解整合素-金属蛋白酶17在肺泡上皮细胞间质转化中的作用研究
2015-03-21周玲玲刘敬禹
周玲玲,刘敬禹
辽宁医学院附属第三医院 呼吸科,辽宁锦州 121000
解整合素-金属蛋白酶17在肺泡上皮细胞间质转化中的作用研究
周玲玲,刘敬禹
辽宁医学院附属第三医院 呼吸科,辽宁锦州 121000
目的探讨解整合素-金属蛋白酶17(a disintegrin and metalloproteinase-17,ADAM 17)过表达和抑制在转化生长因子-β1(transforming growth factor-beta 1,TGF-β1)介导的A549细胞上皮向间质转化(epithelial-to-mesenchymal transition,EMT)过程中的作用,以进一步揭示肺纤维化的机制。方法将A549细胞分为空白对照组、TGF-β1组、PMA(ADAM 17激活剂)组和TNF484(ADAM 17抑制剂)组。各组细胞培养36 h后,应用倒置相差显微镜观察各组细胞形态,Real-time PCR和Western blotting分别检测ADAM 17、上皮及间质细胞标记物在mRNA和蛋白水平上的表达情况。结果镜下观察发现未诱导的A549细胞呈鹅卵石形态,排列比较紧密;经TGF-β1诱导后,细胞形态伸长,出现伪足,排列较松散,细胞与细胞之间的紧密连接消失。Real-time PCR和Western blotting检测结果均显示ADAM 17在PMA组高表达,而在TNF484组低表达,差异有统计学意义;上皮细胞标记物E-cadherin在空白对照组和TNF484组高表达,间质细胞标记物Vimentin在TGF-β1组和PMA组高表达,差异同样有统计学意义。结论ADAM 17的过表达有助于TGF-β1介导的A549细胞EMT进程,提示ADAM 17可作为肺纤维化的标记物之一。
上皮细胞间质转化;解整合素-金属蛋白酶17;肺纤维化
特发性肺纤维化是最常见的肺间质疾病,也是肺间质纤维化的主要原因,多数肺间质疾病病因不明,其发病机制目前也未完全阐明。肺泡上皮细胞通过上皮-间质转化(epithelial-tomesenchymal transition,EMT)作为发病的促动因素,已得到学者们的广泛关注[1-2]。EMT是一种完全分化的上皮细胞经历细胞表型改变转化成完全分化的间质细胞的过程,通常转化成成纤维细胞和肌纤维母细胞[3],参与多种器官的纤维化,包括肺和肾等[4-5]。解整合素-金属蛋白酶17(a disintegrin and metalloproteinase-17,ADAM 17)属于ADAMs家族成员之一,又称肿瘤坏死因子α转化酶,在多种跨膜蛋白分子(如肿瘤坏死因子α、表皮生长因子等)胞外域的剪切脱落过程中起重要作用[6],参与炎症和多种增生型疾病的进程,与肾纤维化和其他慢性肾疾病有关[7-8]。因此,推测ADAM 17可能作用于EMT过程并参与肺纤维化。为此,本研究应用转化生长因子-β1(transforming growthfactor-beta 1,TGF-β1)诱导A549细胞发生EMT,添加PMA(ADAM 17激活剂)或TNF484(ADAM 17抑制剂),Real-time PCR和Western blotting分别检测ADAM 17、上皮(E-cadherin)及间质(Vimentin)细胞标记物在mRNA和蛋白水平上的表达情况,以阐明ADAM 17在EMT过程中的作用。
材料和方法
1 主要试剂 A549细胞株购于中国典型培养物保藏中心,RPMI 1640培养基、胎牛血清(fetal bovine serum,FBS)和胰蛋白酶购于Hyclone公司,重组人TGF-β1购自R&D公司,PMA和TNF484购自Sigma公司,兔抗ADAM 17、E-cadherin和Vimentin多克隆抗体购自Santa Cruz公司,RNAiso Plus、DNaseⅠ、PrimeScript™Ⅱ1st Strand cDNA Synthesis Kit和SYBR®Premix Ex Taq™Ⅱ购于TaKaRa公司。
2 A549细胞的培养与诱导 A549细胞用含10% FBS的RPMI 1640培养基于37℃、5% CO2培养箱中培养,每3~4 d换1次液,待细胞达到80%融合后,用0.25%胰酶和0.02% EDTA消化,收集细胞传代。应用TGF-β1诱导细胞发生EMT,TGF-β1组培养基中仅添加5 ng/ml TGF-β1,PMA组和TNF484组培养基中除添加5 ng/ml TGF-β1外,分别再添加100 ng/ml PMA和TNF 484。培养36 h后,应用倒置相差显微镜观察各组细胞形态。
3 Real-time PCR检测ADAM 17、上皮及间质细胞标记物的表达 取培养36 h后的各组细胞,分别加入RNAiso Plus提取总RNA,常规DNaseⅠ消化,琼脂糖凝胶电泳检测质量,紫外分光光度计检测纯度和浓度,用RNase-free水稀释为1 μg/ μl,按照TaKaRa反转录试剂盒(PrimeScript™Ⅱ1st Strand cDNA Synthesis Kit)说明进行反转录,得到的cDNA样品稀释4倍,加入SYBR®Premix Ex Taq™Ⅱ和检测引物(表1),以GAPDH为内参基因,在Chromo 4™多色实时定量PCR仪(Biorad)上进行40个循环的PCR反应,应用Opticon-3软件对反应结果进行定量分析。每次定量分析检测样品设置3个重复,以3批样品进行独立表达分析。
4 Western blotting检测ADAM 17、上皮及间质细胞标记物的蛋白表达量 取培养36 h后的各组细胞,分别加入RIPA裂解液超声破碎,4℃,12 000× g离心25 min,取上清,用BCA法测定蛋白含量。取样进行SDS-PAGE分离后,转膜,经4% BSA封闭1 h;加入一抗(1∶200稀释),4℃过夜;加入HRP标记的羊抗兔IgG抗体(1∶1 000稀释),4℃摇床孵育1 h。采用UVP全自动凝胶成像系统ECL发光显影,以β-actin作为内参。
5 统计学分析 应用SPSS 13.0统计软件处理数据,统计资料以±s表示,组间比较进行t检验,P<0.05为差异有统计学意义。
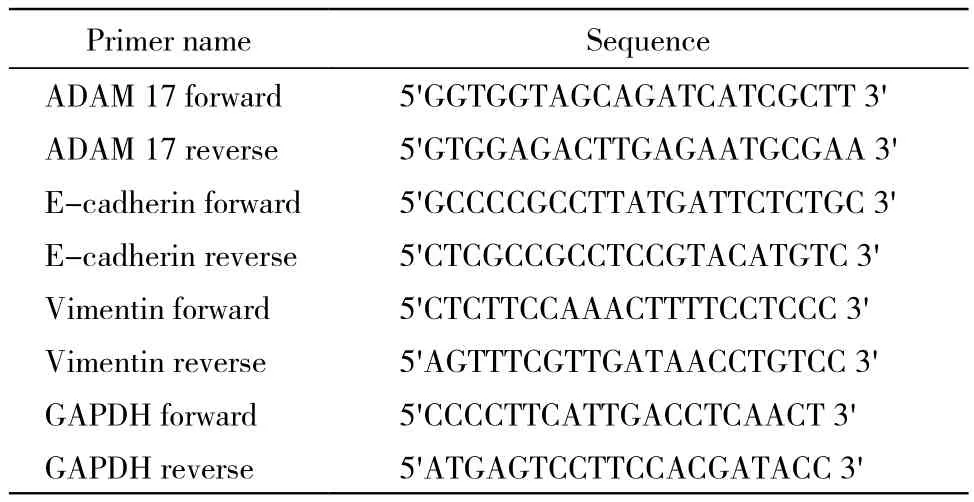
表1 引物序列Tab.1 Primer sequences
结 果
1 光镜下细胞形态学观察 未诱导的A549细胞呈鹅卵石形态,排列比较紧密(图1A);经TGF-β1诱导后,细胞形态伸长,出现伪足,排列较松散,细胞与细胞之间的紧密连接消失(图1B);添加ADAM 17激活剂培养的细胞均匀分布,呈长梭形,出现典型的纤维细胞形态(图1C);而添加ADAM 17抑制剂的细胞仅有少部分呈现间质细胞形态,排列仍较紧密,上皮性明显(图1D)。
2 ADAM 17、E-cadherin和Vimentin mRNA的表达量 Real-time PCR结果显示,如果将空白对照组ADAM 17 mRNA的表达量设定为1,则PMA组是空白对照组的(2.061±0.287)倍,而TNF484组是空白对照组的(0.634±0.172)倍,差异有统计学意义。同样,如果将空白对照组E-cadherin mRNA的表达量设定为1,则TGF-β1组是空白对照组的(0.435±0.141)倍,而PMA组是空白对照组的(0.213±0.097)倍,差异有统计学意义。如果将TGF-β1组也就是阳性对照组Vimentin mRNA的表达量设定为1,则空白对照组和TNF484组分别是阳性对照组的(0.124±0.035)倍和(0.397±0.126)倍,而PMA组是阳性对照组的(1.982±0.225)倍,差异有统计学意义(图2)。结果表明,ADAM 17的过表达有助于TGF-β1介导的A549细胞EMT进程。
3 ADAM 17、E-cadherin和Vimentin蛋白的表达量 Western blotting检测结果显示,ADAM 17蛋白表达量在PMA组最高,在TNF484组最低,表明ADAM 17激动剂和抑制剂的效果明显。上皮细胞标记物E-cadherin蛋白在空白对照组和TNF484组高表达,相反,间质细胞标记物Vimentin蛋白在TGF-β1组和PMA组高表达(图3),这就直观地解释了ADAM 17的过表达和抑制分别对A549细胞EMT进程的促进和削弱,与Real-time PCR结果相吻合。
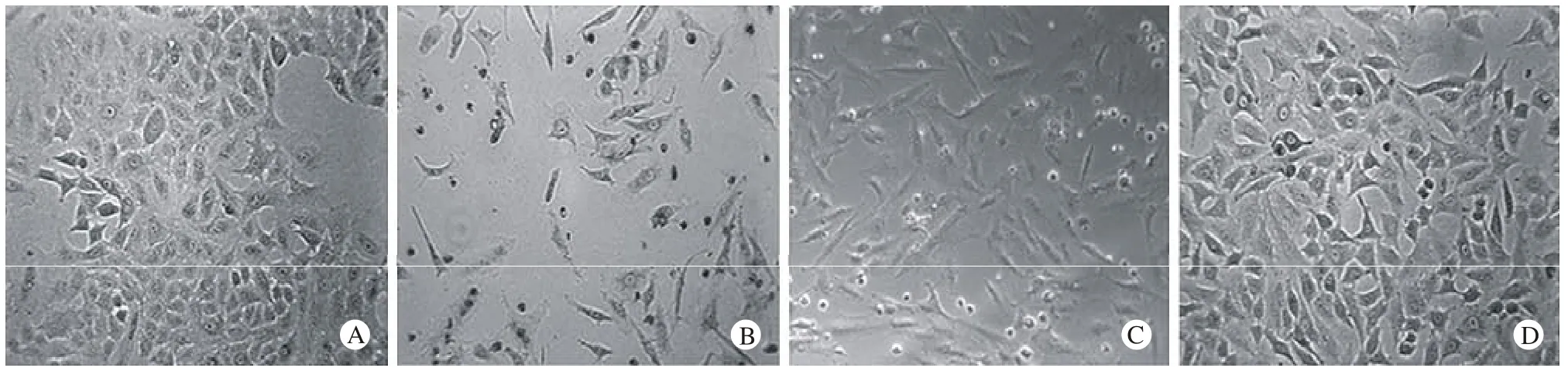
图 1 光镜下A549细胞形态(×200)Fig. 1 A549 cell morphology detected by light microscopy (×200)
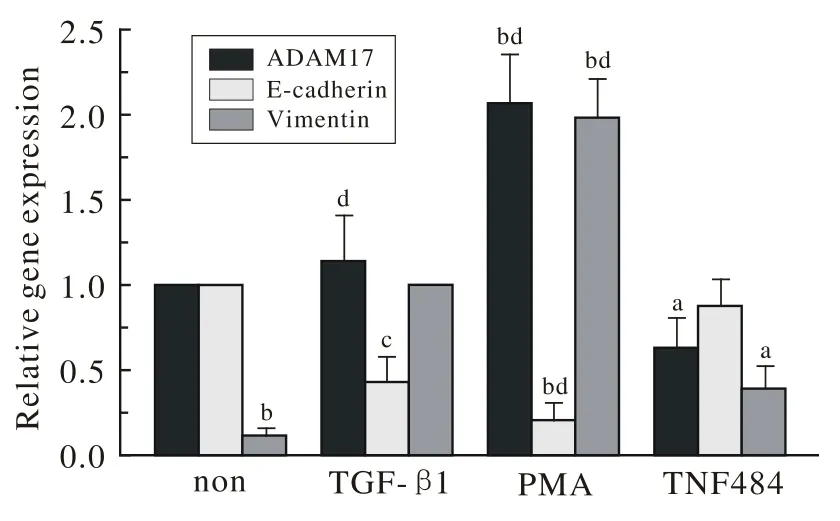
图 2 ADAM 17、 E-cadherin和Vimentin mRNAs在各处理组中的相对表达水平Fig. 2 Relative expression level of ADAM 17, E-cadherin andVimentin mRNAs in all groups
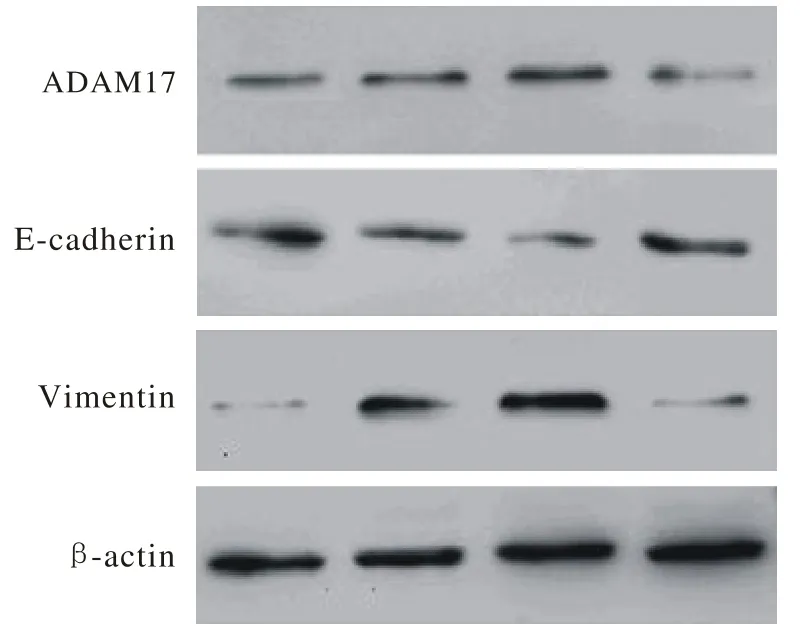
图 3 ADAM 17、 E-cadherin和Vimentin蛋白在各处理组中的表达水平Fig. 3 Expression level of ADAM 17, E-cadherin and Vimentin proteins in all groups
讨 论
特发性肺纤维化肺实质纤维灶内间充质细胞主要包括成纤维细胞和肌纤维母细胞,这些细胞大量增生并伴随着细胞外基质的沉积。但是,对于纤维灶具体的形成机制和细胞来源目前仍不清楚,主要认为先有肺泡上皮细胞的损伤而后出现纤维灶,推测肺泡上皮细胞除了通过自身的损伤与修复反应以外,由诱导或促进损伤的肺泡上皮细胞通过EMT过程发生转化亦起重要作用[9]。发生EMT转化的细胞可促进纤维灶的增生和发展,并产生大量的细胞外基质。TGF-β1是肺纤维化的关键性细胞因子,能刺激未成熟成纤维细胞生长,并促使组织的成纤维细胞聚集、扩散和分化[10-11]。TGF-β1能诱导和促进胶原蛋白的产生和沉积,通过自分泌或旁分泌作用,促进细胞增殖和细胞外基质沉积[12]。本研究应用TGF-β1诱导A549细胞,发现呈鹅卵石形态、排列较紧密的A549细胞诱导后形态伸长,排列较松散,细胞与细胞之间的紧密连接消失;Real-time PCR和Western blotting结果显示,诱导后上皮细胞标记物E-cadherin显著降低,而间质细胞标记物Vimentin明显升高,这表明TGF-β1成功诱导A549细胞发生EMT过程。
ADAM 17作为机体正常发育必不可少的细胞因子,其在炎症中也发挥重要作用,主要通过TNF-α的脱落,细胞黏附分子穿过血管壁引发白细胞迁移[13]。ADAM17参与表皮生长因子受体胞外域的脱落,引发下游级联反应,导致细胞增殖、迁移或凋亡[14]。ADAM17与TGF-α共同存在于肾纤维化中,对于人类肾细胞ADAM17可通过促进TGF-α脱落而发挥致纤维化的作用[15]。本研究在TGF-β1诱导A549细胞过程中过表达和抑制ADAM17,形态学发现添加ADAM 17激活剂培养的细胞呈长梭形,出现典型的纤维细胞形态;而添加ADAM 17抑制剂的细胞仅有少部分呈现间质细胞形态,排列仍较紧密,上皮性明显。Realtime PCR和Western blotting结果显示,TNF484组高表达E-cadherin,而PMA组高表达Vimentin,表明ADAM 17的过表达有助于TGF-β1介导的A549细胞EMT进程,提示ADAM 17可作为肺纤维化的标记物之一。
1 Xi Y, Tan K, Brumwell AN, et al. Inhibition of epithelial-tomesenchymal transition and pulmonary fibrosis by methacycline[J]. Am J Respir Cell Mol Biol, 2014, 50(1): 51-60.
2 Rosas IO, Kottmann RM, Kottman RM, et al. New light is shed on the enigmatic origin of the lung myofibroblast[J]. Am J Respir Crit Care Med, 2013, 188(7): 765-766.
3 Zavadil J, Böttinger EP. TGF-beta and epithelial-to-mesenchymal transitions[J]. Oncogene, 2005, 24(37):5764-5774.
4 Schneider DJ, Wu M, Le TT, et al. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-β production and epithelial to mesenchymal transition[J]. FASEB J, 2012, 26(2):503-512.
5 Kim MK, Maeng YI, Sung WJ, et al. The differential expression of TGF-β1, ILK and wnt signaling inducing epithelial to mesenchymal transition in human renal fibrogenesis: an immunohistochemical study[J]. Int J Clin Exp Pathol, 2013, 6(9): 1747-1758.
6 Allinson TM, Parkin ET, Turner AJ, et al. ADAMs family members as amyloid precursor protein alpha-secretases[J]. J Neurosci Res, 2003, 74(3): 342-352.
7 Dreymueller D, Martin C, Schumacher J, et al. Smooth muscle cells relay acute pulmonary inflammation via distinct Adam17/ErbB axes[J]. J Immunol, 2014, 192(2): 722-731.
8 Mulder GM, Melenhorst WB, Celie JW, et al. Adam17 up-regulation in renal transplant dysfunction and non-transplant-related renal fibrosis[J]. Nephrol Dial Transplant, 2012, 27(5): 2114-2122.
9 Ohbayashi M, Kubota S, Kawase A, et al. Involvement of epithelialmesenchymal transition in methotrexate-induced pulmonary fibrosis[J]. J Toxicol Sci, 2014, 39(2): 319-330.
10 Chen MJ, Gao XJ, Xu LN, et al. Ezrin is required for epithelialmesenchymal transition induced by TGF-β1 in A549 cells[J]. Int J Oncol, 2014, 45(4): 1515-1522.
11 Liu RY, Zeng Y, Lei Z, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells[J]. Int J Oncol, 2014, 44(5): 1643-1651.
12 Deng B, Tan QY, Wang RW, et al. P130cas is required for TGF-β1-mediated epithelial-mesenchymal transition in lung cancer[J]. Oncol Lett, 2014, 8(1): 454-460.
13 Schwarz J, Broder C, Helmstetter A, et al. Short-term TNFα shedding is Independent of cytoplasmic phosphorylation or furin cleavage of Adam17[J]. Biochim Biophys Acta, 2013, 1833(12):3355-3367.
14 Dang M, Armbruster N, Miller MA, et al. Regulated Adam17-dependent EGF family ligand release by substrate-selecting signaling pathways[J]. Proc Natl Acad Sci U S A, 2013, 110(24): 9776-9781.
15 Melenhorst WB, Visser L, Timmer A, et al. Adam17 upregulation in human renal disease: a role in modulating TGF-alpha availability?[J]. Am J Physiol Renal Physiol, 2009, 297(3): F781-F790.
Role of ADAM17 during epithelial - mesenchymal transition in human lung alveolar epithelial cells
ZHOU Lingling, LIU Jingyu
Department of Respiratory, The Third Affiliated Hospital of Liaoning Medical University, Jinzhou 121000, Liaoning Province, China
LIU Jingyu. Email: liujingyu0416@163.com
ObjectiveTo investigate the role of ADAM17 in epithelial - mesenchymal transition (EMT) of A549 cells and the mechanism underlying pulmonary fibrosis.MethodsA549 cells were equally divided into four groups: blank control group,TGF-β1-mediated group, PMA (the activator of ADAM 17) group and TNF484 (the inhibitor of ADAM17) group. A549 cells were cultured in vitro, cellular morphology changes after 36 h were observed by phase contrast microscope. The mRNA and protein expressions of ADAM17 and the markers of epithelial cell and mesenchymal cell were determined by Real-time PCR and Western blotting.ResultsA549 cells lined up tightly in the blank control group, however, after mediated by TGF-β1, the cells became spindle and loosen. Real-time PCR and Western blotting results showed that the expression of ADAM 17 were higher in PMA group and lower in TNF484 group than that in blank control group, which had significant difference. The high expression of E-cadherin in blank control group and TNF484 group and the high expression of Vimentin in TGF-β1-mediated group and PMA group were also of significant difference.ConclusionHigh expression of ADAM 17 contributes to the EMT of alveolar epithelial cells, which suggests that ADAM 17 can be one of fundamental mechanisms of pulmonary fibrosis.
epithelial-to-mesenchymal transition; a disintegrin and metalloproteinase-17; pulmonary fibrosis
R 329.28
A
2095-5227(2015)03-0272-04
10.3969/j.issn.2095-5227.2015.03.019
时间:2014-11-20 15:18
http://www.cnki.net/kcms/detail/11.3275.R.20141120.1518.001.html
2014-09-09
周玲玲,女,硕士。研究方向:间质性肺病。Email: sui feng800sd@163.com
刘敬禹,男,博士,主任医师,主任。Email: liujingyu04 16@163.com
