A fast-response,soluble and multichromic copolymer based on 1,5-diyl(N-(6-hexanoic acid-1-yl)-pyrrole)-naphthalene and 3,4-ethylenedioxythiophene
2015-03-13ShenYANGLeiLILiuyangZHOUWenLUO
Shen YANG,Lei LI,Liuyang ZHOU,Wen LUO
(The Key Laboratory of Rare Earth Functional Materials and Applications,Zhoukou Normal University,Zhoukou 466001,China)
A fast-response,soluble and multichromic copolymer based on 1,5-diyl(N-(6-hexanoic acid-1-yl)-pyrrole)-naphthalene and 3,4-ethylenedioxythiophene
Shen YANG,Lei LI,Liuyang ZHOU,Wen LUO*
(The Key Laboratory of Rare Earth Functional Materials and Applications,Zhoukou Normal University,Zhoukou 466001,China)
A copolymer of 1,5-diyl(N-(6-hexanoic acid-1-yl)pyrrole)-naphthalene(DPN)and 3,4-ethylenedioxythiophene(EDOT)was electrochemically synthesized and characterized.Spectroelectrochemical analysis reveals that the copolymer film not only has distinct electrochromic properties,but also exhibits a maximum optical contrast of 40%at 1 100 nm and fast switching time of 0.6 s.
electrochemically synthesized;electrochromic;optical contrast;switching time
Conducting polymerspossessing electrochromic(EC)properties have drawn extensive attentions due to their widely applications in the fields of thin-film transistors,displays[1],energysaving“smart”windows[2]and memory devices[3].Up to now,many kinds of EC polymers have been developed.However,most polymers are limited with two colors and poor solubility,the exploration of new polymers with multiple colored states,good solubility and fast switching time are still the focus of research.The color change of EC materials is related to doping-dedoping process,which can lead to the variations of band gap[4,5].Besides,structural modification and copolymerization can also adjust the band gap of polymers to achieve different properties of EC materials[6,7].
Structural modification is a common method to control the resulting properties by introducing some functional groups into the main-chain or the pendant-chain of polymers,such as leading to the formation of soluble polymers by introducing the alkyl chains[8,9],getting the polymers of better thermal stability by importing the aromatic groups[10],adjusting the electropolymerization potentials of polymers by bringing the electron withdrawing and donating groups into polymers[11]or forming the conjugatedπ-bonds system Polypyrrole is one of the most stable EC materials and its thin film has widely applications in the fields of photo-electronic technology[12,13].Moreover,different properties can be achieved by structural modification in 2,5 and N-position of pyrrole[14].
On the other hand,copolymerization is an effective way to obtain the desired properties of conducting polymers.Fine tuning in the band gap can be achieved by tailoring the co-monomer feed ratio of copolymerization[15].EDOT is a good choice as a co-monomer since it produces a lowband gap polymer with high stability and good conductivity.However,PEDOT is insoluble,which greatly affects its application.Much effort has been made to overcome such a drawback.In our previous work,copolymer based on pyrrole derivative and EDOT was electrochemically synthesized[16].It isn’t only soluble,but also shows six colors at different potentials and exhibit better performances.
Recently,several researches show that the polymer with a multifold and high surface areas hold the faster switching time due to that the intercalation of ions can be accelerated during the process of doping in this paper,1,5-diyl(N-(6-hexanoic acid-1-yl)-pyrrole)-naphthalene(DPN)was designed and synthesized(Scheme.1),whose structure may make it also have good solubility and get the high surface areas when polymerized.The copolymer based on DPN and EDOT has been also successfully synthesized and characterized.The resultant copolymer not only presents distinct color change but also possesses faster switching response and good electrochemical stability.

Scheme 1 Synthesis route of monomer DPN
1 Experimental
1.1 Materials
1,5-Naphthalenediol(NOL)(aladdin),3,4 -ethylenedioxythiophene(EDOT)(Aldrich),2,5-dimethoxytetrahydrofuran(Aldrich),4-dimethyl-amino-pyridine(DMAP)(aladdin)and dicyclohexylcarbodiimide(DCC)(aladdin).Tetrabutyl ammonium perchlorate(TBAP)(Acros Organics).Dichloromethane and acetonitrile(ACN)were distilled from Ca H2.N-(6-Hexanoic acid-1-yl)-pyrrole was synthesized according to the literature procedure.
1.2 Equipments
A Nicolet 6700 Fourier-transform infrared spectrometer(FTIR)(Thermo Fisher Nicolet,USA)was used to measure the infrared spectra.NMR spectra of the synthesized products were recorded on a Bruker AVANCE III instrument(Bruker,Switzerland)at 500 MHz(1H)and 125 MHz(13C).Mass spectrometry(MS)analysis was recored on a GCT Premier spectrometer(Waters,USA)using the electron impact(EI)mass spectra technique.A CHI660C electrochemical analyzer(CH Instruments,China)was used to perform the electrochemical measurements.UV–vis spectra were recorded on a Varian Cary 100 UV–vis spectrophotometer(Varian,USA).
1.3 Synthesis of DPN
NOL(1.6 g,10 mmol)and DMAP(0.11 g,0.90 mmol)were added to a stirred solution of N -(6-hexanoic acid-1-yl)-pyrrole(3.62 g,20 mmol)in a mixture of anhydrous CH2Cl2(20mL).DCC(4.20 g,20 mmol)was dropped into the reaction mixture at 0℃,which was stirred for 5 min,and then 4 h at room temperature.Precipitated solid was filtered off and the filtrate evaporated down in vacuum,then the residue was purified by elution chromatography on a silica column with hexane∶ethyl acetate(5∶1).2.62 g(54%)of product was obtained as a pale power.1H NMR(500 MHz,CDCl3)δ:7.73(d,2 H),7.48(t,2H),7.26(d,2H),6.67(d,4H),6.16(t,4 H),3.93(t,4 H),2.73(t,4 H),1.94(m,8H),1.81(m,4H).13C NMR(125 MHz,CDCl3)δ:171.79,146.70,128.20,126.05,120.49,119.17,118.78,108.01,49.35,34.16,31.30,26.34,24.60.FT-IR(KBr,cm-1):1 751(C=O);2 928,1 504(C-H);3 097,733[CH(pyr)];1 504[C-C(pyr)];1 600,1 455[C= C(Ar)];1 089,946,795[C-H(Ar)];1 185[C-O -C(as)];1 127[C-O-C(s)].MS(EI):calculat-ed for C30H34N2O4m/z:486.6,found m/z:486.2.For C30H34N2O4(%)calcd:C,74.05;H,7.04;N,5.76;O,13.15.Found:C,74.10;H,7.08;N,5.69;O,13.13.
1.4 Electrochemistry
The electro-syntheses and measurements were performed in a conventional three-electrode cell with an ITO-coated glass(CSG holding Co.LTD,the active area:1.0 cm×2.0 cm)as working electrode which was sequencely washed with ethanol,acetone and deionized water under ultrasonic before use,a platinum sheet and a doublejunction Ag/AgCl electrode(silver wire coated with AgCl in saturated KCl solution,0.1mol/L TBAP in ACN/DCM(1∶1,by volume)solution as the second junction)were applied as the counter electrode and the reference electrode,respectively.All the electrochemistry experiments were carried out at 25℃under N2atmosphere.
1.5 Synthesis of polymer films
The solutions of 5mmol/L DPN,5mmol/L EDOT and 5mmol/L/5mmol/L DPN-EDOT in ACN were prepared respectively.All polymer films were prepared via potentiostatic electrolysis on ITO electrodes,and the films were electrolyzed at negative potential for several minutes in monomer-free electrolytic solution,then the films were washed with clean ACN for several times to remove the residual supporting electrolyte and the monomers.
2 Results and discussion
2.1 Electrochemical polymerization
The successive Cyclic voltammogram(CV)curves of DPN,EDOT and the mixture of the two monomers in ACN containing 0.1mol/L TBAP between-1 and+1.5 V are illustrated in Fig.1.Well-defined polymer oxidation and reduction processes are observed in all the three CVs.The oxidation current increases with the increasing scanning cycle,and the observable polymer films can be formed on the working electrode surface.The increase in peak separation potential reflects the increase in resistance as the thickness of the polymer film increased.The CV of DPN presents the oxidation and reduction peaks at 0.76 and 0.63 V,respectively(Fig.1a).The CV of EDOT(Fig.1c)exhibits broad oxidation and reduction waves,which are ranged from+0.6 to-0.5 V.However,the CV of mixture(Fig.1b)also shows broad oxidation waves between 0.5 and 1.0 V.but an obvious reduction peak appears at 0.43 V,which is different from that of DPN and EDOT,indicating the formation of a new polymer consisting of both DPN and EDOT units.
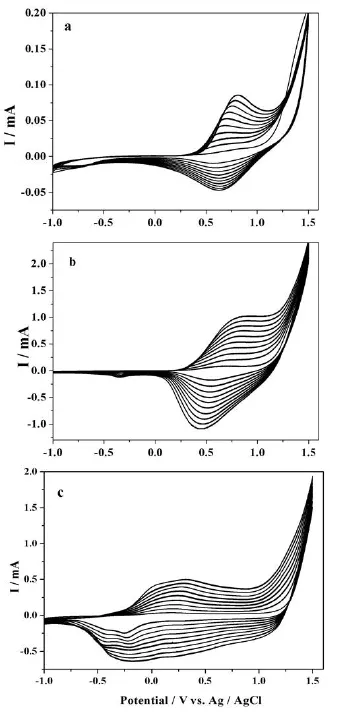
Fig.1 Cyclic voltammogram curves of(a)DPN,(b)DPN-EDOT,(c)EDOT in 0.1mol/L
2.2 Electrochemistry of polymers
The polymer films of P(DPN-EDOT)was prepared by constant potential electrolysis(1.2 V).Fig.2a shows the CV curves of the P(DPNEDOT)at different scanning rates between 50 and 550 m Vs-1.The film presents a well-defined redox process at about 0.55 V.The peak current densities are proportional to the potential scan rates(Fig.2b:inset picture),indicating that the electrochemical processes of the copolymer are reversible and not diffusion limited.
2.3 FT-IR spectra of polymers

Fig.2 (a)cyclic voltammogram curves of the P(DPN-EDOT)film in monomer free solution of 0.1mol/L TBAP/ACN at different scan rates(inset picture:scan rate dependence of the anodic and cathodic peak current densities graph.Ip.aand Ip.cdenote the anodic and cathodic peak current,respectively)
The FT-IR spectra of PDPN,P(DPNEDOT)and PEDOT films are shown in Fig.3.As seen from the spectrum of PDPN(Fig.3a),the bands located at 2 938 and 1 755 cm-1show the stretching modes of C-H in aromatic rings and C =O in ester bonds,respectively.While the band at 780 cm-1is attributed to the bending vibrations of C-H inβ-Carbon atom of pyrrole rings.The peak at 1 104 cm-1is assigned to the presence of Cl O4-.For the spectrum of PEDOT(Fig.3c),stretching vibrations of C=C and C-C in thiophene ring presented at 1 489,1 311 and 1 185 cm-1.The bands at 1 047 and 840 cm-1are attributed to the stretching of C-O-C and C-S -C in EDOT rings respectively.The spectrum of P(DPN-EDOT)(Fig.3b)exhibits almost the characteristic peaks of both PDPN and PEDOT.But the differences still exist.For example,the peak at 960 cm-1in the spectrum of PEDOT doesn’t appear.Besides,some non-neglectable shifts in the apectrum of P(DPN-EDOT)indicate the existence of interaction between pyrrole and thiophene units.For instance,the peak at 1 208 cm-1in the spectrum of P(DPN-EDOT)is not as obvious as the peak at 1 185 cm-1in the spectrum of PEDOT.The peak at 1 357 cm-1in the spectrum of P(DPN-EDOT)is sharper than the peak at 1 311 cm-1in that of PEDOT.
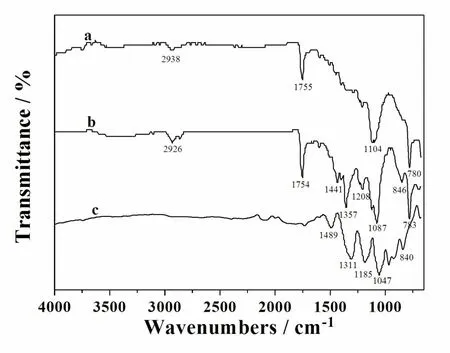
Fig.3 FT-IR spectra of the polymers
2.4 Electrochromic properties of polymer films
Spectroelectrochemistry is the best way of examining the changes in optical properties of a polymer upon applied potentials.The spectrochemical and electrochromic properties of the resultant films are studied in 0.1mol/L TBAP/ACN solution.Fig.4 depicted the different spectroelectrochemical spectra of the copolymer film ranging from-0.4 to+1.2 V,which contains all the peaks in the spectra of PDPN.Besides,a new peak is located at 485 nm,which is due to the introduction of EDOT to the polymer chain.Upon applied voltage,the evolution of polaron and bipolaron can be observed at about 750 nm and> 900 nm,respectively.The P(DPN-EDOT)film could exhibit six colors(from brick red to black),which is shown in inset pictures at different applied potentials.
2.5 Electrochromic switching of copolymer films
Electrochromic switching time and optical contrast are two of the most important characteristics for electrochromic applications.Electrochromic switching response of P(DPN-EDOT)films between-0.6 and 1.0 V with a residence time of 5 s at different wavelength are displayed in Fig.5.The max optical contrast of the copolymer is 40%with the switching time of 0.6 s(95%)at 1 100 nm,which is a little faster than our previous work.The improvement in switching time should be ascribed to the structure of copolymer with a smooth and high surface area.

Fig.4 UV-vis spectroelectrochemical spectra of P(DPNEDOT)films on ITO glass as applied potentials between-0.4 and 1.2 V in 0.1mol/L TBAP/ACN solution(Inset picture:Multichromic behavior copolymer at different potentials)

Fig.5 Electrochromic switching response for copolymer film monitored at 320,380,485 and 1 100 nm in 0.1mol/L TBAP/ACN solution between-0.6 and 1.1 V with a residence time of 5 s
3 Conclusions
The synthesis of anovel multicolored copolymer of DPN with EDOT was achieved in the presence of ACN solution.Spectroelectrochemical studied and electrochromic characterization methods showed that copolymerization with EDOT not only decreased the band gap(Eg),but also enhanced the electochromic properties such as optical contrast and switching time.The copolymer film can be expected to be applied in smart windows and other areas of EC displays.
[1]Mortimer R J,Dyer A L,Reynolds J R.Electrochromic organic and polymeric materials for display applications[J].Displays,2006,27:2-18.
[2]Azens A,Granqvist C G.Electrochromic smart windows:energy ef ficiency and device aspects[J].J.Solid State Electrochem.,2003,7:64-68.
[3]Moller S,Perlov C,Jackson W,et al.A polymer/semiconductor write-once read-many-times memory[J].Nature,2003,426:166-169.
[4]Koyuncu F B,Koyuncu S,Ozdemir E.A new donor-acceptor double-cable carbazole polymer with perylene bisimide penda[J].Electrochim.Acta.,2010,55:4935-4941.
[5]Ertas M,Cirpan A,Toppare L.Synthesis and characterization of conducting copolymer of(N1,N3-bis(thiophene-3 -ylmethylene)benzene-1,3-diamine-co-3,4-ethylenedioxythiophene)[J].Synth.Met.,2004,143:49-58.
[6]Sonmez G,Shen C K F,Rubin Y,et al.A Red,Green,and Blue(RGB)Polymeric Electrochromic Device(PECD):The Dawning of the PECD Era[J].Angew.Chem.Int.Ed.,2004,43:1523-1528.
[7]Kim Y H,Hwang J,Son JI,et al.Synthesis,electrochemical,and spectroelectrochemical properties of conductive poly -[2,5-di-(2-thienyl)-1H-pyrrole-1-(p-benzoic acid)][J].Synth.Met.,2010,160:413-418.
[8]Aubert P H,Beouch L,Van F T,et al.Copolymers based on fluorene dyads mixed to 3,4-ethylenedioxythiophene units:Optical properties of random versus regular structures [J].Synth.Met.,2006,156:898-906.
[9]Cihaner A,Alg F.Processable electrochromic and fluorescent polymers based on N-substituted thienylpyrrole[J].Electrochim.Acta.,2008,54:665-670.
[10]Yin B B,Jiang C Y,Wang Y G,et al.Synthesis and electrochromic properties of oligothiophene derivatives[J].Synth.Met.,2010,160:432-435.
[11]IsO D,Koyuncu F B,Koyuncu S,et al.A new imine coupled pyrrole-carbazole-pyrrole polymer:Electro-optical properties and electrochromism[J].Polymer,2010,51:1663 -1669.
[12]Celebi S,Balan A,Epik B,et al.Donor acceptor type neutral state green polymer bearing pyrrole as the donor unit[J].Org.Electron.,2009,10:631-636.
[13]Varis S,Ak M,Akhmedov I M,et al.A novel multielectrochromic copolymer based on 1-(4-nitrophenyl)-2,5-di(2-thienyl)-1H-pyrrole and EDOT[J].J.Electroanal.Chem.,2007,603:8-14.
[14]Ak M,Tanyeli C,Akhmedov I M,et al.Optoelectrochemical properties of the copolymer of 2,5-di(4-methylthiophen -2-yl)-1-(4-nitrophenyl)-1H-pyrrole monomerwith 3,4-ethylene dioxythiophene[J].Thin Solid Films,2008,516:4334-4341.
[15]Zhang C,Hua C,Wang G H,et al.A novel multichromic copolymer via electrochemical copolymerization of(S)-1,1′-binaphthyl-2,2′-diyl bis(N-(6-hexanoic acid-1-yl)pyrrole)and 3,4-ethylenedioxythiophene[J].Electrochim.Acta.,2010,55:4103-4111.
[16]Berger S,Ghicov A,Nah Y C,et al.Transparent TiO2Nanotube Electrodes via Thin Layer Anodization:Fabrication and Use in Electrochromic Devices[J].Langmuir,2009,25:4841-4844.
O632.7
:A
:1671-9476(2015)05-0085-06
10.13450/j.cnkij.zknu.2015.05.022
Received date:2015-03-17
Foundation item:Youth Fund of Zhoukou Normal University(No.zksyqn201307B);Scientific and Technological Department Project of Henan Province(No.132102210193)
Biography:LUO Wen(1984-),female,teaching assistant,born in Luohe city,Henan province.Researching interest is focused in organic and inorganic hybrid materials.E-mail:luowen@zknu.edu.cn.
