壁虎活性单体衍生物腺嘌呤对人肝癌细胞作用的实验研究
2015-03-29赵荣荣张仕状
韩 明,程 鑫,赵荣荣,张仕状
·论著·
壁虎活性单体衍生物腺嘌呤对人肝癌细胞作用的实验研究
韩 明,程 鑫,赵荣荣,张仕状
目的 探讨壁虎活性单体衍生物腺嘌呤对人肝癌细胞的抑制作用及机制。方法 2014年9月—2015年4月,收集对数生长期的人肝癌Bel-7402细胞进行实验,将96孔板的第2~9列作为腺嘌呤组〔加入腺嘌呤的浓度分别为0.500 00、0.250 00、0.125 00、0.062 50、0.031 25、0.015 63、0.007 81、0.003 91 mg/ml(分别记为A组~H组)〕,第10列作为对照组(加入含10%胎牛血清的RPMI-1640培养液),第11列作为空白组。采用噻唑蓝(MTT)法计算不同浓度腺嘌呤组不同时间(培养24、48、60 h)抑瘤率及腺嘌呤组半抑制浓度(IC50)。将对数生长期的人肝癌Bel-7402细胞分为腺嘌呤组(加入0.500 00 mg/ml的腺嘌呤)和对照组(加入含10%胎牛血清的RPMI-1640培养液),采用透射电镜观察细胞超微结构。将对数生长期的人肝癌Bel-7402细胞分为腺嘌呤组(加入0.500 00 mg/ml的腺嘌呤)和对照组(加入含10%胎牛血清的RPMI-1640培养液),采用流式细胞仪检测细胞周期。结果 24 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,E组~H组抑瘤率均低于D组,E组~G组抑瘤率均高于H组(P<0.05);48 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,D组、E组抑瘤率均高于H组(P<0.05);60 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,D组~F组抑瘤率均高于G组和H组(P<0.05)。C组、E组~H组24 h时抑瘤率均低于48 h时抑瘤率,A组~F组24 h时抑瘤率均低于60 h时抑瘤率,G组24 h时抑瘤率高于60 h时抑瘤率,A组~D组48 h时抑瘤率均低于60 h时抑瘤率,G组、H组48 h时抑瘤率高于60 h时抑瘤率(P<0.05)。不同时间IC50比较,差异有统计学意义(F=16.816,P<0.001);48 h、60 h时IC50低于24 h时,60 h时IC50低于48 h时(P<0.05)。腺嘌呤作用48h后,肝癌细胞出现明显的线粒体改变。对照组G0/G1期、G2/M期人肝癌Bel-7402细胞数高于腺嘌呤组,S期人肝癌Bel-7402细胞数低于腺嘌呤组(P<0.05);腺嘌呤组S期人肝癌Bel-7402细胞数高于G0/G1期,G2/M期人肝癌Bel-7402细胞数低于S期(P<0.05)。结论 腺嘌呤抑制人肝癌细胞的增殖且呈一定的浓度、时间依赖性。腺嘌呤可引起人肝细胞线粒体改变,可将肿瘤细胞阻滞于S期并进一步诱导其凋亡进而发挥抗肿瘤作用。
肝肿瘤;壁虎活性单体;腺嘌呤;细胞凋亡
韩明,程鑫,赵荣荣,等.壁虎活性单体衍生物腺嘌呤对人肝癌细胞作用的实验研究[J].中国全科医学,2015,18(27):3308-3313.[www.chinagp.net]
Han M,Cheng X,Zhao RR,et al.Empirical research of gecko active monomer derivative adenine on human hepatic carcinoma cells[J].Chinese General Practice,2015,18(27):3308-3313.
恶性肿瘤严重危害人类健康,高效低毒的抗肿瘤药物的研发是优化肿瘤治疗的重要课题。目前核苷类抗肿瘤药物种类众多,大部分是将嘌呤、嘧啶等代谢物结构进行改造,通过干扰核酸的代谢,阻止肿瘤细胞的分裂和繁殖,以达到抗肿瘤的作用,如5-氟尿嘧啶、吉西他滨等[1-2]。目前,人体正常核苷成分的抗肿瘤作用国内外鲜见报道,本研究观察壁虎活性单体衍生物腺嘌呤的体外抑瘤作用、肿瘤细胞超微结构及细胞周期改变等,初步探讨腺嘌呤的抗肿瘤机制,为腺嘌呤的开发研究提供科学依据。
1 材料与方法
1.1 材料与仪器 人肝癌Bel-7402细胞购自中国科学院典型培养物保藏委员会细胞库。腺嘌呤(Sigma,美国),RPMI-1640 培养液、胰蛋白酶(Gibco,美国),胎牛血清(Solarbio,中国),噻唑蓝(MTT,Amresco,美国)。CO2培养箱(Thermo,美国),H-7500透射电镜(HITACHI,日本),CKX41倒置显微镜(OLYMPUS,日本),FACSCanto Ⅱ流式细胞仪(美国BD公司)。
1.2 研究方法
1.2.1 细胞复苏与培养 2014年9月—2015年4月,从液氮罐中取出人肝癌Bel-7402细胞,37 ℃水浴中快
本研究背景:
由中药壁虎展开研究,从壁虎中得到抗肿瘤活性单体,经结构鉴定为腺苷。由于腺苷的体内代谢极其迅速,很难制成抗肿瘤药物。根据核苷的结构特点,本课题组对腺嘌呤进行了进一步的研究。腺嘌呤(又称维生素B4)是一种临床应用的升白细胞药物,有望成为一种新的抗肿瘤药物。核苷是DNA和RNA的基本组成单位。目前,临床常用的核苷类抗肿瘤药物如5-氟尿嘧啶、氟达拉滨等,是通过将正常核苷的化学结构进行修饰,掺入DNA或RNA分子中干扰细胞复制,阻止细胞的分裂和繁殖,最终导致肿瘤细胞死亡。正常核苷类抗肿瘤药物具有潜在的研究价值及广阔的市场前景。
速摇动冻存管,直至冻存液完全融化。将细胞悬液移入离心管,加入4 ml含10%胎牛血清的RPMI-1640培养液,以1 000 r/min离心5 min(离心半径10 cm),弃上清液,加入含10%胎牛血清的RPMI-1640培养液重悬细胞,转移至培养瓶中,加适量含10%胎牛血清的RPMI-1640培养液,置于37 ℃、5% CO2培养箱中培养。每2~3 d传代1次,收集对数生长期的细胞进行实验。
1.2.2 计算抑瘤率及半抑制浓度(IC50) 取处于对数生长期的细胞,用胰蛋白酶消化后收集细胞,调整细胞浓度至2.5×104个/ml,接种于96孔培养板,每孔100 μl,37 ℃、5% CO2培养箱中培养24 h。细胞贴壁后,弃去培养液。将96孔板的第2~9列作为腺嘌呤组,第10列作为对照组,第11列作为空白组,第1列和第12列仅加磷酸盐缓冲液(PBS)以维持第2列和第11列的湿度,减少“边缘效应”[3]。腺嘌呤组用含10%胎牛血清的RPMI-1640培养液配制腺嘌呤溶液,并依次倍比稀释,腺嘌呤的浓度分别为0.500 00、0.250 00、0.125 00、0.062 50、0.031 25、0.015 63、0.007 81、0.003 91 mg/ml(分别记为A组~H组),对照组加入含10%胎牛血清的RPMI-1640培养液。分别于37 ℃、5% CO2培养箱中培养24、48、60 h后,采用CKX41倒置显微镜观察细胞形态、数量,加入5 mg/ml的MTT 20 μl,继续孵育4 h,小心吸弃全部上清液,每孔加入150 μl二甲基亚砜(DMSO),轻微震荡至结晶颗粒完全溶解。酶标仪在570 nm处测定各组吸光度值(OD570),设定空白组OD值为零。计算腺嘌呤对肿瘤细胞的抑制率,即抑瘤率〔抑瘤率(%)=(1-腺嘌呤组OD570平均值/对照组OD570平均值)×100%〕和IC50{IC50=lg-1〔Xm-i(ΣP-0.5)〕,其中Xm为设计的最大浓度的对数值,i为各组倍比浓度的对数值,ΣP为各组抑瘤率之和,0.5为经验常数[4]}。实验共重复4次,取均值。
1.2.3 观察人肝癌Bel-7402细胞超微结构 用胰蛋白酶消化1.0×106个对数生长期的人肝癌Bel-7402细胞后,收集细胞,铺于25 cm2培养瓶中,2瓶细胞分别为腺嘌呤组及对照组,腺嘌呤组于37 ℃、5% CO2培养箱中培养24 h后加入浓度为0.500 00 mg/ml的腺嘌呤,对照组加入含10%胎牛血清的RPMI-1640培养液。48 h后用胰蛋白酶消化,收集细胞,2.5%戊二醛固定4 h,1%锇酸4 ℃固定1 h、乙醇梯度脱水、丙酮脱水,包埋处理,超薄切片,经醋酸铀和枸橼酸铅双重染色,将超薄切片置于H-7500透射电镜下观察细胞超微结构并照相。
1.2.4 检测人肝癌Bel-7402细胞周期 采用碘化丙啶(propidiumiodide,PI)单染法。胰蛋白酶消化并收集处于对数生长期的人肝癌Bel-7402细胞,调整每瓶1.3×106个细胞,2瓶细胞分别为腺嘌呤组和对照组,腺嘌呤组培养24 h后加入浓度为0.500 00 mg/ml的腺嘌呤,对照组加入含10%胎牛血清的RPMI-1640培养液,每瓶6 ml。培养24 h后,胰蛋白酶消化,收集细胞于离心管内,2 000 r/min离心5 min(离心半径10 cm),弃上清液;1 ml PBS洗2次,弃上清液,将残余的PBS完全
在美国国防部和商务部列出的关键技术中,有80%是军民重叠的技术。信息网络技术、航空航天技术、新材料技术、新能源技术、核技术等典型的军民两用技术,不仅具有良好的经济技术效益,且能够对相关领域的科技发展起到巨大的带动作用,这就为推进军民融合发展提供十分难得的机遇。两种资源的充分利用,会加快创新与发展,促进核心竞争力的提高。
本研究创新点:
腺嘌呤是一种临床应用的升白细胞药物,关于其抗肿瘤作用鲜见报道。本研究探讨壁虎活性单体衍生物腺嘌呤的体外抗肿瘤作用,发现腺嘌呤可抑制人肝癌细胞的增殖,并在一定程度上呈浓度与时间依赖性;肿瘤细胞出现明显的线粒体改变;G0/G1、G2/M期细胞数减少,S期细胞数增加,表现出S期阻滞。由此可推测腺嘌呤通过诱导其凋亡进而发挥抗肿瘤作用。
吸净;将细胞置于4 ℃ 75%的乙醇(-20 ℃预冷)中固定1 h;2 500 r/min离心5 min(离心半径3 cm),弃固定液,PBS洗3次,加10 μl核糖核酸酶A(RNase A)至终浓度100 μg/ml,37 ℃孵育30 min;加入40 μl终浓度为100 μg/ml的PI,室温避光孵育15 min;过滤后采用FACSCanto Ⅱ流式细胞仪检测细胞周期。实验共重复3次,取均值。
2 结果
2.1 不同浓度腺嘌呤组抑瘤率及IC50比较 加入不同浓度的腺嘌呤后细胞出现不同程度的皱缩,贴壁细胞减少,体积变小,散在生长,细胞呈多形性,细胞质内出现圆形透明颗粒,核分裂象减少,漂浮细胞增多,且随着腺嘌呤浓度的增加、作用时间的延长,细胞形态改变的程度越大。而对照组细胞形态呈上皮细胞样,密集生长,细胞质内未见透明颗粒。不同浓度腺嘌呤组不同时间抑瘤率比较,差异均有统计学意义(P<0.05)。24 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,E组~H组抑瘤率均低于D组,E组~G组抑瘤率均高于H组,差异有统计学意义(P<0.05);48 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,D组、E组抑瘤率均高于H组,差异有统计学意义(P<0.05);60 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,D组~F组抑瘤率均高于G组和H组,差异有统计学意义(P<0.05)。C组、E组~H组24 h时抑瘤率均低于48 h时抑瘤率,A组~F组24 h时抑瘤率均低于60 h时抑瘤率,G组24 h时抑瘤率高于60 h时抑瘤率,A组~D组48 h时抑瘤率均低于60 h时抑瘤率,G组、H组48 h时抑瘤率高于60 h时抑瘤率,差异有统计学意义(P<0.05,见表1)。24 h时IC50为(0.40±0.07)mg/ml,48 h时IC50为(0.34±0.04)mg/ml,60 h时IC50为(0.26±0.03)mg/ml,不同时间IC50比较,差异有统计学意义(F=16.816,P<0.001);48 h、60 h时IC50低于24 h时,差异有统计学意义(q=3.764、8.193,P<0.05);60 h时IC50低于48 h时,差异有统计学意义(q=4.428,P=0.013)。

Table 1 Comparison of tumor inhibition rate at different time points in different levels of adenine group

组别24h48h60hA组4368±4505142±8207818±725hiB组1719±232a2215±325a3294±307ahiC组1116±101ab1491±125abh1822±231abhiD组542±043abc596±046abc776±073abchiE组157±012abcd483±032abch455±033abchF组112±006abcd415±022abch412±028abchG组036±002abcd111±007abch-851±062abcdefhiH组-951±068abcdefg-450±038abcdeh-1089±103abcdefiχ2值305453046030324P值<005<005<005
注:与A组比较,aP<0.05;与B组比较,bP<0.05;与C组比较,cP<0.05;与D组比较,dP<0.05;与E组比较,eP<0.05;与F组比较,fP<0.05;与G组比较,gP<0.05;与24 h比较,hP<0.05;与48 h比较,iP<0.05
2.2 对照组和腺嘌呤组人肝癌Bel-7402细胞超微结构比较 对照组人肝癌Bel-7402细胞结构清晰,细胞器结构完整,核内染色质分布均匀,线粒体较多,细胞表面有微绒毛及伪足突起。腺嘌呤作用48 h后,人肝癌Bel-7402细胞出现细胞核异染色质增多、固缩、凝集于核膜边缘,呈境界分明块状或新月状,细胞核异形变甚至核碎裂,细胞微绒毛减少,细胞质空泡化;线粒体改变最明显,线粒体肿胀,发生空泡变、髓样变;粗面内质网增粗、增宽,核糖体脱颗粒(见图1)。
3 讨论
中药壁虎的临床应用历史久远,壁虎对多种体外培养的肿瘤细胞有明显的抑制作用[5-6]。本课题组选用山东潍坊地区的无蹼壁虎,在体外抑瘤实验示踪下进行抗肿瘤药物筛选,提取得到了鲜无蹼壁虎抗肿瘤活性成分,制备出了鲜无蹼壁虎抗肿瘤活性成分脂质体[7],并获得国家发明专利[8]。进一步研究发现,壁虎活性成分可抑制人肝癌细胞Bel-7402、人肺癌细胞95-C的生长[9],并可以诱导人脐静脉内皮细胞ECV304、人肝癌细胞HepG-2的凋亡[10-11],对小鼠结肠癌细胞CT-26体内、外实验均显示明显的抑制作用[12-13]。在进一步的体外药筛示踪下,对鲜无蹼壁虎抗肿瘤活性成分进行梯级分离纯化,得到抗肿瘤活性较强的单体化合物,经鉴定为腺苷,与李雯等[14]、刘玉军等[15]的研究结果一致。目前腺苷在临床上被用来治疗心律失常[16]。有关腺苷治疗肿瘤的文献报道较多,其抗肿瘤机制与激活A3受体有关[17-18]。腺苷的体内代谢极其迅速[19-20],很难制成抗肿瘤药物。根据真核细胞核苷代谢和化学结构组成的规律,本课题组推断其他核苷及其正常衍生物可能有抗肿瘤作用。

Table 2 Comparison of human liver cancer Bel-7402 cell cycle between control group and adenine group
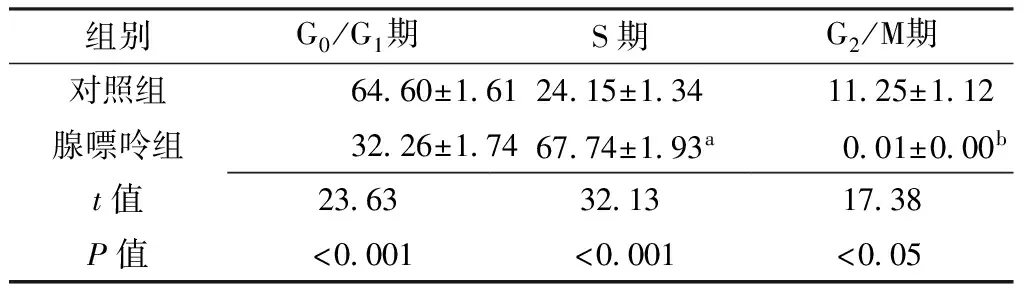
组别G0/G1期S期G2/M期对照组6460±1612415±1341125±112腺嘌呤组3226±1746774±193a001±000bt值236332131738P值<0001<0001<005
注:与G0/G1期比较,aP<0.05;与S期比较,bP<0.05

注:A为对照组(×8 000),B为对照组(×20 000),C为腺嘌呤组(×8 000),D为腺嘌呤组(×20 000)
图1 对照组与腺嘌呤组人肝癌Bel-7402细胞超微结构(醋酸铀和枸橼酸铅双重染色)
Figure 1 The ultrastructure of human liver cancer Bel-7402 cells between control group and adenine group
腺嘌呤(又称维生素B4)是一种临床应用的升白细胞药物,本课题组希望能够将其最终开发成全新机制的一类抗肿瘤药物。本研究结果显示,24 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,E组~H组抑瘤率均低于D组,E组~G组抑瘤率均高于H组;48 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,D组、E组抑瘤率均高于H组;60 h时,B组~H组抑瘤率均低于A组,C组~H组抑瘤率均低于B组,D组~H组抑瘤率均低于C组,D组~F组抑瘤率均高于G组和H组;C组、E组~H组24 h时抑瘤率均低于48 h时抑瘤率,A组~F组24 h时抑瘤率均低于60 h时抑瘤率,A组~D组48 h时抑瘤率均低于60 h时抑瘤率。提示腺嘌呤可显著抑制人肝癌Bel-7402细胞的生长,且具有一定的时效和量效关系。腺嘌呤对Bel-7402细胞作用后48 h、60 h时IC50低于24 h时,60 h时IC50低于48 h时,说明腺嘌呤对人肝癌Bel-7402细胞作用后不同时间点的IC50随时间延长而逐渐降低。
关于肿瘤发生机制的学说很多,经典学说认为,肿瘤是一类细胞周期紊乱和凋亡异常性疾病[21-22]。以调控细胞周期和诱导细胞凋亡为靶点,为抗肿瘤药物的研究提供了新思路。
肿瘤不仅是增殖异常的疾病,同时也是凋亡异常的疾病。形态学观察是检测细胞凋亡最可靠的方法之一。凋亡发生的第一阶段为凋亡的开始,形态学变化表现为细胞表面的特化结构如微绒毛的减少,细胞膜仍完整,未失去选择透过性;线粒体大致完整,核糖体逐渐从内质网上脱离,内质网囊腔膨胀,并逐渐与质膜融合;染色质固缩,形成新月形帽状结构等形态。第二阶段为凋亡小体形成,核染色质断裂,细胞表面产生了许多泡状或芽泡状突起;随后逐渐分离,形成单个的凋亡小体,并逐渐被邻近的细胞所吞噬并消化[23]。本研究结果显示,腺嘌呤作用48 h后,人肝癌Bel-7402细胞出现细胞核异染色质增多、固缩、凝集于核膜边缘,细胞核异形变甚至核碎裂,细胞微绒毛减少,细胞质空泡化;线粒体改变最明显,线粒体肿胀,发生空泡变、髓样变。提示人肝癌Bel-7402细胞发生了凋亡的起始改变。腺嘌呤对肿瘤细胞作用的形态学表现符合细胞凋亡的形态学变化特征。
许多抗肿瘤药物可使细胞周期停滞在G1或G2/M期,大部分为G2/M期阻滞[24-26]。G2/M期转换在细胞周期进展及凋亡启动中起重要作用,G2/M期阻滞的细胞容易发生凋亡,其增殖能力明显降低。本实验采用PI单染法检测腺嘌呤对人肝癌Bel-7402细胞周期分布的影响,结果显示,对照组G0/G1期、G2/M期人肝癌Bel-7402细胞数高于腺嘌呤组,S期人肝癌Bel-7402细胞数低于腺嘌呤组,且腺嘌呤组S期人肝癌Bel-7402细胞数高于G0/G1期,G2/M期人肝癌Bel-7402细胞数低于S期,提示腺嘌呤对肿瘤细胞有S期阻滞作用,导致肿瘤细胞增殖周期停止,随后发生细胞凋亡。
综上所述,壁虎活性单体衍生物腺嘌呤可显著抑制肿瘤细胞的增殖,且呈一定的浓度、时间依赖性。腺嘌呤作用肿瘤细胞48 h后,透射电镜下呈现凋亡细胞的形态改变;可通过将肿瘤细胞阻滞于S期并进一步诱导肿瘤细胞凋亡进而发挥抗肿瘤作用,但其分子机制有待于进一步研究。
[1]He Y,Peng YZ,Ji ZN,et al.Clinical study of oxaliplatin plus raltitrexed versus oxaliplatin plus fluorouracil as first-line treatment for advanced gastric cancer[J].Chinese Journal of Clinical Pharmacology and Therapeutics,2015,20(2):194-198.(in Chinese) 何杨,彭玉珍,吉兆宁,等.雷替曲塞或5-氟尿嘧啶联合奥沙利铂一线治疗晚期胃癌的对比性研究[J].中国临床药理学与治疗学,2015,20(2):194-198.
[2]Wan YY,Hui HX,Wang XW,et al.Clinical observation on chemotherapeutic efficacy and safety of gemcitabine in a fixed dose rate containing regimen in treatment of relapsed or refractory diffuse large B-cell lymphoma patients[J].Chinese Journal of Cancer Prevention and Treatment,2015,22(11):880-884.(in Chinese) 万一元,惠红霞,王晓炜,等.吉西他滨固定剂量率输注联合方案治疗复发难治性弥漫大B细胞淋巴瘤临床观察[J].中华肿瘤防治杂志,2015,22(11):880-884.
[3]Zhang ZY.Antitumor effect of grateloupia filicina on U87 cell and its xenografts in nude mice[J].Journal of Chongqing Medical University,2011,36(9):1051-1053.(in Chinese) 张治业.蜈蚣藻多糖对人神经胶质瘤U87细胞及其裸鼠移植瘤生长的抑制作用[J].重庆医科大学学报,2011,36(9):1051-1053.
[4]Gharavi M,Nobakht M,Khademvatan Sh,et al.The effect of garlic extract on expression of INFγ and inos genes in macrophages infected with leishmania major[J].Iran J Parasitol,2011,6(3):74-81.
[5]Song P,Wang XM,Xie S,et al.Experimental study on mechanisms of lyophilized powder of fresh gekko chinenis in inhibiting H22 hepatocarcinoma angiogenesis[J].Chinese Journal of Integrated Traditional and Western Medicine,2006,26(1):58-62.(in Chinese) 宋萍,王学美,谢爽,等.鲜壁虎冻干粉抑制H22肿瘤血管生成机理的实验研究[J].中国中西医结合杂志,2006,26(1):58-62.
[6]Xie S,Wang XM,Xie DZ,et al.Effects of natural extraction of gecko in inducing apoptosis and antiproliferation of C6 glioma cells[J].Cancer Research on Prevention and Treatment,2003,30(6):458-461.(in Chinese) 谢爽,王学美,谢东泽,等.鲜壁虎提取物抑制C6胶质瘤细胞增殖和诱导凋亡的研究[J].肿瘤防治研究,2003,30(6):458-461.
[7]Kang JG,Zhang SZ,Li ZZ,et al.Preparation of fresh Gekko.swinhonis gunther anti-neoplasm active component alkaloid liposomes and its quality evaluation[J].Chinese Journal of New Drugs,2009,18(15):1453-1459.(in Chinese) 康建功,张仕状,李真真,等.鲜无蹼壁虎抗肿瘤活性成分生物碱脂质体的研制及其质量评价[J].中国新药杂志,2009,18(15):1453-1459.
[8]张仕状,康建功,段全红.一种抗癌新药的制备方法:中国,CN1256101C[P].(2006-05-17)[2015-05-14].http://epub.sipo.gov.cn/patentoutline.action.
[9]Gao LJ,Cheng X,Zhao RR,et al.Experimental study of separating gekko swinhonis active constitutent using MTT method in vitro[J].Journal of Weifang Medical College,2011,33(1):1-3,35.(in Chinese) 高丽娟,程鑫,赵荣荣,等.MTT法指导分离壁虎抗肿瘤活性成分的实验研究[J].潍坊医学院学报,2011,33(1):1-3,35.
[10]Zhao J,Wu T,Gao ZQ,et al.Effects of the fresh GEKKO SWINHONIS anti-neoplasm active component on the proliferation and apoptosis of ECV304 cells[J].Journal of Weifang Medical College,2010,32(5):328-330,insert 1.(in Chinese) 赵静,吴潼,高志芹,等.无蹼壁虎抗肿瘤活性成分对ECV304细胞增殖和凋亡的影响[J].潍坊医学院学报,2010,32(5):328-330,后插1.
[11]Xie B,Gao ZQ,Shi JF,et al.Effects of the Gekko Swinhonis anti-neoplasm active component on the proliferation,migration and apoptosis of HepG2 cells[J].Chinese Pharmacological Bulletin,2012,28(1):101-105.(in Chinese) 谢斌,高志芹,石剑飞,等.无蹼壁虎抗肿瘤活性成分对HepG2细胞增殖、迁移及凋亡的影响[J].中国药理学通报,2012,28(1):101-105.
[12]Li YH,Liu DM,Sheng JW,et al.Extraction of anti-neoplasm active component from Gekko swinhonis and its inhibitory effects on CT-26 murine colon carcinoma[J].Journal of the Fourth Military Medical University,2009,30(12):1103-1106.(in Chinese) 李耀辉,刘冬梅,盛继文,等.无蹼壁虎抗肿瘤成分的提取及其对CT-26小鼠结肠腺癌的抑制作用[J].第四军医大学学报,2009,30(12):1103-1106.
[13]Kang JG,Zhang SZ,Li YH,et al.Experimental study of the effects of fresh Gekko swinhonis anti-neoplasm active component on inhibiting CT-26 tumor growth[J].Chinese Journal of Hospital Pharmacy,2007,27(4):441-444.(in Chinese) 康建功,张仕状,李耀辉,等.鲜无蹼壁虎抗肿瘤活性成分抑制CT-26肿瘤细胞生长实验研究[J].中国医院药学杂志,2007,27(4):441-444.
[14]Li W,Wang GC,Zhang XQ,et al.Chemical constituents of Gekko swinhonis[J].China Journal of Chinese Materia Medica,2010,35(18):2412-2415.(in Chinese) 李雯,王国才,张晓琦,等.中药壁虎化学成分研究[J].中国中药杂志,2010,35(18):2412-2415.
[15]Liu YJ,Yang PM,Gao P,et al.Determination of uridine,adenine and adenosine in gecko by HPLC[J].China Journal of Traditional Chinese Medicine and Pharmacy,2013,28(3):679-681.(in Chinese) 刘玉军,杨培民,高鹏,等.HPLC法测定壁虎药材中3种核苷类成分[J].中华中医药杂志,2013,28(3):679-681.
[16]Intravenous Adenosine Versus Verapamil in Terminating Episodes of Paroxysmal Supraventricular Tachycardia Study Grou.A randomized,multicenter trial to compare the safety and efficacy of adenosine versus verapamil for termination of paroxysmal supraventricular tachycardia[J].Chinese Journal of Internal Medicine,2003,42(11):773-776.(in Chinese) 国产腺苷与维拉帕米终止室上性心动过速试验组.国产腺苷和维拉帕米终止室上性心动过速的临床研究[J].中华内科杂志,2003,42(11):773-776.
[17]Vincenzi F,Targa M,Corciulo C,et al.The anti-tumor effect of A3 adenosine receptors is potentiated by pulsed electromagnetic fields in cultured neural cancer cells[J].PLoS One,2012,7(6):e39317.
[18]Cohen S,Stemmer SM,Zozulya G,et al.CF102 an A3 adenosine receptor agonist mediates anti-tumor and anti-inflammatory effects in the liver[J].J Cell Physiol,2011,226(9):2438-2447.
[19]Scherer EB,Schmitz F,Vuaden FC,et al.Mild hyperhomocysteinemia alters extracellular adenine metabolism in rat brain[J].Neuroscience,2012,223:28-34.
[20]Sukrong S,Yun KY,Stadler P,et al.Improved growth and stress tolerance in the arabidopsis oxt1 mutant triggered by altered adenine metabolism[J].Mol Plant,2012,5(6):1310-1332.
[21]Xiang T,Li L,Yin X,et al.The ubiquitin peptidase UCHL1 induces G0/G1cell cycle arrest and apoptosis through stabilizing p53 and is frequently silenced in breast cancer[J].PLoS One,2012,7(1):e29783.
[22]Tenga MJ,Lazar IM.Proteomic study reveals a functional network of cancer markers in the G1-Stage of the breast cancer cell cycle[J].BMC Cancer,2014,14:710.
[23]Kurosaka K,Takahashi M,Watanabe N,et al.Silent cleanup of very early apoptotic cells by macrophages[J].J Immunol,2003,171(9):4672-4679.
[24]Zhang L,Tong ZS,Li SF,et al.Inhibitory effects of paclitaxel liposome on ovarian cancer cell[J].Journal of Practical Oncology,2012,27(1):22-28.(in Chinese) 张丽,佟仲生,李淑芬,等.紫杉醇脂质体对卵巢癌细胞生长抑制作用的实验研究[J].实用肿瘤杂志,2012,27(1):22-28.
[25]Lu YS,Kashida Y,Kulp SK,et al.Efficacy of a novel histone deacetylase inhibitor in murine models of hepatocellular carcinoma[J].Hepatology,2007,46(4):1119-1130.
[26]Wang XJ,Xiang SL,Tang HL,et al.Effects of diallyl disulfide on proliferation and cell cycle of human glioblastoma U251 cells[J].Journal of Practical Oncology,2014,29(2):126-132.(in Chinese) 汪雪菁,向姝霖,唐海林,等.二烯丙基二硫对人胶质瘤U251细胞增殖及细胞周期的影响[J].实用肿瘤杂志,2014,29(2):126-132.
(本文编辑:崔丽红)
Empirical Research of Gecko Active Monomer Derivative Adenine on Human Hepatic Carcinoma Cells
HANMing,CHENGXin,ZHAORong-rong,etal.
BasicMedicalCollege,WeifangMedicalUniversity,Weifang261053,China
Objective To study the inhibitory effect of gecko active monomer derivative adenine on human hepatic carcinoma cells.Methods Human liver cancer Bel-7402 cells in logarithmic phase and conducted experiments were collected in the study from September 2014 to April 2015.The 2nd to 9th columns of the 96 hole plate was assigned into the adenine group (adenine concentrations were 0.500 00,0.250 00,0.125 00,0.062 50,0.031 25,0.015 63,0.007 81 and 0.003 91 mg/ml,which were respectively recorded as group A to group H).The 10th column was taken as the control group (RPMI-1640 culture medium containing 10% fetal bovine serum).The 11th column was taken as the blank group.Thiazolyl blue method (MTT) was used to observe the tumor inhibition rate of each group (at 24 h,48 h and 60 h of the culture) and the 50% inhibiting concentration (IC50) of the adenine group.The human liver cancer Bel-7402 cells in logarithmic phase were divided into adenine group (0.500 00 mg/ml adenine) and control group (RPMI-1640 culture medium containing 10% fetal bovine serum),and the cell ultrastructure was observed by transmission electron microscope.The collected human liver cancer Bel-7402 cells in logarithmic phase were divided into adenine group (0.500 00 mg/ml adenine) and control group (RPMI-1640 culture medium containing 10% fetal bovine serum),and tumor cell cycle was detected by flow cytometry.Results At 24 h,tumor inhibition rates of group B-group H were lower than those of group A;tumor inhibition rates of group C-group H were lower than group B;tumor inhibition rates of group D-group H were lower than group C;tumor inhibition rates of group E-group H were lower than group D;tumor inhibition rates of group E-group G were higher than H group (P<0.05).At 48 h,tumor inhibition rates of group B -group H were lower than those of group A;tumor inhibition rates of group C-group H were lower than group B;tumor inhibition rates group D-group H were lower than group C;tumor inhibition rates of group D and group E were higher than group H (P<0.05).At 60 h,tumor inhibition rates of group B-group H were lower than that of group A;tumor inhibition rates of group C-group H were lower than group B;tumor inhibition rate of group D-group H were lower than group C;tumor inhibition rates of group D-group F were higher than group G and group H (P<0.05).Tumor inhibition rates of group C and group E-group H at 24 h were lower than those at 48 h.Tumor inhibition rates of group A-group F at 24 h were lower than those at 60 h.Tumor inhibition rate of group G at 24 h was higher than that at 60 h.Tumor inhibition rates of group A-group D at 48 h were lower than those at 60 h.Tumor inhibition rates of group G and goupr H at 48 h were higher than those at 60 h(P<0.05).IC50varied significantly at different time points (F=16.816,P=0.000).The IC50at 48 h and 60 h was lower than that at 24 h;the IC50at 60 h was lower than that at 48 h (P<0.05).After 48h,the changes of mitochondria in liver cancer cells were obvious.The numbers of cells in G0/G1and G2/M phase in control group were higher than those in adenine group;the number of cells in S phase in control group was lower than that in adenine group (P<0.05).In adenine group,the number of cells in S phrase was higher than that in G0/G1phrase,and the number of cells in G2/M phrase was lower than that in S phrase (P<0.05).Conclusion Adenine could inhibit the proliferation of human hepatic carcinoma cells in a time and dose dependent manner.Adenine lead to changes of mitochondria.Adenine could inhibit tumor cells in S phase and further induce tumor cell apoptosis,thus playing an anti-tumor effect.
Liver neoplasms;Gecko active monomer;Adenine;Apoptosis
国家自然科学基金资助项目(81202992,81102735);山东省自然科学基金资助项目(ZR2011HQ023,ZR2014HP008);山东省医药卫生科技发展计划项目(2011QZ026)
261053 山东省潍坊市,潍坊医学院基础医学院(韩明),医学影像学系(程鑫,赵荣荣,张仕状)
张仕状,261053 山东省潍坊市,潍坊医学院医学影像学系;E-mail:zhangsz5712@163.com
R 735.7
A
10.3969/j.issn.1007-9572.2015.27.011
2015-05-14;
2015-07-02)
