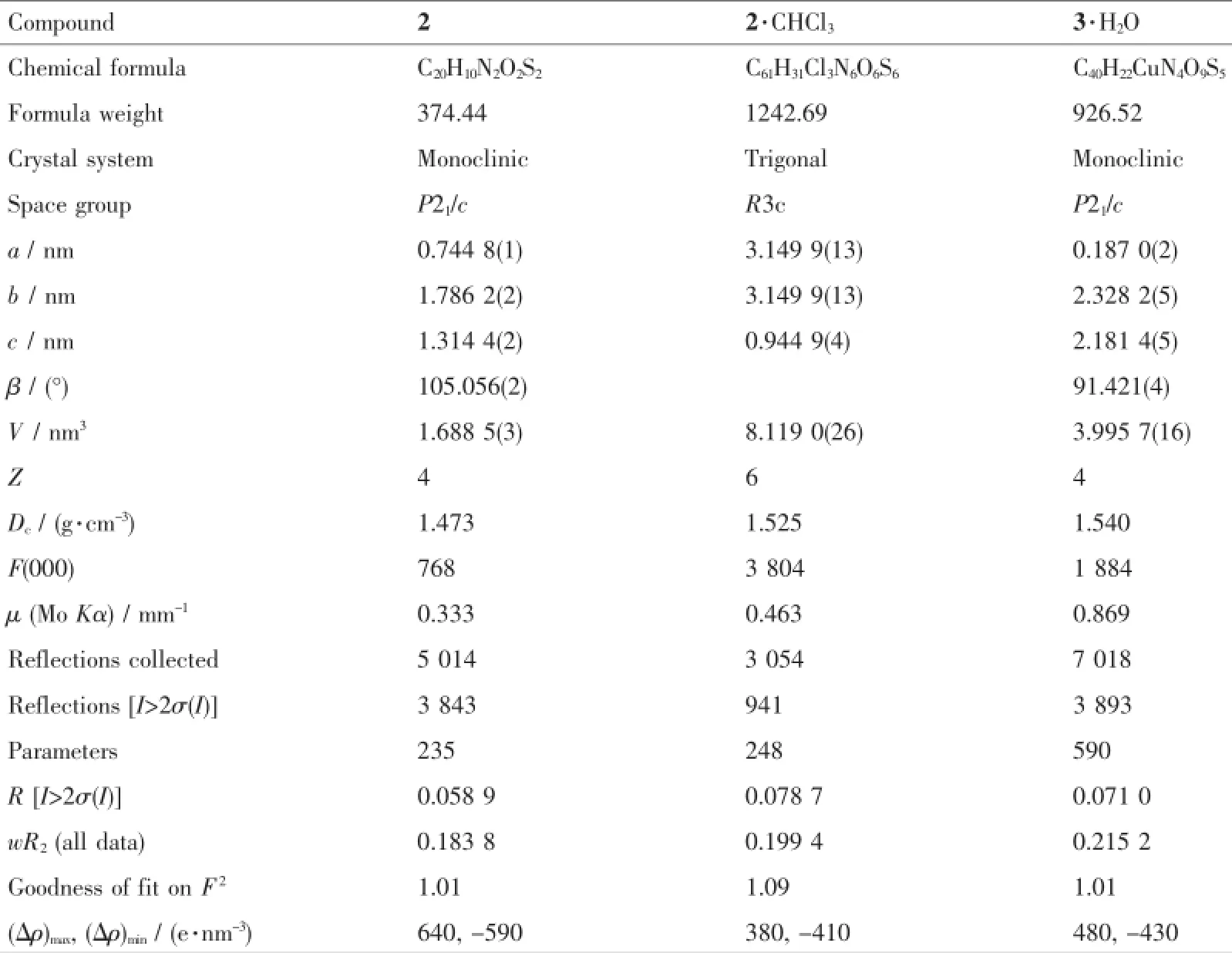
Table 1Crystal data and structure refinement parameters for 2,2·CHCl3and 3·H2O
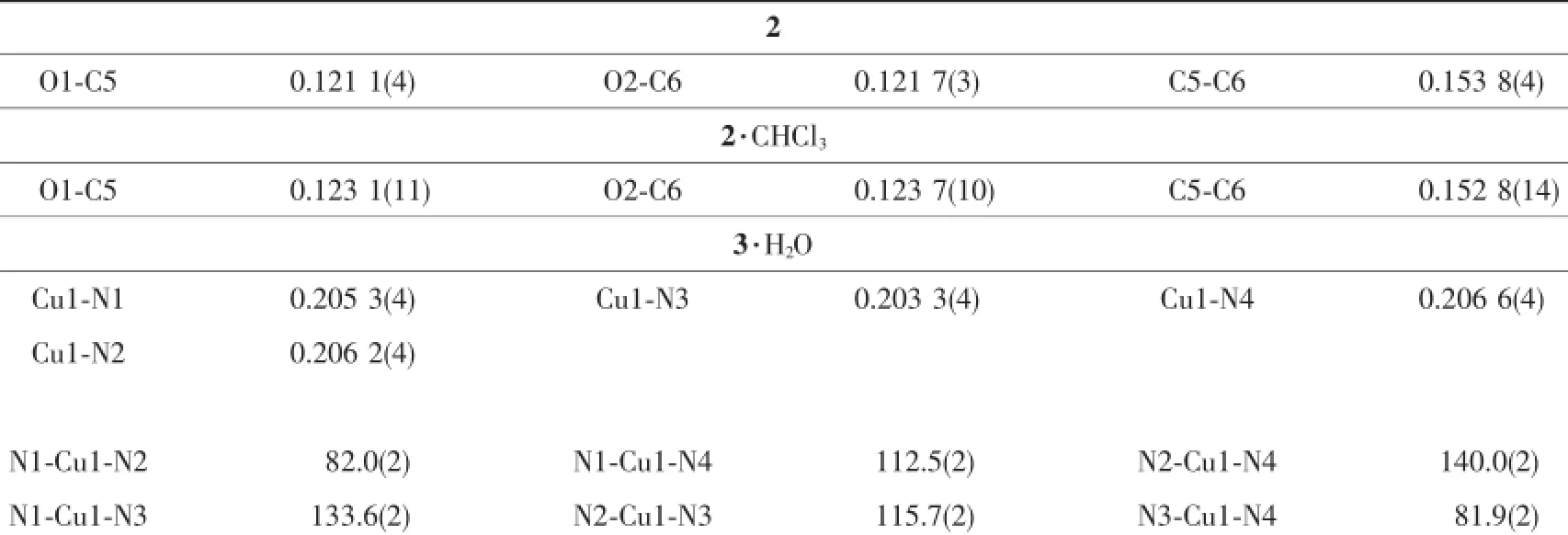
Table 2 Selected bond distances(nm)and angles(°)for 2,2·CHCl3and 3·H2O
2 Results and discussion
2.1 Syntheses and spectral characterizations
Our strategy here is to synthesize 2 and 9 thiophenesubstituted1,10-phenanthroline-5,6-dione derivatives and their possible metal complexes,and relative synthetic strategy is based on the routes shown in Scheme 1,Different synthetic methods,such as Suzuki-Miyaura and Grignard reagents[25-26],have been used to optimize the experimental conditions for obtaining2,9-dithiophenesubstituted1,10-phenanthroline-5,6-dione compound 2,and it is found that Suzuki-Miyaura coupling could give a higher yield. However,the protection of 1,10-phenanthroline-5,6-dione by 2-nitropropane is necessary to guarantee the smoothproceedingofSuzuki-Miyauracoupling between6,9-dibromo-2,2-dimethyl-1,3-dioxolo[4,5-f] [1,10]-phenanthroline-5,6-dione and 2-thiophenylboric acid.
Mononuclear Cu(Ⅱ)complex 3 with a molar rationmetal∶nligandof 1∶2 was synthesized under reflux 4 h in themixtureofacetonitrileandchloroform. Considering that 2 is a bidentate chelating ligand with large steric hindrance,the formation of its metal complexes is difficult since 2 and 9 substituted thiophene rings will block the metal ion complexation. A series of metal ions with different charge and size, such as Fe2+,Mn2+,Co2+,Ni2+,Cu2+,Zn2+,Fe3+,Ag+and Cd2+ions,has been used to react with compound 2, but only the Cu(Ⅱ)ion can overcome the steric hindrance by pushing two side thiophene rings to larger dihedral angles and a mononuclear four-coordinate Cu(Ⅱ)complex 3·H2O can be isolated successfully.
UV-Vis spectra of 1~3 have been recorded and plotted together in Fig.SI1 for comparison.They show characteristic absorptions at 300~450 nm corresponding to the π-π*transitions between adjacent aromatic heterocycles.The difference of λmaxmay arise from the variation of delocalized π system of 1,2 and 3. Compared with 2-nitropropane protected compound 1, 2,9-dithiophene substituted 1,10-phenanthroline-5,6-dione compound 2 and 3 display similar UV-Vis spectra,and the interring π-π*transitions of 2 and 3 have 69 and 68 nm hypochromatic shifts,indicative of worse conjugating system after the dissociation of acetal.
2.2 Crystal structures
2.2.1 Compound 2 and 2·CHCl3
X-ray single-crystal diffraction studies for 2 and its chloroform solvate compound 2·CHCl3reveal that both of the thiophene rings at 2 and 9 positions of 1,10-phenanthroline-5,6-dione centre adopt the same cis conformation,as shown in Fig.1a and Fig.1b, respectively.
Compound 2 crystallizes in the monoclinic space group P2/c,while 2·CHCl3belongs to the trigonal space group R3c.The C-C bond distance between two carbonylgroupsat5and6positionsof1,10-phenanthroline unit in 2 is 0.153 8(4)nm,indicative of character of single bond,which is similar to that in 2·CHCl3(0.152 8(14)nm).The 1,10-phenanthroline-5,6-dione ring is essentially coplanar with adjacent two thiophene rings in both 2 and 2·CHCl3,where the dihedral angles between the central 1,10-phenanthroline-5,6-dione and its neighboring two thiophene rings in 2 are 5.6(2)°and 1.9(2)°,as depicted in Fig. 1a.In contrast,the corresponding dihedral angles in 2·CHCl3are 6.5(6)°and 5.2(6)°,respectively.Moreover,intermolecularπ-π stackinginteractions are found for both structures.In compound 2,the centroid -to-centroidseparationsbetweenadjacent1,10-phenanthroline-5,6-dione and thiophene rings from different molecules are 0.382 9(9)and 0.387 3(9)nm (Fig.2a),while there are four types of π-π stacking interactions in 2·CHCl3with the centroid-to-centroid separations of 0.383 3(3),0.361 8(3),0.309 4(3)and 0.356 6(3)nm in 2(Fig.2b).
2.2.2 Cu(Ⅱ)complex 3·H2O
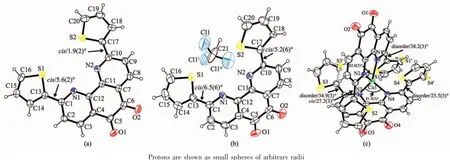
Fig.1ORTEP diagrams(30%thermal probability level ellipsoids)of the molecular structures of three compound 2(a),2·CHCl3(b)and 3·H2O(c)with the atom-numbering scheme
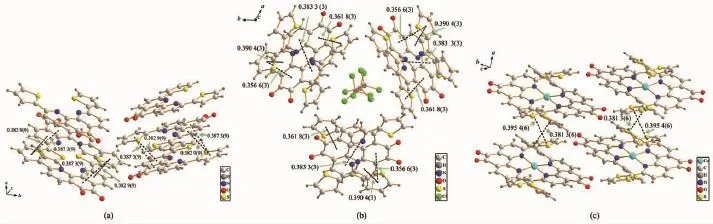
Fig.2Perspective view of the packing structures of 2(a),2·CHCl3(b)and 3·H2O(c)showing-stacking interactions
The structural analysis of 3·H2O indicates that it consists of one Cu(Ⅱ)coordination centre and two bidentate chelating ligands 2,and the coordination geometry around central Cu(Ⅱ)ion can be described as a slightly distorted tetrahedron,as depicted in Fig.1c. Interestingly,it is found that the conformation of each 2,9-dithienyl-1,10-phenanthroline-5,6-dione ligand has changed after Cu(Ⅱ)ion complexation.In comparison with the free ligand 2 and its chloroform solvate compound 2·CHCl3,which have essentially planar molecular structures,the dihedral angles between the central 1,10-phenanthroline-5,6-dione and its neighboring two thiophene rings are significantly increased from 1.9(2)°/5.6(2)°in 2 and 5.2(6)°/6.5(6)°in 2· CHCl3to 25.3(3)°/27.2(3)°and 34.9(3)°/38.2(3)°in 3· H2O because of the geometric requirement of fourcoordinate Cu(Ⅱ)centre.In addition,three of four thiophene rings of two ligands are found to be disordered with different site occupancy factors to further reduce the spacial crowding around the Cu(Ⅱ)centre.The dihedral angle between two chelating 1,10 -phenanthroline-5,6-dione rings in 3·H2O is 55.43(3)°. Furthermore,strong π-π stacking interactions are observed in the crystal packing of 3·H2O between adjacent two thiophene rings from different molecules with the centroid-to-centroid separations of 0.381 3(6) and 0.395 4(6)nm,as can be seen in Fig.2c.
3 Conclusions
In summary,planar 2,9-dithienyl-1,10-phenanthroline-5,6-dione(2)and its entwined concave-shaped mononuclear four-coordinate Cu(Ⅱ)complex 3 have been firstly synthesized and structurally characterized. Because of the large spacial crowding effect of two thiophene rings at 2 and 9 positions of 1,10-phenanthroline-5,6-dione,our experimental results reveal that only its Cu(Ⅱ)coordination complex can be isolated successfully,in comparison with other transition-metal ions such as Fe2+,Mn2+,Co2+,Ni2+,Cu2+,Zn2+,Fe3+,Ag+and Cd2+.Further studies are being undertaken on the aromatic heterocyclic extension of 5,6-dione unit of 2 viaRadziszewskireactionwithcertainthiophene based aldehydes.
[1]Carlin R L,Dejongh L J.Chem.Rev.,1986,86:659-680
[2]Bauerle P,Mullen K.Electronic Materials.Weinheim:Wiley -VCH,1998:105-197
[3]Ma Z,Jacobsen F E,Giedroc D P.Chem.Rev.,2009,109: 4644-4681
[4]Torre G,Vazquez P,Agullo-Lopez F,et al.Chem.Rev.,2004, 104:3723-3750
[5]Campbell C T,Sellers J R V.Chem.Rev.,2013,113:4106-4135
[6]Brandt W W,Dwyer F P,Gyarfas E D.Chem.Rev.,1954, 54:959-1017
[7]Sivula K,Luscombe C K,Thompson B C,et al.J.Am.Chem. Soc.,2006,128:13988-13989
[8]Narutaki M,Takimiya K,Otsubo T,et al.J.Org.Chem., 2006,71:1761-1768
[9]Huang W,Masuda G,Maeda S,et al.Chem.Eur.J.,2006,12: 607-619
[10]Huang W,Masuda G,Maeda S,et al.Inorg.Chem.,2008, 47:468-480
[11]Margapoti E,Shukla V,Valore A,et al.J.Phys.Chem.C.,2009,113:12517-12522
[12]Ammann M,Bauerle P.Org.Biomol.Chem.,2005,3:4143-4152
[13]Vidal P L,Blohorn B D,Bidan G,et al.Chem.Eur.J., 2000,6:1663-1673
[14]Hu B,Fu S J,Xu F,et al.J.Org.Chem.,2011,76:4444-4456
[15]Xu F,Peng Y X,Hu B.CrystEngComm,2012,14:8023-8032
[16]Huang W,Tanaka H,Ogawa T.J.Phys.Chem.C,2008, 112:11513-11526
[17]Wang L,You W,Huang W,et al.Inorg.Chem.,2009,48: 4295-4305
[18]Huang W,Wang L,Tanaka H,et al.Eur.J.Inorg.Chem., 2009:1321-1331
[19]Zhang B,Cao K S,Xu Z A,et al.Eur.J.Inorg.Chem., 2012:3844-3851
[20]Wang X X,Tao T,Geng J,et al.Chem.Asian J.,2014,9: 514-525
[21]Peng Y X,Tao T,Wang X X,et al.Chem.Asian J.,2014,9: 3593-3603
[22]SAINT,Version 6.02.Bruker AXS,Inc.,Madisson,WI, 2001.
[23]Sheldrick G M.SHELXTL,Version 6.10,Bruker Analytical X-ray Systems,Madison,WI,2001.
[24]Brandenburg K.Diamond Version 3.2c,Crystal and Molecular Structure Visualization,Crystal Impact,K.Brandenburg& H.Putz Gbr,Bonn,Germany,2009.
[25]Mellace M G,Fagalde F,Katz N E,et al.Inorg.Chem., 2004,43:1100-1106
[26]Ashby E C.J.Am.Chem.Soc.,1997,119:9085-9086
A Cu(Ⅱ)Complex with 2,9-Dithienyl-1,10-phenanthroline-5,6-dione Ligand with Large Steric Hindrance
JIN Chao1WANG Yin-Ge1QIAN Hui-Fen*,1,2HUANG Wei*,2
(1College of Sciences,Nanjing Tech University,Nanjing 210009,China)
(2State Key Laboratory of Coordination Chemistry,Nanjing National Laboratory of Microstructures, School of Chemistry and Chemical Engineering,Nanjing University,Nanjing 210093,China)
1,10-Phenanthroline-5,6-dione based molecules(1 and 2),extended by two thiophene rings at 2 and 9 positions,have been designed and prepared successfully.It is interesting to mention that the study of coordination chemistry of this large steric ligand 2 with some transition-metal ions reveals that only a Cu(Ⅱ)complex 3·H2O can be yielded.The conformation of ligand 2 undergoes great alteration after Cu(Ⅱ)ion complexation,where the dihedral angles between the central 1,10-phenanthroline-5,6-dione and its neighboring two thiophene rings are significantly increased from 1.9(2)°/5.6(2)°in 2 and 5.2(6)°/6.5(6)°in 2·CHCl3to 25.3(3)°/27.2(3)°and 34.9(3)°/ 38.2(3)°in 3·H2O in order to meet the geometric requirement of four-coordinate Cu(Ⅱ)centre.CCDC:1013452, 2;1013453,2·CHCl3;1013454,3·H2O.
copper(Ⅱ)complex;1,10-phenanthroline-5,6-dione;thiophene derivative;coupling reaction
O614.121
A
1001-4861(2015)02-0413-07
10.11862/CJIC.2015.054
2014-09-07。收修改稿日期:2014-10-07。
国家自然科学基金(No.21171088);“青蓝工程”资助项目。*
。E-mail:qhf@njtech.edu.cn,whuang@nju.edu.cn
